Abstract
Face recognition abilities improve between adolescences and adulthood over typical development (TD), but plateau in autism, leading to increasing face recognition deficits in autism later in life. Developmental differences between autism and TD may reflect changes between neural systems involved in the development of face encoding and recognition. Here, we focused on whole-brain connectivity with the fusiform face area (FFA), a well-established face-preferential brain region. Older children, adolescents, and adults with and without autism completed the Cambridge Face Memory Test, and a matched car memory test, during fMRI scanning. We then examined task-based functional connectivity between the FFA and the rest of the brain, comparing autism and TD groups during encoding and recognition of face and car stimuli. The autism group exhibited underconnectivity, relative to the TD group, between the FFA and frontal and primary visual cortices, independent of age. Underconnectivity with the medial and rostral lateral prefrontal cortex was face-specific during encoding and recognition, respectively. Conversely, underconnectivity with the L orbitofrontal cortex was evident for both face and car encoding. Atypical age-related changes in connectivity emerged between the FFA and the R temporoparietal junction, and R dorsal striatum for face stimuli only. Similar differences in age-related changes in autism emerged for FFA connectivity with the amygdala across both face and car recognition. Thus, underconnectivity and atypical development of functional connectivity may lead to a less optimal face-processing network in the context of increasing general and social cognitive deficits in autism.
Keywords: autism, development, face recognition, fMRI, functional connectivity, FFA
Introduction
Autism is a neurodevelopmental disorder characterized by deficits in social cognition. One frequently studied social cognitive skill that is disrupted in autism is face recognition—the everyday social task of accurately recognizing a previously encoded individual face (see Weigelt, Koldewyn, & Kanwisher, 2013). Improvements in face recognition occur between late childhood/adolescence and adulthood in typical development (TD), but plateau between adolescence and adulthood in autism (Germine, Duchaine, & Nakayama, 2011; Greimel et al., 2014; O’Hearn, Schroer, Minshew, & Luna, 2010; Rump, Giovannelli, Minshew, & Strauss, 2009). Little is currently known about the neural substrates underlying this developmental difference in face recognition abilities between autism and TD. As face recognition likely involves multiple brain regions acting in concert, one outstanding question is: how might functional brain connectivity contribute to developmental face recognition impairments in autism?
The neural substrates underlying typical face recognition can be divided into core and extended face-processing regions. Core regions include the fusiform face area (FFA), superior temporal sulcus (STS), and occipital face area (OFA) (Haxby, Hoffman, & Gobbini, 2000). Of these regions, the FFA is the most face selective and its development is the most protracted, suggesting that the FFA may support fine-tuned, mature face recognition performance (Golarai et al., 2007; Scherf, Behrmann, Humphreys, & Luna, 2007). Extended face-processing regions include the amygdala (AMY), temporoparietal junction (TPJ), intraparietal sulcus (IPS), inferior frontal gyrus (IFG), anterior insula (AI), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and medial prefrontal cortex (mPFC) (Frith & Frith, 2007; Haxby et al., 2000; Haxby, Hoffman, & Gobbini, 2002). Recent research suggests that activation in extended face-processing regions may decrease with age in TD (Haist, Adamo, Han, Lee, & Stiles, 2013; Joseph, Gathers, & Bhatt, 2011), while the connectivity between these regions may increase with age (Joseph et al., 2012).
Functional magnetic resonance imaging (fMRI) studies examining developmental face processing in both TD and autism generally focus on neural activation of core face-processing regions. Developmental studies focusing on TD have demonstrated that, with age, FFA activation becomes more face-specific (i.e., activation to faces is maintained, while activation to other stimuli decreases) and the size of the FFA increases (i.e., number of voxels), especially from childhood through adolescence (Golarai et al., 2007; Golarai, Liberman, Yoon, & Grill-Spector, 2010; Haist et al., 2013; Scherf et al., 2007; Scherf, Luna, Avidan, & Behrmann, 2011; Scherf, Thomas, Doyle, & Behrmann, 2013). Furthermore, these changes may be linked to increases in face recognition performance (Golarai et al., 2007, 2010). Recent work examining age-related changes in activation of extended face-processing regions suggests neural activation may decrease with age in TD (Haist et al., 2013). In autism, reduced FFA activation, independent of age, in response to face stimuli has been reported (Humphreys, Hasson, Avidan, Minshew, & Behrmann, 2008; Pelphrey, Morris, McCarthy, & Labar, 2007; Pierce, Müller, Ambrose, Allen, & Courchesne, 2001; Pierce & Redcay, 2008; Schultz et al., 2000). Other studies, including those involving passive face viewing and working memory for faces, have reported no differences in FFA activation in adults with autism, relative to neurotypical adults (Hadjikhani et al., 2004; Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2007; Kleinhans et al., 2008). Taken together, from a developmental perspective, findings of FFA activation differences in adolescence with autism, but no differences in adulthood, suggests that development is delayed in autism during adolescence, but leads to a relatively typical endpoint (Scherf, Luna, Minshew, & Behrmann, 2010).
While local FFA function, as measured by neuronal activation magnitude, may eventually reach typical levels by adulthood in individuals with autism, age-related changes in the functional connectivity between the FFA and other core and extended face-processing regions may continue to be atypical and underlie the late increase in face recognition deficits in autism. In TD individuals, functional connectivity between core face-processing regions increases with age (Cohen Kadosh, Cohen Kadosh, Dick, & Johnson, 2011; Joseph et al., 2012) and may be associated with face recognition improvements (Cohen Kadosh et al., 2011). Functional connectivity between core and extended face-processing regions also change with age in TD and may play an important role in integrating information between early visual processing and emotional, and social cognitive regions (Fairhall & Ishai, 2007; Zhen, Fang, & Liu, 2013). In adults with autism, connectivity between core and extended face-processing regions is reduced relative to neurotypical adults (Kleinhans et al., 2008; Koshino et al., 2008). However, differences in connectivity between core face-processing regions and bottom-up visual processing regions, and top-down frontal control regions, have also been found between adults with autism and neurotypical adults (Kleinhans et al., 2008; Koshino et al., 2008). Recent studies focused on age-related functional connectivity changes in autism have also demonstrated that connectivity between core face-processing regions (e.g., STS and fusiform gyrus) and between core face-processing and subcortical reward-processing regions (e.g., striatum and fusiform gyrus) decreases with age in TD, but increases with age in autism, potentially leading to relative underconnectivity in childhood and overconnectivity in adulthood (Alaerts et al., 2015; Padmanabhan, Lynn, Foran, Luna, & O’Hearn, 2013). Thus, the current fMRI literature suggests that while neural activation within core face-processing regions may support typical face recognition development, connectivity between and within core and extended face-processing regions may provide insight into the neural mechanism underlying the atypical face recognition development in autism (Johnson, Grossmann, & Cohen, 2009). Together, this literature also suggests that atypical functional connectivity underlying disrupted face recognition development in autism may be more widespread than canonical face-processing regions.
Here, we examine age-related changes in task-related functional connectivity of the FFA during face and car stimuli encoding and recognition in autism relative to TD. First, we predict that face recognition performance will improve with age in TD, but not in autism, resulting in robust recognition differences in adulthood (O’Hearn et al., 2010). Next, we predict that, relative to the TD group, individuals with autism will demonstrate (a) reduced connectivity independent of age, (b) atypical age-related changes in connectivity, and (c) face-specific atypical age-related changes (i.e., more striking age-related differences for faces than cars) in connectivity between the FFA and other core and extended face-processing regions. This study provides the first examination of how age-related changes in FFA connectivity differ in autism during a developmental period when face recognition becomes increasingly impaired.
Method
Participants
We tested 14 older children, 16 adolescents, and 14 adults diagnosed with autism and an equal number of TD controls, matched for age, IQ and gender (See Table 1). Age groups were chosen, instead of treating age as a continuous variable, based on previous results showing differences in face recognition development between adolescence and adulthood (O’Hearn et al., 2010). This approach also increased power and avoided specifying an age-related trajectory that may be asymptotic or occur stepwise. One adolescent with autism was excluded from all analyses due to poor face recognition performance (> 2 SD from group mean). Additionally, two older children (one with autism and one TD) and one adult with autism were excluded from all analyses due to poor car recognition performance (> 2 SD from group mean).
Table 1.
| Variable | Demographic information
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Older Children | Adolescents | Adults | ||||||||||
|
| ||||||||||||
| Autism (N=13) | TD (N=13) | Autism (N=15) | TD (N=16) | Autism (N=13) | TD (N=14) | |||||||
|
| ||||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| Age (years) | 11.7 | 1.2 | 12.0 | 1.4 | 15.3 | 1.8 | 15.3 | 1.7 | 24.9 | 4.9 | 24.1 | 5.5 |
| FSIQ | 111.4 | 11.2 | 110.7 | 8.4 | 110.9 | 10.5 | 110.3 | 9.2 | 115.3 | 13.6 | 114.9 | 11.0 |
| VIQ | 107.1 | 10.7 | 107.3 | 10.0 | 108.3 | 12.6 | 108.0 | 11.0 | 113.9 | 12.9 | 111.7 | 11.6 |
| PIQ | 112.8 | 12.2 | 111.4 | 8.2 | 111.2 | 11.4 | 110.3 | 9.6 | 112.9 | 13.6 | 114.5 | 10.0 |
| ADOS | ||||||||||||
| Communication | 3.3 | 1.3 | 4.1 | 1.2 | 4.6 | 1.3 | ||||||
| Social | 7.1 | 1.8 | 8.1 | 2.3 | 8.3 | 3.0 | ||||||
| Full Score | 10.4 | 3.0 | 12.5 | 3.4 | 13.1 | 3.6 | ||||||
| ADI | ||||||||||||
| Social | 20.9 | 4.7 | 19.5 | 5.6 | 22.4 | 2.9 | ||||||
| Communication | 17.2 | 3.7 | 15.1 | 4.0 | 16.9 | 3.7 | ||||||
| Behaviors | 6.2 | 1.7 | 6.2 | 2.3 | 6.4 | 2.6 | ||||||
| Abnormality | 3.5 | 1.5 | 2.7 | 1.3 | 2.9 | 0.8 | ||||||
ADOS, Autism Diagnosis Observation Schedule; ADI, Autism Diagnostic Interview; M, Mean; SD, Standard Deviation; TD, Typical Development. FSIQ, Full Scale Intelligence Quotient; VIQ, Verbal Intelligence Quotient; PIQ, Performance Intelligence Quotient.
Participants were recruited through the University of Pittsburgh Autism Center for Excellence subject core (HD#055748) and other projects in the Laboratory of Neurocognitive Development. Participants were diagnosed with autism by an expert clinician using the Autism Diagnostic Interview (ADI) (Lord, Rutter, & Couteur, 1994) and the Autism Observation Schedule-G (ADOS) (Lord et al., 2000). Autism participants met cut-offs for autism on the ADI1 and autism or autism spectrum disorder on the ADOS. Exclusion criteria included: brain injury, uncorrected vision problems, drug abuse, neurological disorders or MRI contraindications (e.g., metal plates). Participants with a known autism etiology (e.g., Fragile X) were excluded. TD participants with a diagnosed learning disability or psychiatric disorder, or a first-degree relative with a psychiatric disorder, were excluded.
All adult participants and legal guardians of child and adolescent participants provided consent prior to being enrolled in the study; all older children and adolescent participants provided assent. The Institutional Review Board at the University of Pittsburgh approved this study.
Stimuli and procedure
Participants were first acclimated to the MRI environment in a mock scanner and practiced encoding and recognition trials with cartoon characters. Participants completed three tasks during fMRI acquisition, the Cambridge Face Memory Test (CFMT), the Cambridge Face Memory Test Australian (CFMT-Australian), and a matched in-house Car Memory Test (Duchaine & Nakayama, 2006; McKone et al., 2011). The order of these tasks was counterbalanced across participants. Tasks were modified from the original to allow for jittered trials in the scanner environment. Participants viewed the task presented on a translucent screen visible via a mirror mounted to the head coil. To simplify the current study and increase power, we examine the first condition only for face and car tasks, and for faces, we collapsed across CFMT and CFMT-Australian. The tasks included encoding and recognition trials from condition 1, which were analyzed separately. Participants had previously completed the CFMT and Car Memory Test during a prior behavioral visit. Stimuli were black and white images of young Caucasian men (hair cropped with neutral expressions) and early 2000s sedans (make and model information removed).
Participants were asked to encode and subsequently recognize a total of 12 ‘target’ faces and 6 ‘target’ cars. First, during three encoding trials, a target stimulus was presented from 3 angles sequentially (left 1/3 profile, front, right 1/3 profile), for 3s each (9s total). Next, during recognition, three stimuli were presented simultaneously, one of which was the target stimulus, in a view identical to that shown during the encoding phase. Three recognition trials examined recognition of the left 1/3 side view, front, and right 1/3 side view separately. Participants were instructed to choose the target stimulus, from among the distractor stimuli, by pressing the corresponding button on a MRI-safe response glove. Recognition trials in which participants did not respond were excluded from both behavioral and fMRI analyses. A jittered (1500–12000ms) inter-trial fixation cross stimuli was presented between each encoding and recognition trial. In total, there were 12 face encoding trials and 36 face recognition trials, and 6 car encoding trials and 18 car recognition trials.
Behavioral analysis
On the basis of a priori hypotheses, we examined recognition performance (percent correct) using ANOVAs with diagnosis group (TD, autism) and age group (older children, adolescents, adults) as between subject factors, for each object category (faces, cars) separately. We also examined age-related changes for each category in each diagnosis group separately. All behavioral analyses were conducted on arcsine-transformed data to correct for a negatively skewed distribution (face accuracy skewness: Z = −5.55, car accuracy skewness: Z = −3.60). After transformation, behavioral data were normally distributed (arcsine-transformed face accuracy skewness: Z = −1.28, arcsine-transformed car accuracy skewness: Z = 1.33). Non-transformed group means are presented in the figures to aid in interpretation.
fMRI analysis
Scans were performed on a Siemens Allegra 3T MRI scanner at the Neuroscience Imaging Center at the McGowan Institute for Regenerative Medicine at the University of Pittsburgh. Structural images were collected with magnetization-prepared rapid gradient echo (MPRAGE) sequences (TR = 2100 ms, TE = 3.93 ms, flip angle = 7°, 176 1 mm axial slices, voxel size = 1.05 mm3). Functional images were obtained using a gradient echo, echo-planar imaging (EPI) sequence sensitive to blood-oxygen-level-dependent (BOLD) contrast (TR = 1500 ms, TE = 25 ms, flip angle = 70°, 29 4 mm axial slices, voxel size = 3.1 × 3.1 × 4 mm3, 188 volumes per run, in-plane field of view = 200 mm).
Preprocessing
Imaging data were preprocessed using FSL (Smith et al., 2004) and AFNI (Cox, 1996). Structural scans were nonlinearly transformed into Montreal Neurological Institute (MNI) space using fnirt. Functional scans were first corrected for slice acquisition time using slicetimer, then corrected for motion with mcflirt, co-registering to the middle functional volume. Functional volumes were then co-registered to the structural scan and warped into MNI space using the transformation defined by the previous structural-to-MNI nonlinear warp. This step also resulted in resampling of the functional data into 3mm isocubic voxels. Data were then spatially smoothed with a 5 mm full-width-at-half-maximum kernel. Next, low frequency drift was removed with a high-pass temporal filter (0.0167Hz). Each time series was normalized to have a mean of 100 to approximate percent signal change.
A general linear model (GLM) was used to fit voxel-wise functional data using AFNI’s 3dDeconvolve program (Ward, 1998). The hemodynamic response was modeled using a gamma function convolved with separate boxcar functions at the time of trial onset for encoding and recognition trials separately. Correct trials were modeled separately from incorrect and non-responsive trials. Six motion parameters and their derivatives were included as regressors of no interest (3 translations: X, Y, Z; 3 rotations: roll, pitch, yaw). To further correct for motion artifact, volumes including rapid movement (>0.8mm, relative to the previous volume) and the volume preceding this movement, were censored (Siegel et al., 2014). The number of volumes removed did not differ between diagnosis groups (p=0.71). However, more volumes were censored in younger participants [main effect of age group, F(2,81)=6.464, p<0.002, ; older children: M=7.32, adolescents: M=2.55, adults: M=1.11]. The number of censored volumes across age did not differ between diagnosis groups (p=0.92). No more than 20% of volumes were removed from any subject.
Connectivity measures were generated by obtaining separate parameter estimates for encoding trials and correct recognition trials separately, and then separately concatenated to form two beta series per subject (Rissman, Gazzaley, & D’Esposito, 2004). R and L FFA ROIs were used as seed regions (see Seed ROI selection section below). For each subject, the average beta series from each seed region and trial type were entered into a GLM model to calculate correlation (i.e., functional connectivity) between each respective beta series and every other voxel in the brain. To minimize the influence of non-grey matter signal, mean beta-series estimates were calculated using cerebrospinal fluid (CSF) and white matter (WM) masks separately and included as regressors-of-no-interest. Beta outliers were removed using the following procedure:
The minimum and maximum beta values across all voxels for each trial were calculated.
Trials with beta values more than 2.5 standard deviations from the mean of the minima or maxima were censored.
Resulting correlation maps were z-transformed (Fisher’s r to z transformation) and used as a measure of functional connectivity strength between regions.
Functional connectivity
Seed ROI selection (Supplementary Fig. 1)
To identify the FFA functionally and independent of the current data set, a reverse inference map based on a meta-analytic search of the term “ffa” in the online NeuroSynth software (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011) was used, which included data from 63 studies. Spheres with a radius of 6mm were centered on the peak activation coordinates (minimum distance between peaks: 24mm) and were then masked to include only significant voxels in the NeuroSynth meta-analysis. These regions were subsequently restricted to the fusiform gyrus according to the Harvard-Oxford cortical atlas. This resulted in R (25 voxels, 675mm3) and L (21 voxels, 567 mm3) FFA ROIs.
Analysis
Functional connectivity strength with each seed region for each trial type was extracted and submitted to group-level analyses for each voxel. Using ANOVA tests (AFNI’s 3dMVM program; Chen, Adleman, Saad, Leibenluft, & Cox, 2014), functional connectivity was assessed as the dependent variable in a 2 (diagnosis group) × 3 (age group) × 2 (category) design. Four ANOVAs examined the encoding and recognition conditions separately in the R and then L FFA. Diagnosis group (autism, TD) and age group (older children, adolescents and adults) served as between-group factors, with object category (faces, cars) as a within-group factor. Cluster size was determined using a Monte-Carlo simulation (AFNI’s 3dClustSim program) to correct for multiple comparisons. A voxel threshold of p < .025, at a cluster threshold of p < .05, yielded in a cluster size of 73 voxels (1971 mm3). In order to account for medication effects on functional connectivity, we re-ran our analyses with medication status as a covariate. The results were generally the same, with connectivity clusters remaining significant at p<0.05 (uncorrected).
We first report regions showing connectivity differences between diagnosis groups, independent of age and category (diagnosis main effects), with R FFA, followed by L FFA. Next, we report regions showing diagnosis group differences that vary across stimuli, independent of age (diagnosis by category interaction). We then report regions showing differences between diagnosis groups that differ across age groups, independent of category (diagnosis by age group interaction). Finally, we report regions that exhibit age-related differences between diagnosis groups that are category-specific (diagnosis by age group by category interaction). For regions showing an interaction with category, follow-up simple effect ANOVAs were performed for each category separately to determine whether diagnosis differences or age-related change differences were specific to face stimuli.
Results
Behavioral performance (Fig. 1)
Fig 1.
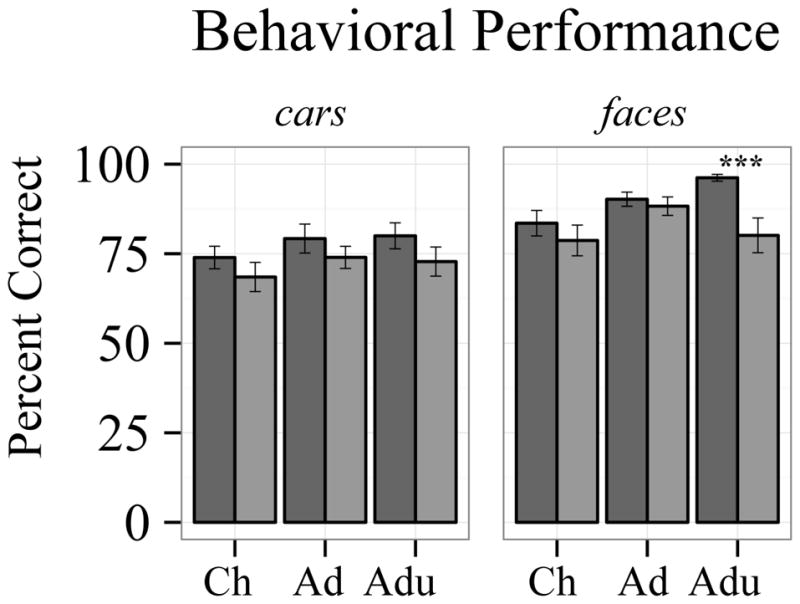
Behavioral performance. Face recognition performance increased across age groups in TD, but not autism, and only differed between diagnosis groups in adults. Car recognition performance was better in the TD group, but age-related changes were not different between diagnosis groups. Dark grey bars represent the TD group, light grey bars represent the autism group. Error bars represent standard error of the mean. ***p=0.001
ANOVAs were utilized to test diagnosis and age group effects for faces and cars separately. The TD group performed better than the autism group in both face [diagnosis main effect: F(1,78)=9.589, p=.003, ] and car recognition [diagnosis main effect: F(1,78)=4.030, p=.048, ]. Performance across diagnosis groups improved with age for face [age main effect: F(2,78)=4.739, p=.011, ], but not car recognition [age main effect: F(2,78)=1.683, p=.193, ]. Age-related performance improvements for face recognition [diagnosis by age group interaction: F(2,78)=3.129, p=.049, ], but not for car recognition [diagnosis by age group interaction: F(2,78)=0.104, p=.902, ], differed between diagnosis groups.
Follow-up simple effects tests comparing diagnosis groups in each age group separately revealed that the TD group performed better than the autism group, for face stimuli [t(25)=3.683, p=0.001], but not car stimuli [t(25)=1.366, p=0.184] in the adult group only. Performance did not differ between diagnosis groups during adolescence or childhood for either faces or cars (all p’s>.2). These results support our predictions, providing further evidence that face recognition improves into adulthood typically, but not in autism (Germine et al., 2011; O’Hearn et al., 2010). In the current study, car recognition does not exhibit this developmental pattern.
Functional connectivity
Diagnosis group differences independent of age
Overall diagnosis differences (diagnosis main effect)
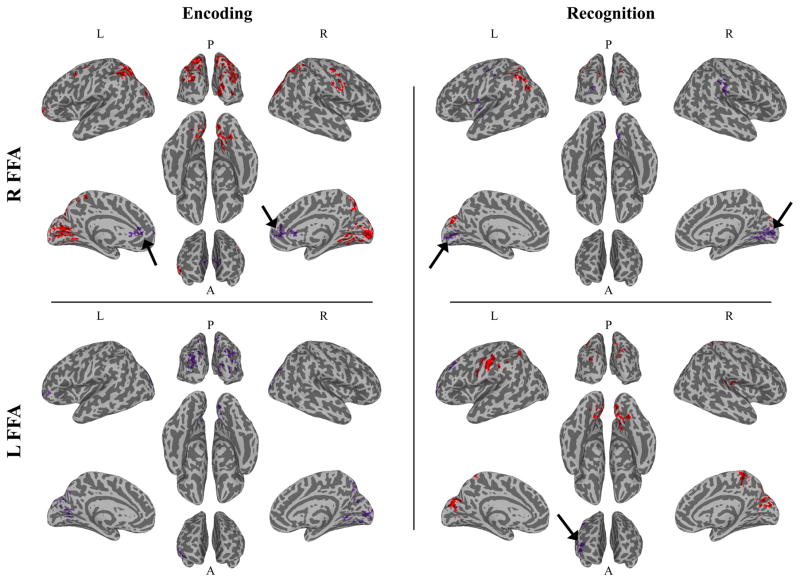
Connectivity between the FFA and visual, attention, extended face-processing, and frontal cognitive control regions was reduced in the group with autism, compared to the TD group, across both categories (Figure 2, Table 2).
Fig 2.
Connectivity: Diagnosis group differences. Top: R FFA Connectivity. Bottom: L FFA Connectivity. Left: Encoding. Right: Encoding. Red represents a diagnosis main effect. Purple represents diagnosis by category interaction. In all regions showing a diagnosis main effect, connectivity was reduced in the group with autism, compared to the TD group. Black arrows highlight regions showing underconnectivity specific to face stimuli.
Table 2.
| General diagnosis differences
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Center of Mass | F-Stat | Peak F-Stat | ||||||||||||
|
| ||||||||||||||
| Seed | Cluster | Volume | No. Voxels | X | Y | Z | Mean | SEM | Value | X | Y | Z | ||
| Encoding | ||||||||||||||
| R FFA | BL PPC | 55566 | 2058 | 4.5 | 69.5 | 30.1 | 8.37 | 0.06 | 19.92 | 37.5 | 55.5 | 52.5 | ||
| L TPJ | ||||||||||||||
| BL visual cortex | ||||||||||||||
| L premotor cortex | 2862 | 106 | 37.8 | −3.8 | 56.2 | 7.40 | 0.20 | 13.65 | 37.5 | −1.5 | 64.5 | |||
| L lPFC | ||||||||||||||
| L lateral OFC | 2025 | 75 | 46.3 | −49 | −4.2 | 8.40 | 0.33 | 21.50 | 49.5 | −52.5 | −1.5 | |||
| R somatosensory cortex | 2025 | 75 | −55.8 | 3.3 | 34.9 | 7.45 | 0.24 | 13.82 | −61.5 | 1.5 | 34.5 | |||
| R motor cortex | ||||||||||||||
| L FFA | BL visual cortex | 11178 | 414 | −10 | 77.9 | 11.4 | 7.47 | 0.10 | 18.50 | −25.5 | 85.5 | 22.5 | ||
| L visual cortex | 5535 | 205 | 12 | 86.7 | 26.8 | 7.44 | 0.12 | 14.69 | 22.5 | 91.5 | 25.5 | |||
| L medial parietal | ||||||||||||||
| L lateral OFC | 2592 | 96 | 45.1 | −49.2 | −4.7 | 8.13 | 0.28 | 16.56 | 49.5 | −49.5 | −1.5 | |||
| L medial parietal | 2187 | 81 | 2.6 | 69.3 | 53 | 7.30 | 0.16 | 11.20 | 10.5 | 70.5 | 55.5 | |||
| L PPC | ||||||||||||||
| Recognition | ||||||||||||||
| R FFA | L TPJ | 5157 | 191 | 47.7 | 54.6 | 44.2 | 6.89 | 0.11 | 15.66 | 37.5 | 61.5 | 28.5 | ||
| L PPC | ||||||||||||||
| BL visual cortex | 2943 | 109 | 2.4 | 83.3 | 29.9 | 7.15 | 0.14 | 11.12 | −1.5 | 85.5 | 31.5 | |||
| L FFA | BL visual cortex | 8937 | 331 | 0.8 | 78 | 25.8 | 7.54 | 0.11 | 16.68 | −1.5 | 79.5 | 28.5 | ||
| L somatosensory cortex | 4752 | 176 | 53.6 | 15.7 | 32.9 | 8.34 | 0.20 | 16.44 | 49.5 | 16.5 | 34.5 | |||
| R medial parietal | 3294 | 122 | −9.9 | 40.2 | 62.9 | 7.21 | 0.17 | 13.30 | −4.5 | 46.5 | 64.5 | |||
| R dorsal posterior insula lobe | 2565 | 95 | −32.6 | 22.5 | 11.7 | 8.33 | 0.34 | 20.80 | −34.5 | 37.5 | 13.5 | |||
| L PPC | 2052 | 76 | 35.4 | 53.2 | 51.6 | 6.94 | 0.17 | 12.81 | 37.5 | 55.5 | 61.5 | |||
|
| ||||||||||||||
| Category-specific diagnosis differences | ||||||||||||||
|
| ||||||||||||||
| Seed | Cluster | Volume | No. Voxels | Center of Mass | F-Stat | Peak F-Stat | Follow-up t-test (TD-Autism) | |||||||
| X | Y | Z | Mean | SEM | Value | X | Y | Z | Faces | Cars | ||||
|
| ||||||||||||||
| Encoding | ||||||||||||||
| R FFA | BL mPFC | 5886 | 218 | −2.2 | −43.5 | 6 | 8.11 | 0.18 | 21.60 | −16.5 | −40.5 | −1.5 | 2.36* | −0.79 |
| BL ACC | ||||||||||||||
| L FFA | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Recognition | ||||||||||||||
| R FFA | BL visual cortex | 7884 | 292 | −2.9 | 79.4 | 3.7 | 8.43 | 0.18 | 33.11 | −13.5 | 85.5 | 13.5 | 3.00** | −1.57 |
| L somatosensory cortex | 3267 | 121 | 32.2 | 5.1 | 50 | 7.62 | 0.23 | 24.53 | 34.5 | 16.5 | 37.5 | 2.20* | −0.85 | |
| L lPFC | ||||||||||||||
| R TPJ | 2295 | 85 | −58.4 | 28.1 | 24.2 | 7.78 | 0.23 | 13.49 | −49.5 | 28.5 | 16.5 | 1.55 | −1.29 | |
| L anterior superior temporal gyrus | 2052 | 76 | 55.3 | 0.2 | 0 | 7.68 | 0.29 | 17.80 | 64.5 | 1.5 | 4.5 | 2.13* | −0.17 | |
| L FFA | L rostral lPFC | 2727 | 101 | 37.3 | −53.8 | 1.4 | 8.12 | 0.26 | 18.49 | 31.5 | −46.5 | 1.5 | 2.28* | −1.33 |
| L dorsal lPFC | 2646 | 98 | 42.9 | −32.4 | 36.8 | 8.30 | 0.28 | 21.02 | 40.5 | −31.5 | 37.5 | 1.90# | −2.20* | |
SEM, Standard Error of the Mean; TD, Typical Development; R, Right; L, Left; BL, Bilateral; PPC, posterior parietal cortex; TPJ, temporoparietal junction; lPFC, lateral prefrontal cortex; OFC, orbitofrontal cortex; mPFC, medial prefrontal cortex; ACC, anterior cingulate cortex;
p<0.01;
p<0.05;
p<0.1
Encoding
The autism group displayed reduced functional connectivity between the R FFA and bilateral visual and posterior parietal (PPC) cortices, the L temporoparietal junction (TPJ), L lateral prefrontal (lPFC) and orbitofrontal (OFC) cortices, and unexpectedly the R somatosensory and motor cortices. Participants with autism also displayed reduced functional connectivity between L FFA and a smaller set of regions, including bilateral visual cortex, L medial parietal cortex, PPC, and OFC.
Recognition
During recognition, a pattern of results emerged that was similar to, but less robust than diagnosis differences during encoding. The autism group exhibited reduced connectivity between the R FFA and bilateral visual cortex, L PPC, and the L TPJ. Additionally, participants with autism displayed reduced functional connectivity between the L FFA and bilateral visual cortex, R medial parietal and L PPC, L somatosensory cortex, and R dorsal posterior insula.
Category-specific diagnosis differences (diagnosis by category interaction)
Connectivity between the FFA and frontal and temporal extended-face processing, and frontal cognitive control regions exhibited diagnosis group differences depending on the object category (Table 2, Figure 2, Supplementary Figure 2). Generally, significant diagnosis by category interactions reflected reduced connectivity in the autism group, relative to the TD group, for faces, but not cars.
Encoding
Underconnectivity between the R FFA and bilateral medial PFC (mPFC), and ACC was category specific in autism. Follow-up analyses (See Table 2) comparing diagnosis groups in each category separately revealed that R FFA connectivity with the mPFC was reduced for faces, but not cars, in the autism group when compared to the TD group. No diagnosis by category differences emerged for L FFA connectivity.
Recognition
Diagnosis groups differed depending on category for connectivity between the R FFA and R visual cortex, R TPJ, L somatosensory cortex extending to the L lPFC, and L anterior superior temporal gyrus. Follow-up analyses (See Table 2) revealed that connectivity was reduced in the autism group, relative to the TD group, for faces, but not cars, for all clusters except the R TPJ. Though R FFA—R TPJ connectivity was quantitatively less for faces in the autism group, compared to the TD group, these differences did not reach statistical significance.
Additionally, diagnosis groups differed depending on category for connectivity between the L FFA and L rostral lPFC, and L dorsal lPFC. Follow up analyses (See Table 2) revealed that L FFA—L rostral lPFC connectivity was reduced in the autism group, relative to the TD group, for faces, but not cars. L FFA—L dorsal lPFC connectivity in the autism group was marginally reduced for faces, relative to the TD group, but significantly greater for cars.
Age-related change differences between diagnosis groups
Overall age-related differences (diagnosis by age interaction)
Age-related changes in connectivity between the FFA and subcortical extended face-processing and cognitive control regions, and cortical visual regions differed between diagnosis groups for both face and car recognition (Table 3, Figure 3, Supplementary Figure 3). The TD group exhibited decreasing connectivity with age, whereas the group with autism exhibited increases or quadratic changes with age.
Table 3.
| General age-related differences
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seed | Cluster | Volume | No. Voxels | Center of Mass | F-Stat | Peak F-Stat | ||||||||||
| X | Y | Z | Mean | SEM | Value | X | Y | Z | ||||||||
| Encoding | ||||||||||||||||
| R FFA | L motor cortex | 4482 | 166 | 38.4 | 28.7 | 58.8 | 5.73 | 0.12 | 10.97 | 34.5 | 19.5 | 61.5 | ||||
| L somatosensory cortex | ||||||||||||||||
| L FFA | - | - | - | - | - | - | - | - | - | - | - | - | ||||
| Recognition | ||||||||||||||||
| R FFA | - | - | - | - | - | - | - | - | - | - | - | - | ||||
| L FFA | R dorsal striatum (R caudate nucleus/putamen) | 6669 | 247 | −22.3 | −7.4 | −9.7 | 5.64 | 0.11 | 12.37 | −10.5 | −16.5 | −7.5 | ||||
| R amygdala | ||||||||||||||||
| L amygdala | 2754 | 102 | 24 | −4.9 | −10.3 | 4.95 | 0.09 | 8.54 | 25.5 | 4.5 | −13.5 | |||||
| L dorsal striatum (L putamen) | ||||||||||||||||
| R visual cortex | 2241 | 83 | −5.6 | 50.7 | 6.2 | 5.22 | 0.14 | 9.76 | −4.5 | 49.5 | 10.5 | |||||
|
| ||||||||||||||||
| Category-specific age-related differences | ||||||||||||||||
|
| ||||||||||||||||
| Seed | Cluster | Volume | No. Voxels | Center of Mass | F-Stat | Peak F-Stat | Follow-up ANOVA | |||||||||
| Faces | Cars | |||||||||||||||
| X | Y | Z | Mean | SEM | Value | X | Y | Z | F-value | partial η2 | F-value | partial η2 | ||||
|
| ||||||||||||||||
| Encoding | ||||||||||||||||
| R FFA | L TPJ | 2214 | 82 | 50.2 | 28.6 | 11.7 | 5.57 | 0.14 | 10.20 | 43.5 | 37.5 | 16.5 | 1.05 | 0.03 | 3.97* | 0.09 |
| L visual cortex | 2214 | 82 | 12.5 | 70.2 | 33.2 | 5.55 | 0.17 | 11.53 | 13.5 | 64.5 | 28.5 | 1.07 | 0.03 | 2.89# | 0.07 | |
| L medial parietal | ||||||||||||||||
| L FFA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Recognition | ||||||||||||||||
| R FFA | BL thalamus | 8019 | 297 | −0.8 | 6.9 | 4.7 | 5.45 | 0.08 | 10.91 | −4.5 | 13.5 | 4.5 | 7.06** | 0.15 | 0.86 | 0.02 |
| BL dorsal striatum (BL caudate) | ||||||||||||||||
| R dorsal striatum (R putamen) | 4752 | 176 | −30.2 | −12.4 | 3.2 | 5.90 | 0.13 | 11.27 | −25.5 | −7.5 | 4.5 | 6.35** | 0.14 | 1.31 | 0.03 | |
| R anterior insula | ||||||||||||||||
| L dorsal ACC | 3834 | 142 | −1.2 | −17.5 | 36.9 | 5.12 | 0.09 | 9.01 | 13.5 | −22.5 | 25.5 | 6.99** | 0.15 | 0.40 | 0.01 | |
| BL preSMA | ||||||||||||||||
| R TPJ | 3024 | 112 | −58.5 | 28.6 | 22.8 | 6.06 | 0.18 | 12.95 | −61.5 | 34.5 | 22.5 | 3.88* | 0.10 | 0.71 | 0.02 | |
| L FFA | R dorsal striatum (R putamen) | 2430 | 90 | −32.1 | −6.9 | 5.7 | 5.46 | 0.15 | 10.09 | −28.5 | −7.5 | 1.5 | 4.67* | 0.11 | 5.06** | 0.12 |
| R insula | ||||||||||||||||
SEM, Standard Error of the Mean; R, Right; L, Left; BL, Bilateral; TPJ, temporoparietal junction; ACC, anterior cingulate cortex; preSMA, pre-supplementary motor area
p<0.01;
p<0.05;
p<0.1
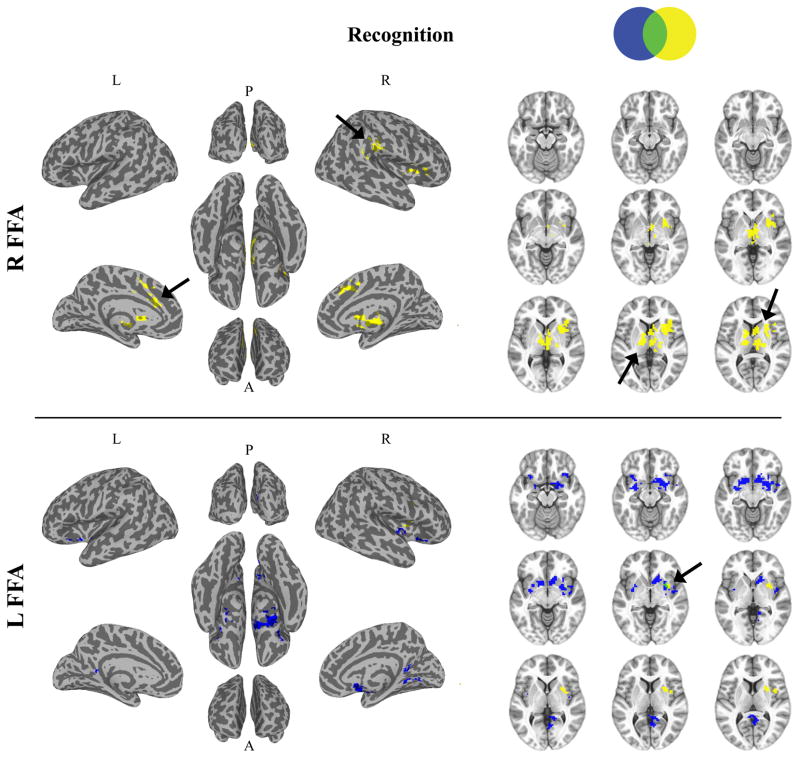
Fig 3.
Connectivity: Age-related change differences between diagnosis groups during recognition. Top: R FFA Connectivity. Bottom: L FFA Connectivity. Left: Cortical. Right: Subcortical. Blue represents a diagnosis by age interaction. Yellow represents diagnosis by age by category interaction. Black arrows highlight regions showing a diagnosis by age interaction specific to face stimuli.
Encoding
Age-related changes differed between diagnosis groups in connectivity between the R FFA and L motor cortex. However, no regions displaying a diagnosis by age group interactions were evident for L FFA connectivity.
Recognition
No regions displayed diagnosis by age group interaction for R FFA connectivity. However, diagnosis groups differed in age-related changes for connectivity between the L FFA and R visual cortex, R dorsal striatum (caudate nucleus and putamen) extending to the R amygdala, and L amygdala extending to the L dorsal striatum (putamen).
Category-specific age-related differences (diagnosis by age by category interactions)
Diagnosis groups displayed different age-related changes, depending on the object category, for connectivity between the FFA and cortical visual, frontal and temporal extended face-processing, and subcortical sensory and cognitive control regions (Table 3, Figure 3, Figure 4). As hypothesized, most age-related change differences were face-specific. However, unexpectedly, some regions also showed age-related change differences to car stimuli.
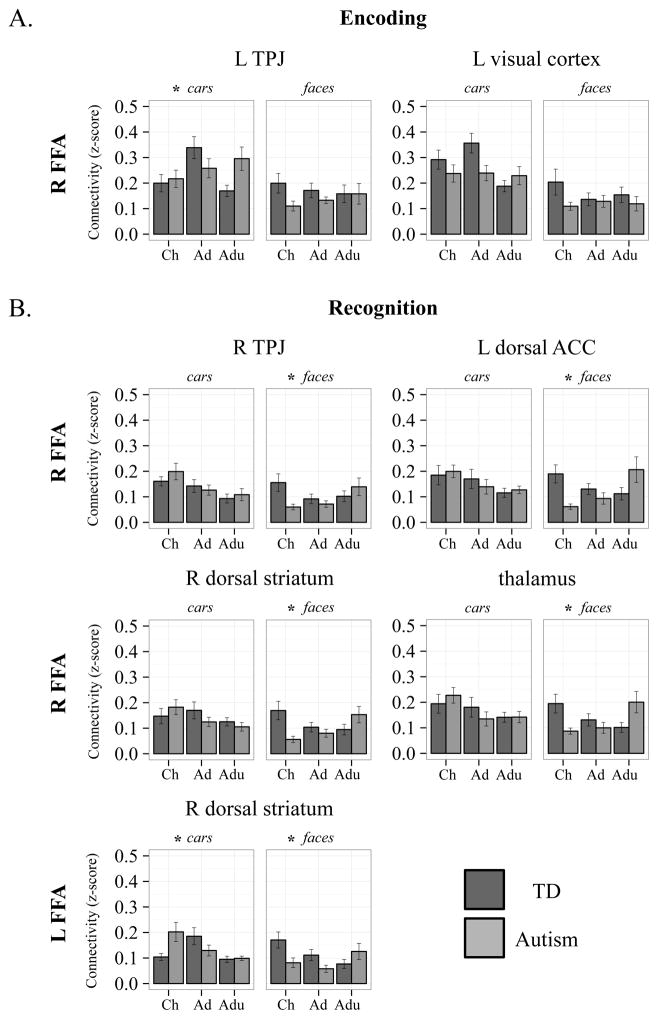
Fig 4.
Graphs: Category-specific age-related change differences between diagnosis groups. A. Category-specific age-related change differences in R FFA connectivity during encoding. B. Category-specific age-related change differences in R and L FFA connectivity during recognition. Generally, age-related change differences between diagnosis groups were evident for face recognition. Dark grey bars represent the TD group, light grey bars represent the autism group. R, right; L, left; Ch, children; Ad, adolescents; Adu, adults; TPJ, temporoparietal junction; ACC, anterior cingulate cortex. *significant category-specific age-related change differences
Encoding
Age-related change differences between diagnosis groups depended on category for connectivity between the R FFA and L visual and medial parietal cortices, and L TPJ. Follow-up analyses (See Table 3) revealed that R FFA—L visual and medial parietal cortex connectivity showed marginally different age-related changes in the autism group, relative to the TD group, for cars, but not faces. Similarly, R FFA—L TPJ connectivity changed differently with age in the autism group, relative to the TD group, for cars, but not faces. No regions exhibited age-related change differences depending on category for L FFA.
Recognition
Diagnosis groups displayed different age-related changes depending on the object category for connectivity between the R FFA and bilateral thalamus extending to the dorsal striatum, R putamen (dorsal striatum) extending to the R anterior insula, L dorsal ACC (dACC) extending to the bilateral pre-supplementary motor area (preSMA), and R TPJ. Follow-up analyses (See Table 3) for all significant regions revealed that connectivity for faces, but not cars, changed with age differently in the autism group, relative to the TD group.
Similarly, age-related changes differed between diagnosis groups depending on the object category for connectivity between the L FFA and R dorsal striatum (putamen) extending to the R insula. Follow-up analyses (See Table 3) revealed that L FFA—R dorsal striatum connectivity changed with age differently for both faces and cars in the autism group, relative to the TD group. However, these changes were not the same across faces and cars: L FFA—R dorsal striatum connectivity decreased with age with age in the TD group, but increased with age in the autism group during face recognition, while connectivity increased from childhood to adolescences and decreased from adolescences to adulthood in the TD group, but decrease with age overall in the autism group during car recognition.
Discussion
Here we provided unique insight into the developmental plateau of face recognition in autism by examining functional connectivity between the FFA, a core face-processing region, and the rest of the brain. Consistent with previous work, we found that face recognition performance improves with age typically, but not in autism, which may result in greater face recognition deficits in adults with autism (O’Hearn et al., 2010). In addition, FFA connectivity differed between TD and autism groups for a distributed set of extended face-processing regions, as well as domain-general regions. More specifically, FFA underconnectivity in autism was evident independent of age, sometimes for faces only, with frontal extended face-processing and domain-general regions, as well as primary visual regions. Age-related differences between TD and autism groups were found for FFA connectivity with temporoparietal and subcortical extended-face processing regions, as well as subcortical cognitive control regions. Generally, in regions exhibiting age-related connectivity differences, decreases with age were found in the TD group, while increases with age were found in autism. In most, but not all regions, these effects were specific to face stimuli.
Connectivity with the extended face-processing network
Frontal regions
Connectivity was reduced in autism between the FFA and frontal regions included in the extended face-processing network (Frith & Frith, 2007; Haxby et al., 2000, 2002). More specifically, independent of age, bilateral FFA connectivity with the lOFC displayed underconnectivity in the autism group, relative to the TD group, for both face and car encoding, and R FFA connectivity with the mPFC/ACC displayed underconnectivity in the autism group, relative to the TD group for face encoding only. Underconnectivity with the mPFC may reflect specific social deficits such as thinking about others’ attributes when viewing a face (Amodio & Frith, 2006; Frith & Frith, 2006; Schurz, Radua, Aichhorn, Richlan, & Perner, 2014), and therefore be specific to R FFA connectivity and face stimuli. Moreover, reduced FFA—mPFC connectivity specifically during the encoding, but not recognition, condition suggests that integration of information from temporal and occipital regions that are necessary for object representation (Haxby et al., 1991, 1994; Hillebrandt et al., 2013) may be atypical, leading to an overall less reliable representation in autism. Overall, these data provide further support for reduced long-range corticocortical functional connectivity in autism (Belmonte et al., 2004; Khan et al., 2013), that may be critical for object recognition, especially faces.
Temporoparietal regions
In autism, connectivity between the FFA and both the R and L TPJ, which are included in extended face-processing network, was reduced relative to TD individuals. More specifically, while connectivity between the R FFA and L TPJ was reduced in autism independent of age for both face and car encoding and recognition, connectivity between the R FFA and the R TPJ exhibited atypical age-related changes in the autism group relative to the TD group for face recognition only. We found that FFA—R TPJ connectivity is reduced in older children with autism (p<0.05), but not adolescents or adults with autism (all p’s>0.3), during face recognition. It is possible that these disruptions in FFA—TPJ connectivity may reflect abnormal attention allocation and control, with the FFA—R TPJ connectivity development more protracted and face specific, and FFA—L TPJ connectivity more general across age and object processing. Indeed, visual attention is often atypical in individuals with autism (Amso, Haas, Tenenbaum, Markant, & Sheinkopf, 2014; Keehn, Müller, & Townsend, 2013). And interestingly, even when performance on simple attention tasks is typical in those with autism, functional connectivity between attention and cognitive control, and visual processing networks appears atypical (Fitzgerald et al., 2014), highlighting a neurobiological basis of face processing deficits in autism. Given that atypical age-related changes in connectivity between the R FFA and R TPJ were evident in autism during face recognition only, provides further support for the notion that attention to faces develops differently in autism.
Evidence also suggests the TPJ is important for determining stimulus relevance (i.e., importance to the task at hand) and selecting appropriate behavioral responses (Han & Marois, 2014). This view of TPJ is consistent with our results of atypical R FFA—R TPJ connectivity during face recognition trials when participants were asked to identify the target face from among two distractors. Thus, atypical development of attention and relevance detection in autism may contribute at least partially to the plateau in the development of face recognition in autism, from adolescence into adulthood
Our findings also highlight the importance of considering age when assessing connectivity in autism. For example, had we not considered age, we may have found that FFA—R TPJ connectivity is reduced during face recognition. Thus, fully considering the developmental trajectory of functional connectivity in autism is necessary in order to fully characterize the nature of this disorder and its implications.
Subcortical regions: Amygdala
Connectivity between the L FFA and bilateral amygdala exhibited atypical age-related changes for both face and car recognition. Specifically, we found underconnectivity between the L FFA and bilateral amygdala in adolescents with autism, consistent with previous research examining social cognition using vignettes in adolescents with autism (Weisberg et al., 2014). Furthermore, we also found overconnectivity in autism by adulthood, unlike previous studies examining social cognition that found underconnectivity in adults with autism (Kleinhans et al., 2008; Weisberg et al., 2014). Differences between our results and previous studies reporting reduced functional connectivity between the FFA and amygdala in adults with autism are likely to reflect a number of study differences. First, we found differences in L FFA connectivity while other studies only reported differences in R FFA functional connectivity (Kleinhans et al., 2008). Second, we considered age as a factor while other studies combined both adolescents and adults into one group and do not consider age as a factor (Kleinhans et al., 2008, 2015; Weisberg et al., 2014). Third, we did not limit our analysis to face-specific connectivity (Kleinhans et al., 2008) and included non-social stimuli (e.g., cars). Our results show that L FFA—amygdala connectivity does not decrease with age in individuals with autism, as it does in TD individuals, but may follow a different developmental trajectory, regardless of object category. Similar to the TPJ (Han & Marois, 2014), the amygdala is also involved in the detection of relevant stimuli, which increases visual processing for such a stimulus at the cortical level (Sander, Grafman, & Zalla, 2003; Zalla & Sperduti, 2013). Thus, we speculate that atypical FFA—amygdala connectivity in individuals with autism may underlie deficits in detecting relevant target features for both social and non-social categories. Behavioral studies have sown that recognition deficits exist across object categories in autism and may emerge in adolescences, although these effects are more robust face recognition deficits (Berhmann et al., 2006; Blair, Frith, Smith, Abell, & Cipolotti, 2002; O’Hearn et al., 2014). Future work should explore the causal relationship between cortico-amygdala connectivity and the detection of relevant or salient stimuli and how these connections may be altered in autism, affecting social processing specifically or object processing generally.
Connectivity with domain-general regions
Frontal regions
We also found reduced connectivity between the FFA and frontal regions not included in the extended face-processing network. This included face-specific FFA underconnectivity and car-specific FFA overconnectivity with the rostral and dorsal lPFC in autism, respectively, independent of age. Atypical functional connectivity between temporal (e.g., the FFA) and frontal regions (e.g., the lPFC) is consistent with previous studies, although these studies were specific to social cognition, including emotion recognition (Wicker et al., 2008), face working memory (Koshino et al., 2008), and theory of mind (ToM; Kana, Libero, Hu, Deshpande, & Colburn, 2014).
Our findings suggest that atypical connectivity may be domain general in autism and future studies should also include non-social stimuli to better characterize the nature of these differences. Furthermore, as lateral frontal regions are important for cognitive control and working memory (Cole & Schneider, 2007) it is possible that atypical connectivity between ventral object processing regions, such as the FFA, and working memory areas impact object recognition in autism.
Visual cortex
One of our most striking results was reduced bilateral FFA connectivity with the visual cortex in autism for both face and car encoding and recognition, independent of age, and reduced R FFA connectivity with the visual cortex for face recognition only. Previous studies suggest that individuals with autism exhibit enhanced or more local, compared to global, perceptual processing of objects (Hubl et al., 2003; Samson, Mottron, Soulières, & Zeffiro, 2012). Other studies suggest that adults with autism exhibit reduced functional temporal-occipital connectivity (Kana, Keller, Cherkassky, Minshew, & Just, 2009; Sato, Toichi, Uono, & Kochiyama, 2012; Wicker et al., 2008) during social cognition tasks, and these visual processing differences in autism may contribute to disrupted face processing/recognition (Samson et al., 2012). General underconnectivity, independent of age, between the FFA and the visual cortex suggests that abnormalities in perceptual systems underling object encoding may contribute to recognition deficits in autism, especially faces (Greimel et al., 2012; Solomon, McCauley, Iosif, Carter, & Ragland, 2016). It is also possible that perceptual complexity, or the added social component of faces, impairs recognition abilities more than cars in individuals with autism, despite similar connectivity profiles for face and car encoding. Atypical changes in face-specific connectivity in the ventral stream during recognition, over development, may also contribute to atypical social cognitive development in autism.
Subcortical regions: Dorsal striatum
We also found age-related differences in connectivity between FFA and subcortical regions not included in the extended face-processing network. Specifically, FFA connectivity with the R caudate nucleus, extending to the R putamen, exhibited age-related changes that differed between autism and TD groups during recognition, for both faces and cars. Additionally, R FFA connectivity with a small cluster within the R putamen exhibited atypical age-related changes in autism during face recognition only, with connectivity decreasing with age in the TD group, but increasing with age in the autism group. This atypical connectivity between the FFA and dorsal striatal regions is consistent with our previous work using resting-state fMRI, where we found that connectivity between the fusiform gyrus and bilateral dorsal striatum demonstrated atypical age-related change between childhood and adulthood in autism (Padmanabhan et al., 2013), with a decrease with age in the TD group, and an increase with age in the autism group. Here, we also find greater L FFA—R dorsal striatum connectivity in children with autism during car recognition, but reduced connectivity during face recognition. Thus, differences in striatal connectivity in autism may be task- or stimulus-specific. These discrepancies also call for future studies to directly compare task-dependent and resting-state corticostriatal connectivity across development in autism.
Limitations and future directions
Given the novelty of our study, future research is necessary to address a number of limitations. First, longitudinal data analyses are necessary to more accurately characterize the development of functional connectivity in both TD and autism. In addition, small sample sizes within each age group limit our ability to fully characterize the nature of developmental change in functional connectivity during face and car recognition. While we think that the face recognition developmental trajectory is neither continuous nor linear, future studies with larger age groups are needed to confirm our associated connectivity findings. In addition, our results reflect the particular FFA region we identified based on an independent meta-analysis of mostly neurotypical adults; results may vary depending on the specific FFA seed region used. For example, since the FFA increases in size with age (Golarai et al., 2007, 2010; Haist et al., 2013), the average beta-series may include voxels that are not face-specific in older children and adolescents, but that are face-specific in adults. This suggests a face localizer to determine each subject’s FFA might be helpful, although it adds more variability to the study. Additionally, further research is necessary to determine if the results presented in this study are limited to working and/or short-term memory, or if long-term memory for faces is also affected. Finally, research should also examine how both face recognition, and the activity in the face-processing network, is modulated during different attentional strategies (i.e., looking to eyes, nose, etc.) across development.
Conclusions
Face recognition impairments in autism, especially those that increase between adolescents and adulthood, may be related to atypical connectivity between the FFA and a distributed set of extended face-processing and domain-general brain regions. Together our findings suggest that overall underconnectivity accompanied by atypical development of connectivity is associated with increasing face recognition deficits between adolescents and adulthood in autism. Specifically, atypical cortico-subcortical and temporoparietal connectivity development may be important contributors to atypical face recognition development autism. Underconnectivity and atypical development of connectivity may lead to a less optimal face-processing brain network in the context of increasing general cognitive and social cognitive deficits that are characteristic of autism. Further, social cognitive deficits may manifest due to a combination of deficits in perceptual processing, attention allocation, relevance detection, and cognitive control. These results also identify the adolescent period as one of dynamic developmental change in autism that may reflect a unique period of plasticity that can inform treatment strategies.
Supplementary Material
Supplementary Fig 1: FFA Regions of Interest. A. Axial view. B. Coronal view. The left side of the image depicts the left hemisphere of the brain for easier viewing. R FFA = 25 voxels (675mm3). L FFA =21 voxels (567mm3).
Supplementary Fig 2: Graphs: Category-specific diagnosis group differences. Regions showing object-specific diagnosis main effect. Connectivity was generally reduced in the group with autism, compared to the TD group for faces, but not cars (except for L FFA—L dorsal lPFC connectivity during recognition). Dark grey bars represent the TD group, light grey bars represent the autism group. R, right; L, left; TD, typical development; mPFC, medial prefrontal cortex; STG, superior temporal gyrus; TPJ, temporoparietal junction; lPFC, lateral prefrontal cortex. *significant diagnosis group differences
Supplementary Fig 3. Graphs: Age-related change differences between diagnosis groups. A. Age-related change differences in R FFA connectivity across both face and car encoding. B. Age-related change differences in L FFA connectivity across both face and car recognition. Generally, age-related change differences between diagnosis groups were evident for face recognition. Dark grey bars represent the TD group, light grey bars represent the autism group. R, right; L, left; Ch, children; Ad, adolescents; Adu, adults. *significant category-specific age-related change differences
Research highlights.
Overall, functional connectivity between the FFA and frontal and visual cortices was reduced independent of age for face, but not car, recognition in autism compared to typically developing (TD) individuals.
Connectivity between the FFA and L orbitofrontal cortex was reduced in individuals with autism, relative to TD individuals, for both face and car recognition.
Atypical age-related changes in autism, relative to TD individuals, were evident in connectivity between the FFA and both domain-general subcortical regions and temporoparietal regions included in the extended face-processing network. In most cases, connectivity decreased with age in TD individuals, but increased with age in individuals with autism.
FFA—amygdala connectivity also exhibited atypical age-related changes in those with autism, relative to TD individuals, during both face and car recognition.
Acknowledgments
This work was completed at the University of Pittsburgh and supported by NIMH K01MH081191 (PI O’Hearn), and NIH HD055748 (PI Minshew) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. Recruitment was supported by NICHD ACE grant HD055648 and CPEA grant HD35469. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the participants, their families, Jennifer Fedor, Heidi Baumgartner, and the staff at the Autism Center for Excellence for their generous help. We also thank two anonymous reviewers for their critical feedback and suggestions that resulted in a much improved manuscript. Preliminary results were presented at the Association for Psychological Science meeting in Washington, DC in 2013 and The International Congress for Integrative Developmental Cognitive Neuroscience (FLUX Congress) in Pittsburgh, PA in 2013.
Footnotes
Except one individual on section D (Abnormality of Development Evident at or Before 36 Months
Contributor Information
Andrew Lynn, Department of Cognitive, Linguistic and Psychological Sciences, Brown University.
Aarthi Padmanabhan, Department of Psychiatry, Stanford University.
Daniel Simmonds, Laboratory of Neurocognitive Development, University of Pittsburgh.
William Foran, Laboratory of Neurocognitive Development, University of Pittsburgh.
Michael N. Hallquist, Laboratory of Neurocognitive Development, University of Pittsburgh. Department of Psychiatry, University of Pittsburgh
Beatriz Luna, Laboratory of Neurocognitive Development, University of Pittsburgh. Department of Psychiatry, University of Pittsburgh. Department of Psychology, University of Pittsburgh.
Kirsten O’Hearn, Laboratory of Neurocognitive Development, University of Pittsburgh. Department of Psychiatry, University of Pittsburgh. Department of Psychology, University of Pittsburgh.
References
- Alaerts K, Nayar K, Kelly C, Raithel J, Milham MP, Di Martino A. Age-related changes in intrinsic function of the superior temporal sulcus in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2015;10(10):1413–1423. doi: 10.1093/scan/nsv029. http://doi.org/10.1093/scan/nsv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. http://doi.org/10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Amso D, Haas S, Tenenbaum E, Markant J, Sheinkopf SJ. Bottom-up attention orienting in young children with autism. Journal of Autism and Developmental Disorders. 2014;44(3):664–673. doi: 10.1007/s10803-013-1925-5. http://doi.org/10.1007/s10803-013-1925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. http://doi.org/10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhmann M, Avidan G, Leonard GL, Kimchi R, Luna B, Humphreys K, Minshew N. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006;44(1):110–129. doi: 10.1016/j.neuropsychologia.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Frith U, Smith N, Abell F, Cipolotti L. Fractionation of visual memory: agency detection and its impairment in autism. Neuropsychologia. 2002;40(1):108–118. doi: 10.1016/s0028-3932(01)00069-0. [DOI] [PubMed] [Google Scholar]
- Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. NeuroImage. 2014;99:571–588. doi: 10.1016/j.neuroimage.2014.06.027. http://doi.org/10.1016/j.neuroimage.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K, Cohen Kadosh R, Dick F, Johnson MH. Developmental changes in effective connectivity in the emerging core face network. Cerebral Cortex (New York, NY: 1991) 2011;21(6):1389–1394. doi: 10.1093/cercor/bhq215. http://doi.org/10.1093/cercor/bhq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37(1):343–360. doi: 10.1016/j.neuroimage.2007.03.071. http://doi.org/10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia. 2006;44(4):576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex (New York, NY: 1991) 2007;17(10):2400–2406. doi: 10.1093/cercor/bhl148. http://doi.org/10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J, Johnson K, Kehoe E, Bokde ALW, Garavan H, Gallagher L, McGrath J. Disrupted Functional Connectivity in Dorsal and Ventral Attention Networks During Attention Orienting in Autism Spectrum Disorders. Autism Research: Official Journal of the International Society for Autism Research. 2014 doi: 10.1002/aur.1430. http://doi.org/10.1002/aur.1430. [DOI] [PubMed]
- Frith CD, Frith U. The Neural Basis of Mentalizing. Neuron. 2006;50(4):531–534. doi: 10.1016/j.neuron.2006.05.001. http://doi.org/10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social Cognition in Humans. Current Biology. 2007;17(16):R724–R732. doi: 10.1016/j.cub.2007.05.068. http://doi.org/10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Germine LT, Duchaine B, Nakayama K. Where cognitive development and aging meet: face learning ability peaks after age 30. Cognition. 2011;118(2):201–210. doi: 10.1016/j.cognition.2010.11.002. http://doi.org/10.1016/j.cognition.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JDE, Grill-Spector K. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature Neuroscience. 2007;10(4):512–522. doi: 10.1038/nn1865. http://doi.org/10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Liberman A, Yoon JMD, Grill-Spector K. Differential development of the ventral visual cortex extends through adolescence. Frontiers in Human Neuroscience. 2010;3:80. doi: 10.3389/neuro.09.080.2009. http://doi.org/10.3389/neuro.09.080.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greimel E, Nehrkorn B, Fink GR, Kukolja J, Kohls G, Müller K, … Schulte-Rüther M. Neural mechanisms of encoding social and non-social context information in autism spectrum disorder. Neuropsychologia. 2012;50(14):3440–3449. doi: 10.1016/j.neuropsychologia.2012.09.029. http://doi.org/10.1016/j.neuropsychologia.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Greimel E, Schulte-Rüther M, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, Konrad K. Impairment in face processing in autism spectrum disorder: a developmental perspective. Journal of Neural Transmission. 2014:1–11. doi: 10.1007/s00702-014-1206-2. http://doi.org/10.1007/s00702-014-1206-2. [DOI] [PubMed]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, … Tager-Flusberg H. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22(3):1141–50. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2007;28(5):441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haist F, Adamo M, Han J, Lee K, Stiles J. The functional architecture for face-processing expertise: FMRI evidence of the developmental trajectory of the core and the extended face systems. Neuropsychologia. 2013 doi: 10.1016/j.neuropsychologia.2013.08.005. http://doi.org/10.1016/j.neuropsychologia.2013.08.005. [DOI] [PMC free article] [PubMed]
- Han SW, Marois R. Functional Fractionation of the Stimulus-Driven Attention Network. The Journal of Neuroscience. 2014;34(20):6958–6969. doi: 10.1523/JNEUROSCI.4975-13.2014. http://doi.org/10.1523/JNEUROSCI.4975-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby Hoffman, Gobbini The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, … Rapoport SI. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(5):1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51(1):59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14(11 Pt 1):6336–53. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrandt H, Dumontheil I, Blakemore SJ, Roiser JP. Dynamic causal modelling of effective connectivity during perspective taking in a communicative task. NeuroImage. 2013;76:116–124. doi: 10.1016/j.neuroimage.2013.02.072. http://doi.org/10.1016/j.neuroimage.2013.02.072. [DOI] [PubMed] [Google Scholar]
- Hubl D, Bölte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, … Dierks T. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61(9):1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Hasson U, Avidan G, Minshew N, Behrmann M. Cortical patterns of category-selective activation for faces, places and objects in adults with autism. Autism Research: Official Journal of the International Society for Autism Research. 2008;1(1):52–63. doi: 10.1002/aur.1. http://doi.org/10.1002/aur.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Grossmann T, Cohen KK. Mapping functional brain development: Building a social brain through interactive specialization. Developmental Psychology. 2009;45(1):151–159. doi: 10.1037/a0014548. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Gathers AD, Bhatt RS. Progressive and regressive developmental changes in neural substrates for face processing: testing specific predictions of the Interactive Specialization account. Developmental Science. 2011;14(2):227–241. doi: 10.1111/j.1467-7687.2010.00963.x. http://doi.org/10.1111/j.1467-7687.2010.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JE, Swearingen JE, Clark JD, Benca CE, Collins HR, Corbly CR, … Bhatt RS. The changing landscape of functional brain networks for face processing in typical development. NeuroImage. 2012;63(3):1223–1236. doi: 10.1016/j.neuroimage.2012.08.021. http://doi.org/10.1016/j.neuroimage.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Social Neuroscience. 2009;4(2):135–152. doi: 10.1080/17470910802198510. http://doi.org/10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Hu CP, Deshpande HD, Colburn JS. Functional Brain Networks and White Matter Underlying Theory-of-Mind in Autism. Social Cognitive and Affective Neuroscience. 2014;9(1):98–105. doi: 10.1093/scan/nss106. http://doi.org/10.1093/scan/nss106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Müller RA, Townsend J. Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews. 2013;37(2):164–183. doi: 10.1016/j.neubiorev.2012.11.014. http://doi.org/10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Gramfort A, Shetty NR, Kitzbichler MG, Ganesan S, Moran JM, … Kenet T. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(8):3107–3112. doi: 10.1073/pnas.1214533110. http://doi.org/10.1073/pnas.1214533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Reiter MA, Neuhaus E, Pauley G, Martin N, Dager S, Estes A. Subregional differences in intrinsic amygdala hyperconnectivity and hypoconnectivity in autism spectrum disorder. Autism Research: Official Journal of the International Society for Autism Research. 2015 doi: 10.1002/aur.1589. http://doi.org/10.1002/aur.1589. [DOI] [PMC free article] [PubMed]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, … Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain: A Journal of Neurology. 2008;131(Pt 4):1000–1012. doi: 10.1093/brain/awm334. http://doi.org/10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cerebral Cortex (New York, NY: 1991) 2008;18(2):289–300. doi: 10.1093/cercor/bhm054. http://doi.org/10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EHJ, Leventhal BL, DiLavore PC, … Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur AL. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- McKone E, Hall A, Pidcock M, Palermo R, Wilkinson RB, Rivolta D, … O’Connor KB. Face ethnicity and measurement reliability affect face recognition performance in developmental prosopagnosia: Evidence from the Cambridge Face Memory Test–Australian. Cognitive Neuropsychology. 2011;28(2):109–146. doi: 10.1080/02643294.2011.616880. http://doi.org/10.1080/02643294.2011.616880. [DOI] [PubMed] [Google Scholar]
- O’Hearn K, Schroer E, Minshew N, Luna B. Lack of developmental improvement on a face memory task during adolescence in autism. Neuropsychologia. 2010;48(13):3955–3960. doi: 10.1016/j.neuropsychologia.2010.08.024. http://doi.org/10.1016/j.neuropsychologia.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hearn K, Tanaka J, Lynn A, Fedor J, Minshew N, Luna B. Developmental plateau in visual object processing from adolescence to adulthood in autism. Brain and Cognition. 2014;90:124–134. doi: 10.1016/j.bandc.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Lynn A, Foran W, Luna B, O’Hearn K. Age related changes in striatal resting state functional connectivity in autism. Frontiers in Human Neuroscience. 2013;7:814. doi: 10.3389/fnhum.2013.00814. http://doi.org/10.3389/fnhum.2013.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G, Labar KS. Perception of dynamic changes in facial affect and identity in autism. Soc Cogn Affect Neurosci. 2007;2(2):140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Müller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform “face area” in autism: evidence from functional MRI. Brain: A Journal of Neurology. 2001;124(Pt 10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pierce K, Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of “who. Biological Psychiatry. 2008;64(7):552–560. doi: 10.1016/j.biopsych.2008.05.013. http://doi.org/10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–63. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rump KM, Giovannelli JL, Minshew NJ, Strauss MS. The Development of Emotion Recognition in Individuals With Autism. Child Development. 2009;80(5):1434–1447. doi: 10.1111/j.1467-8624.2009.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Mottron L, Soulières I, Zeffiro TA. Enhanced visual functioning in autism: An ALE meta-analysis. Human Brain Mapping. 2012;33(7):1553–1581. doi: 10.1002/hbm.21307. http://doi.org/10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14(4):303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Sato W, Toichi M, Uono S, Kochiyama T. Impaired social brain network for processing dynamic facial expressions in autism spectrum disorders. BMC Neuroscience. 2012;13:99. doi: 10.1186/1471-2202-13-99. http://doi.org/10.1186/1471-2202-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K, Behrmann M, Humphreys K, Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Developmental Science. 2007;10(4):F15–30. doi: 10.1111/j.1467-7687.2007.00595.x. http://doi.org/10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Scherf K, Luna B, Avidan G, Behrmann M. “What” precedes “which”: developmental neural tuning in face- and place-related cortex. Cerebral Cortex (New York, NY: 1991) 2011;21(9):1963–1980. doi: 10.1093/cercor/bhq269. http://doi.org/10.1093/cercor/bhq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K, Luna B, Minshew N, Behrmann M. Location, Location, Location: Alterations in the Functional Topography of Face- but not Object- or Place-Related Cortex in Adolescents with Autism. Frontiers in Human Neuroscience. 2010;4:26. doi: 10.3389/fnhum.2010.00026. http://doi.org/10.3389/fnhum.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K, Thomas C, Doyle J, Behrmann M. Emerging Structure-Function Relations in the Developing Face Processing System. Cerebral Cortex (New York, NY: 1991) 2013 doi: 10.1093/cercor/bht152. http://doi.org/10.1093/cercor/bht152. [DOI] [PMC free article] [PubMed]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, … Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57(4):331–40. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. http://doi.org/10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, Petersen SE. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Human Brain Mapping. 2014;35(5):1981–1996. doi: 10.1002/hbm.22307. http://doi.org/10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, … Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. http://doi.org/10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Solomon M, McCauley JB, Iosif AM, Carter CS, Ragland JD. Cognitive control and episodic memory in adolescents with autism spectrum disorders. Neuropsychologia. 2016;89:31–41. doi: 10.1016/j.neuropsychologia.2016.05.013. http://doi.org/10.1016/j.neuropsychologia.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Deconvolution analysis of fMRI time series data: Documentation for the AFNI software package. 1998. [Google Scholar]
- Weigelt S, Koldewyn K, Kanwisher N. Face recognition deficits in autism spectrum disorders are both domain specific and process specific. PloS One. 2013;8(9):e74541. doi: 10.1371/journal.pone.0074541. http://doi.org/10.1371/journal.pone.0074541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg J, Milleville SC, Kenworthy L, Wallace GL, Gotts SJ, Beauchamp MS, Martin A. Social Perception in Autism Spectrum Disorders: Impaired Category Selectivity for Dynamic but not Static Images in Ventral Temporal Cortex. Cerebral Cortex. 2014;24(1):37–48. doi: 10.1093/cercor/bhs276. http://doi.org/10.1093/cercor/bhs276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Social Cognitive and Affective Neuroscience. 2008;3(2):135–143. doi: 10.1093/scan/nsn007. http://doi.org/10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. http://doi.org/10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalla T, Sperduti M. The amygdala and the relevance detection theory of autism: an evolutionary perspective. Frontiers in Human Neuroscience. 2013;7:894. doi: 10.3389/fnhum.2013.00894. http://doi.org/10.3389/fnhum.2013.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Z, Fang H, Liu J. The hierarchical brain network for face recognition. PloS One. 2013;8(3):e59886. doi: 10.1371/journal.pone.0059886. http://doi.org/10.1371/journal.pone.0059886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1: FFA Regions of Interest. A. Axial view. B. Coronal view. The left side of the image depicts the left hemisphere of the brain for easier viewing. R FFA = 25 voxels (675mm3). L FFA =21 voxels (567mm3).
Supplementary Fig 2: Graphs: Category-specific diagnosis group differences. Regions showing object-specific diagnosis main effect. Connectivity was generally reduced in the group with autism, compared to the TD group for faces, but not cars (except for L FFA—L dorsal lPFC connectivity during recognition). Dark grey bars represent the TD group, light grey bars represent the autism group. R, right; L, left; TD, typical development; mPFC, medial prefrontal cortex; STG, superior temporal gyrus; TPJ, temporoparietal junction; lPFC, lateral prefrontal cortex. *significant diagnosis group differences
Supplementary Fig 3. Graphs: Age-related change differences between diagnosis groups. A. Age-related change differences in R FFA connectivity across both face and car encoding. B. Age-related change differences in L FFA connectivity across both face and car recognition. Generally, age-related change differences between diagnosis groups were evident for face recognition. Dark grey bars represent the TD group, light grey bars represent the autism group. R, right; L, left; Ch, children; Ad, adolescents; Adu, adults. *significant category-specific age-related change differences