Abstract
The gut microbiota interact with innate immune cells and play an important role in shaping the immune system. Many factors may influence the composition of the microbiota such as mode of birth, diet, infections and medication including antibiotics. In diseases with a multifactorial etiology, like type 1 diabetes, manipulation and alterations of the microbiota in animal models has been shown to influence the incidence and onset of disease. The microbiota are an important part of the internal environment and understanding how these bacteria interact with the innate immune cells to generate immune tolerance may open up opportunities for development of new therapeutic strategies. In this review, we discuss recent findings in relation to the microbiota, particularly in the context of type 1 diabetes.
Keywords: gut microbiota, neonatal immune response, type 1 diabetes, immune regulation
Graphical Abstract
How do antibiotics (Abx) affect pancreatic beta cell autoimmunity; gut microbiota composition is altered after Abx treatment and antigen presenting cells exposed to the altered bacteria in the gut display impaired antigen presenting ability to CD8 T cells, which in turn alleviate insulitis in the pancreas and protect the host from diabetes development
1. Gut microbiota and type 1 diabetes
Type 1 diabetes (T1D) is a T cell-mediated autoimmune metabolic disease which is commonly seen in children and young adults (1) although it can also present in older adults. The insulin-producing beta cells of the pancreatic islets are damaged and destroyed by activated autoreactive T cells resulting in disordered blood glucose regulation (2). This destruction is the result of a complex interaction between genetic susceptibility genes and environmental factors (3, 4). Genetic screening has shown that certain major histocompatibility complex (MHC) class II genes, also called human leukocyte antigen (HLA) genes, DQA1*0301 (DQ2), DQB1*0302 (DQ8), DRB1*DR301 (DR3) and a number of DR4 alleles are associated with susceptibility to T1D in patients (5, 6). However, only a small portion of individuals carrying those alleles will develop T1D (7). Yet, a sharp rise of T1D incidence has been seen in recent years (8) in a time frame that is not sufficient for genetic change, indicating that environmental factors may play a crucial role in diabetes development (9). Prenatal influence, viral infections, dietary factors in the young as well as “hygiene” can all affect the disease onset (10). More recently, several studies have shown commensal microbiota to be connected with the development of this autoimmune disease (11). Although triggering factors for T1D have not yet been clearly identified, the gut microbiota are believed to play an important role in the development of the disease (12, 13).
The gut microbiota are associated with the development of several diseases including obesity and type 2 diabetes (14), liver disorders (15), intestinal inflammatory syndromes (16), allergic diseases (17), disorders in the central nervous system (18), and especially, autoimmune diseases (19–22). We, and others, have recently reported that alteration of gut microbiota by pharmacological means can protect from or accelerate T1D development in non-obese diabetic (NOD) mice (23–27), a well established animal model for T1D research (28).
We were among the first to demonstrate that the gut microbiota shape the NOD mouse innate immune system (11). MyD88 is a central adaptor in most innate immune Toll-like receptor signaling pathways and MyD88-deficient NOD mice do not develop autoimmune diabetes in a clean, but not sterile, housing environment; however, germ-free MyD88-deficient mice develop full-blown diabetes (11). This indicates that commensal bacteria, especially gut bacteria play a very important role in triggering the autoimmune disease. When a defined microbial mixture was introduced orally into the germ-free MyD88-deficient mice, diabetes development in these mice was attenuated (11). Similar results were later observed in different mouse models of human diseases including Celiac Disease (29), obesity/type 2 diabetes (30), and autoimmune uveitis (31). There are 10-fold more microorganisms residing in the gut than the total number of human cells (32), and they protect the host from infection by various pathogens (33). The main roles of gut bacteria are to aid in nutrition derived from the diet and to generate energy. A healthy microbiota composition helps to keep the gut epithelia intact and reduce permeability (34, 35). Furthermore, the interaction between gut epithelia and the bacteria promotes the development of a normal immune system (36, 37). Several reports have demonstrated that colonization by some specific bacteria in the gut can protect mice from developing type 1 diabetes; these bacteria include SFB (Segmented Filamentous Bacteria) (38), Lactobacillus johnsonii N6.2 (39), as well as some Streptococcal species (40), and glycoprotein extracts from Klebsiella pneumoniae (41).
2. Modification of the gut microbiota
Although controversial, germ-free mice (11, 42–44) may have accelerated T1D. Conversely, there has been speculation that gut bacteria may trigger T1D development in genetically susceptible humans (45) and mouse models of T1D (46). One possible means by which this could occur could be transfer of metabolites or cell components of the bacteria through a “leaky” gut wall and uptake by antigen presenting cells, processing and presentation of the antigen to activate T cells (47). Tight junctions represent the major barrier within the paracellular pathway between intestinal epithelial cells. Alterations in intestinal permeability allow access of bacterial toxin (45), infectious agents and dietary antigens from the lumen to mucosal immune elements (48, 49). Another possible mechanism is some bacterial product(s) share the molecular homology with islet autoantigen(s) and the islet beta cells are attacked by the immune cells that are reactive to the bactierial antigens (46).
Autoantibodies have been observed in T1D patients as young as several months old (50, 51). Animal model studies have also shown that alteration of gut microbiota early in life, and gut permeability are important in shaping the host immune system (25, 52, 53), especially at the prenatal or neonatal stages.
Efforts have been made to investigate which bacteria in the gut may be beneficial or harmful in the development of T1D (45, 46, 54–57). Researchers have studied altered gut microbiota in experimental mice after treating with a combination of 4 antibiotics, including Ampicillin, Metronidazole, Neomycin and Vancomycin (58). Although there are studies using germ-free (GF) mice to test whether one or more species of bacteria introduced into the mice has an impact on diabetes development (42, 59), which species are probiotic and which are detrimental have not been conclusively determined, as most of the bacteria in the gut are non-culturable.
Other studies have been conducted using vancomycin, a specific gram-positive bacterial inhibitor, to modify the gut bacteria. Antibiotic intervention during the prenatal period revealed an acceleration of diabetes onset (27, 60), whereas NOD mice receiving vancomycin from birth onwards gave the opposite result (52). Recently, Brown and colleagues showed that using Neomycin and Vancomycin to treat NOD mouse pups from the neonatal period for their lifetime (61) accelerated diabetes development. These studies indicated that the time at which antibiotic treatment is commenced is crucial and that treating the mothers may be a way of having an effect while avoiding direct administration of the antibiotics to the pups. Many of these studies used an approach giving long-term antibiotic treatment, although long-term antibiotic treatment rarely occurs in humans. Thus, the advantage of studying short-term treatment makes the studies in animals closer to humans (25, 27). In addition, human studies have shown that approximately 30% of pregnant women in the USA have had a short-course of antibiotic medication during their pregnancy (62) and the number could be higher in other countries. It should be noted that long-term antibiotic treatment could cause resistant bacteria to propagate in the gut (63).
3. Protective bacteria that arise from pharmacological alteration of gut microbiota in early life
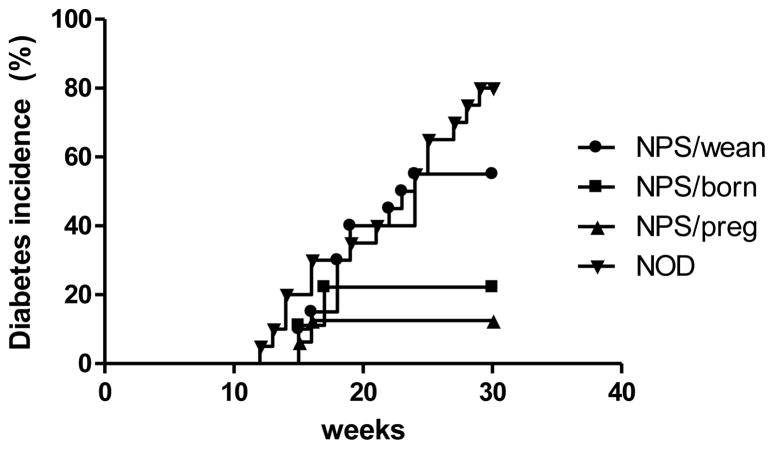
The colonization of gut microbiota is strongly influenced by microbial exposure at birth (64). When antibiotic treatment is used to study the effect of gut microbiota on disease, it is clear that the timing of administration, duration of treatment, as well as the type of antibiotic used must be taken into account. We have published a study showing that Neomycin/Polymyxin B/Streptomycin- treated NOD mice were protected from T1D development (25). This protection was more significant when mice were treated at the prenantal stage (Figure 1, adapted from reference (25))
Figure 1.
Maternal antibiotic treatment protects offspring from diabetes development in NOD mice. Antibiotic treatment (3 week) starting at different times of life led to a different phenotype of T1D development. NPS (Neomycin/Polymyxin B/Streptomycin); NPS/preg (NOD offspring from mothers treated with NPS during pregnancy); NPS/born (NOD treated with NPS from birth to weaning); NPS/wean (NOD treated with NPS immediately after weaning). The change in diabetes incidence was dependent on the time of antibiotic treatment.
It is clear that the earlier the NOD mice received the antibiotics, the better the protection from diabetes development. In our study, NOD mice treated with NPS at different time points early in life delayed and overall reduced T1D onset. When pregnant mothers were treated with antibiotics, the offspring were most protected from diabetes, while mice receiving antibiotics from birth or weaning were also protected from disease development although this was not statistically significant. Here, not only did the NPS treatment generate a gut bacterial composition that was protective but it was also clear that the timing of treatment was very important in inducing the effects. Overall, these studies suggest that gut microbiota in very early life (prenatal or neonatal) may have the most positive impact on the host immune system. Other studies have demonstrated that microbial exposure during early life is important for development and maintenance of the immune system and germ-free mice are more susceptible to developing T1D (42, 43).
Considering which bacteria have protective effects in relation to diabetes development, we have observed that the Gram-positive Firmucutes Lachnospiraceae and Coriobacteriaceae significantly increased in prenatally NPS treated mice (25). Several studies in mice and human case reports indicated that, in both BioBreeding Diabetes-Prone rats (65–67) and diabetic children (68, 69), decreased Bacteroidetes, together with an increase in other Gram-positive Firmicutes such as Lactobacillus, Bifidobacterium, were found, compared to BioBreeding Diabetes-Resistant rats and healthy children, respectively.
SFB (Segmented Filamentous Bacteria) are a group of bacteria within the Genus Candidatus Arthromitus which belongs to the Phylum of Firmicutes, Class of Clostridia and Family of Lachnospiraceae(70). SFB were found to induce intestinal Th17 cells in Lamina Propria (LP) (71) and there are reports showing that colonization of the gut of NOD mice with SFB can induce a substantial population of Th17 cells in the LP and protect the female NOD mice from diabetes development (38). However, we did not find that SFB conferred diabetes protection in NOD mice (27). A study by Yurkovetskiy and colleagues also showed that SFB did not protect GF NOD mice from diabetes development; however, SFB reduced T1D development in male GF NOD mice after colonization with other gut bacteria (72). Since SFB are a group of bacteria, genomic sequencing results from several SFB strains have shown that they are different from each other (73–76). It is, thereofre, conceivable that different strains may have different biological effects. Nevertheless, it is still important to study the role of SFB in mice because these gram-positive, anaerobic, commensal bacteria are capable of inducing the postnatal maturation of homeostatic innate and adaptive immune responses in the gut. A recent publication also showed that not only can colonization with SFB induce IL-17A but CXCR2-dependent recruitment of neutrophils in the gut also occurs (77).
4. Shaping the immune system via alterations in gut bacteria
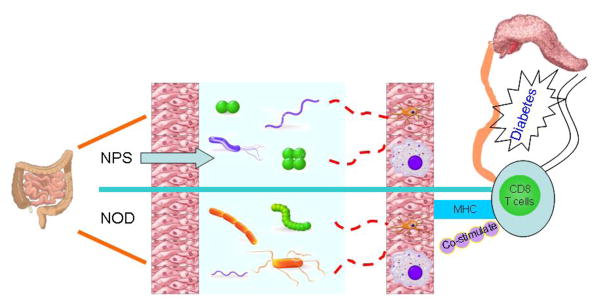
How does alteration of the gut bacteria lead to protection from diseases like type 1 diabetes? There are a number of proposed mechanisms which are associated with genetics, gut microbiota (related to antibiotic usage, mode of delivery, diet) and infection in animal models and humans (78, 79). Our recent studies indicate that protection can be mediated by tolerogenic antigne presenting cells (APCs) originating in the gut associated lymphoid tissue (GALT), which have reduced ability to stimulate cytotoxic CD8+ T cells. (Figure 2, Cover figure)
Figure 2.
How do antibiotics affect pancreatic beta cell autoimmunity: gut microbiota composition is altered after antibiotic (NPS) treatment and antigen presenting cells exposed to the altered bacteria in the gut display impaired antigen presenting ability to CD8 T cells, which in turn alleviate insulitis in the pancreas and protect the host from diabetes development.
In supporting the mechanism of tolerogenic APCs as a result of altered gut microbiota due to antibiotic usage, Umenai and coauthors showed hyporesponsiveness of macrophages, a potent subset of APCs, in response to LPS stimulation in mice after Streptomycin treatment (80). Similarly, a recent study demonstrated that dendritic cells became hyporesponsive to LPS stimulation and reduced inflammatory cytokine production upon exposure to the gut bacterium Lactobacillus reuteri (81). Dolpady and co-authors reported that administration of a mixture of Bifidobacteriaceae, Lactobacillaceae and Streptococcus in 4-wk old mice promoted tolerogenic CD103+ dendritic cells and reduced Th1 and Th17 cells in mucosal and PLN sites (82). Thus, alteration of gut microbiota by different means including antibiotic treatment can affect APCs lead to acceleration of or protection from diabetes development. The changes in APC function could be the result of direct contact between APCs and gut microbiota in the gut or indirect contact, mediated by metabolites from altered gut microbiota. Due to the immature nature of gut barrier and the changing, maturing community of gut microbiota in early-life, timing becomes critical.
Reduction in regulatory T cell markers have also been postulated as a mechanism for protection of NOD mice after treating with a mixture of antibiotics – metronidazole, streptomycin and polymyxin prenatally (53, 60). These studies demonstrated that the changes in the immune system occurred when antibiotic treatment was given prenatally, a particularly important time for immune system development. Livanos and coauthors reported recently that NOD mice receiving antibiotics (penicillin V) from lactation until the age of 40 days had earlier diabetes onset and overall higher incidence of diabetes (83). This early-life treatment reduced the percentage of Treg and Th17 cells in lamina propria (LP), which may have contributed to the accelerated diabetes development. In the Streptozotocin (STZ)-induced type 1 diabetes model, mice treated with antibiotics were fully protected from diabetes (84). This was attributed to blocking pro-diabetic bacteria translocation to PLN (84).
Early-life treatment using vancomycin reduced the incidence of diabetes in one study (52) but accelerated diabetes in another (27). The discrepancy may be attributed to different treatment protocols. However, the protection in Hansen’s study was accompanied by an increase in the level of Akkermansia, which was later reported to be correlated with a pro-diabetic effect (85, 86). A gluten-free diet can also reduce inflammation and diabetes incidence in NOD, with elevated abundance of Akkermansia. Adding gluten to the gluten-free diet reversed the protection, accompanied by a decreased level of Akkermansia (86). More recently, a study showed that Akkermansia mediate glucose tolerance via IFNγ (87) using loss-and-gain-of-Akkermansia muciniphila approaches in IFNγ knock-out mice. In a clinical study, a 4-day treatment with broad-spectrum antibiotics (Vancomycin, Gentamicin and Meropenem) significantly shifted the gut microbiota composition but this did not have a clinical impact in respect of metabolic markers such as glucose tolerance or insulin secretion (88). However, in a study by Endesfelder and coauthors a change of gut microbiota occurring as a result of dietary change had an impact on islet autoimmunity. The authors stratified the children in the study based on the microbial communities identified. They found that it was possible to detect functional associations between the diet consumed, the microbiome and development of autoimmunity. They identified a subgroup of children where Bacteroides was dominant, with low Akkermansia in the gut microbiota and this was associated with early introduction of a non-milk diet, lower abundance of genes for the production of butyrate and early autoantibody development (89, 90). They postulated that low butyrate generation by the bacteria contributed to increased risk of the development of islet autoantibodies (90).
It is clear that the effect of gut microbiota on host immune responses early in life is much stronger than later in life. This was observed in human studies demonstrating that the development of type 1 diabetes was closely related to gut microbiota, gut permeability and immune system in early-life. Amarri and colleagues have previously found that gut bacteria and gut permeability as well as other immune markers were significantly altered in breast-fed infants (89). Other early influences have been demonstrated in a Danish study where the authors found that antibiotics used in early-life increased the incidence of type 1 diabetes in young children: however, this was also related to the mode of birth delivery, which has major effects on determining the composition of gut microbiota at the time of birth (91).
Antibiotic treatment in adult mice is not as effective as prenatal or neonatal treatment in altering the course of type 1 diabetes. However, later life antibiotic treatment can significantly alter the gut bacteria and it can have clear impact on diseases other than T1D, including Crohn’s disease (92), colitis (93), obesity and type 2 diabetes (94).
In addition to antibiotics, it is known that innate immune system can also alter gut microbiota, which affect both type 1 and type 2 diabetes development (11, 30). However, it was not clear which type of bacteria contribute to the protection or promotion of diabetes. Using a MyD88-deficient NOD mouse that has a defined T cell receptor reperotire, we recently found that one type of bacterium, Leptotrichia goodfellowii, in the gut can trigger diabetes development in islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)-reactive CD8 T cell receptor NY8.3 transgenic NOD model. L. goodfellowii is a member of Fusobacteria, and expresses a protein peptide sharing a homologous sequence with IGRP and its abundance is correlated with the progression of diabetes (46). This is the first evidence of molecular mimicry that relates to a bacterial antigen in initiation of diabetes onset, due to T cell cross-reactivity to the microbial peptide and leading to an activated autoimmune response.
5. Fecal microbiota transfer (FMT) as a potential therapy
Gut bacterial composition may be altered by diet, antibiotics, probiotics, or by direct microbiota transfer. Fecal microbiota transfer (FMT) has proven to be a effective way to transfer “healthy” microbiota, that may have beneficial effects on metabolism and the immune system in recipient mice (30, 95). FMT has also been used in patients with colitis (96–98) and recently in cancer patients (99, 100). We recently demonstrated that FMT could restore the gut microbiota and rebalance the gut hemeostasis, which in turns delayed diabetes onset in NOD mice (26, 29). In other disease models, including obesity, the gut microbiota have been shown to contribute to generation of the metabolic syndrome (30). FMT from lean mice to obese mice can ameliorate the metabolic syndrome in the obese mice due to the rebalancing of gut microbiota (101). Treating metabolic syndrome with FMT has also been tested in humans [98].
The beneficial effects of FMT have been particularly used in patients suffering from Clostridium difficile infection (102, 103). Although antibiotic treatment has been the mainstay of treatment of Clostridium difficile infection, some patients have developed recurrent infection later on and importantly antibiotic treatment for other medical conditions can lead to persistent infection with Clostridium difficile (104–106). Since the clinical trial on FMT in patients with recurrent Clostridium difficile infection (103), considerable progress has been made in this field, including formulation of the microbiota and delivery route of the bacteria. Oral administration is very common using capsules containing the gut microbiota of healthy donors after pathogen-free screening, stool purification and preservation processes (107). In addition, FMT has also been effective in treating diseases like ulcerative colitis (108) and chronic pouchitis (109) in clinical practice. Although FMT has not been used in T1D treatment, if specific combinations of beneficial bacteria could be identified, this could be a promising therapy, given that fecal filtrate has a similar efficacy to fecal microbiota in treating other medical conditions (110). However, more comprehensive studies need to be performed to understand which what bacterium (or bacteria) and metabolites can trigger the imbalance of immune system at early-life in humans at high-risk of developing T1D.
6. Perspectives and conclusion
It is clear that many factors can affect the composition of gut microbiota, which modulate the immune system contributing to health or disease including T1D. Birth delivery mode (cesarean section vs. vaginal delivery), breast vs. formulation feeding and antibiotic usage, all can have a strong impact on the health of newborn infants (111–113). The period in utero, as well as post-partum, is a crucial time for establishment of the immune system of the babies. Intervention during early life may lead to novel and more effective therapeutic strategies in treating T1D. There is no doubt that gut microbiota are associated with particular metabolites and can influence both metabolic pathways and the development of the immune system. Many studies have shown that depletion or alteration of intestinal microbiota has a significant impact on gut mucosal and epithelial gene expression, as well as immune responses (114). Gram-negative and Gram-positive bacteria can stimulate different immune responses and induce Th1 or Th2 type cytokines, as well as proinflammatory or anti-inflammatory mediators (115–118). We summarized some of the bacteria studied in association with T1D (Table 1).
Table 1.
Summary of bacteria studied in association with T1D
| Bacteria | Gram-positive or -negative | Mode of action | Protection from or acceleration of T1D | References |
|---|---|---|---|---|
| SFB (Segmented filamentous bacteria), Candidatus Arthromitus | positive | Induce Th17 response | Protection No effect |
38 27 |
| Lactobacillus johnsonii & L. reuteri | positive | Healthy probiotics | Protection | 39, 81 |
| Klebsiella pneumoniae | negative | Pathogen causing pneumonia | Protection | 41 |
| Bifidobacterium | positive | Often as healthy probiotics | Acceleration | 67–69 |
| Streptococcus | positive | Some pathogens, some commensal bacteria in mouth, skin, intestine and upper respiratory tract | Protection | 40, 82 |
| Akkermansia muciniphila | negative | Mucin-degrading, often anti-inflammatory effect | Protection | 52, 90 |
| Bacteroides | negative | processing complex molecules to simpler compounds in the host intestine | Acceleration | 89, 90 |
| Leptotrichia goodfellowii | negative | Oral commensal, pathogen in immune compromised patients | Acceleration in NY8.3 NOD | 46 |
APC dysfunction has been found to be associated with T1D development (119, 120), but previously this had not been correlated with alteration of gut bacteria. Our recent studies provide evidence that tolerogenic APC are generated as the results of altered gut microbiota (25, 27). Interestingly, the tolerogenic APCs can confer T1D protection and this protection can also be transferred to a second host and to the offspring. Probiotics and other strategies altering the gut microbiota, including FMT, could be a promising approach to modulate gut microbiota and rebalance the homeostasis of mucosal and systemic immune systems. This “bug for drug” approach, also called “Bacteriotherapy” is being tested in different clinical trials for other medical conditions (121). The prospect of this approach for T1D could be on the horizon. Type 1 diabetes has not only a genetically inherited component that determines susceptibility to disease, but the interaction with the environment to precipitate disease is a very important part of the pathogenesis. Identifying modifiable environmental factors, such as the gut microbiota would provide a therapeutic target that could be modified by treatments that could be easy to administer. It would be important to identify safe means of doing this that could potentially be administered very early in life. Probiotics have already been tested in infants and young children but the appropriate composition should be identified. Whether gut bacteria are involved in the initiation of the process leading to T1D or in the progression of β-cell autoimmunity is still unclear (122). A pure, culturable bacterial cocktail would need to be identified before this kind of regimen could be used in clinical practice.
A number of immunotherapeutic treatments have been trialed in human type 1 diabetes, some of which have had a transient effect, but none as yet have been long-lasting (123). It has been argued that for a treatment to be successful, the innate immune system should present antigens in a tolerogenic manner to T regulatory cells (123) and this is potentially one of the ways in which altering the gut microbiota could work if the right combination could be identified. Ideally, this type of treatment would be combined with others that could be synergistic in maintaining a tolerogenic environment.
In conclusion, both probiotic and antibiotic treatment can significantly alter the gut bacteria, as well as the bacteria composition in other sites of the body including the oral cavity. The altered bacteria interact with the host immune system and reduce inflammatory cytokines as well as the expression of costimlatory molecules in APCs, inducing a tolerogenic environment. However, further investigation is required and, in particular, more work done to identify less disease- promoting gut microbiota and how to maintain this type of gut microbiome.
Acknowledgments
The authors thank all the members in Wen laboratory, past and the present. This work was funded by research grants from NIH (DK092882, DK100500 and P30 DK945735), American Diabetes Association (14-13-BS-222) and JDRF (2015-136).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Babaya N, Nakayama M, Eisenbarth GS. The stages of type 1A diabetes. Ann N Y Acad Sci. 2005;1051:194–204. doi: 10.1196/annals.1361.061. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe KA, Hitman GA, Rowe RE, Hawa M, Huang X, Stewart T, Leslie RD. Concordance for type 1 diabetes in identical twins is affected by insulin genotype. Diabetes Care. 2001;24(5):838–842. doi: 10.2337/diacare.24.5.838. [DOI] [PubMed] [Google Scholar]
- 4.Redondo MJ, Yu L, Hawa M, Mackenzie T, Pyke DA, Eisenbarth GS, Leslie RD. Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia. 2001;44(3):354–362. doi: 10.1007/s001250051626. [DOI] [PubMed] [Google Scholar]
- 5.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F, Lowe CE, Szeszko JS, Hafler JP, Zeitels L, Yang JH, Vella A, Nutland S, Stevens HE, Schuilenburg H, Coleman G, Maisuria M, Meadows W, Smink LJ, Healy B, Burren OS, Lam AA, Ovington NR, Allen J, Adlem E, Leung HT, Wallace C, Howson JM, Guja C, Ionescu-Tirgoviste C, Simmonds MJ, Heward JM, Gough SC, Dunger DB, Wicker LS, Clayton DG. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39(7):857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 7.Achenbach P, Bonifacio E, Koczwara K, Ziegler AG. Natural history of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S25–31. doi: 10.2337/diabetes.54.suppl_2.s25. [DOI] [PubMed] [Google Scholar]
- 8.Patterson CC, Gyurus E, Rosenbauer J, Cinek O, Neu A, Schober E, Parslow RC, Joner G, Svensson J, Castell C, Bingley PJ, Schoenle E, Jarosz-Chobot P, Urbonaite B, Rothe U, Krzisnik C, Ionescu-Tirgoviste C, Weets I, Kocova M, Stipancic G, Samardzic M, de Beaufort CE, Green A, Dahlquist GG, Soltesz G. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55(8):2142–2147. doi: 10.1007/s00125-012-2571-8. [DOI] [PubMed] [Google Scholar]
- 9.Knip M, Veijola R, Virtanen SM, Hyoty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S125–136. doi: 10.2337/diabetes.54.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- 10.Knip M, Simell O. Environmental triggers of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(7):a007690. doi: 10.1101/cshperspect.a007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455(7216):1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10(10):735–744. doi: 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- 13.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord. 2015;16(1):55–65. doi: 10.1007/s11154-015-9309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llorente C, Schnabl B. The gut microbiota and liver disease. Cell Mol Gastroenterol Hepatol. 2015;1(3):275–284. doi: 10.1016/j.jcmgh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto Y, Kurashima Y, Kiyono H. The gut microbiota and inflammatory bowel disease. Curr Opin Rheumatol. 2015;27(4):388–396. doi: 10.1097/BOR.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 17.Vital M, Harkema JR, Rizzo M, Tiedje J, Brandenberger C. Alterations of the Murine Gut Microbiome with Age and Allergic Airway Disease. J Immunol Res. 2015;2015:892568. doi: 10.1155/2015/892568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Lukiw WJ. Microbiome-generated amyloid and potential impact on amyloidogenesis in Alzheimer’s disease (AD) J Nat Sci. 2015;1(7):e138. [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson BM, Gaudreau MC, Al-Gadban MM, Gudi R, Vasu C. Impact of dietary deviation on disease progression and gut microbiome composition in lupus-prone SNF1 mice. Clin Exp Immunol. 2015;181(2):323–337. doi: 10.1111/cei.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verbeke KA, Boesmans L, Boets E. Modulating the microbiota in inflammatory bowel diseases: prebiotics, probiotics or faecal transplantation? Proc Nutr Soc. 2014;73(4):490–497. doi: 10.1017/S0029665114000639. [DOI] [PubMed] [Google Scholar]
- 21.Walujkar SA, Dhotre DP, Marathe NP, Lawate PS, Bharadwaj RS, Shouche YS. Characterization of bacterial community shift in human Ulcerative Colitis patients revealed by Illumina based 16S rRNA gene amplicon sequencing. Gut Pathog. 2014;6:22. doi: 10.1186/1757-4749-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alkanani AK, Hara N, Gottlieb PA, Ir D, Robertson CE, Wagner BD, Frank DN, Zipris D. Alterations in Intestinal Microbiota Correlate With Susceptibility to Type 1 Diabetes. Diabetes. 2015;64(10):3510–3520. doi: 10.2337/db14-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burrows MP, Volchkov P, Kobayashi KS, Chervonsky AV. Microbiota regulates type 1 diabetes through Toll-like receptors. Proc Natl Acad Sci U S A. 2015;112(32):9973–9977. doi: 10.1073/pnas.1508740112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Y, Peng J, Tai N, Hu C, Zhang X, Wong FS, Wen L. Maternal Antibiotic Treatment Protects Offspring from Diabetes Development in Nonobese Diabetic Mice by Generation of Tolerogenic APCs. J Immunol. 2015;195(9):4176–4184. doi: 10.4049/jimmunol.1500884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng J, Narasimhan S, Marchesi JR, Benson A, Wong FS, Wen L. Long term effect of gut microbiota transfer on diabetes development. J Autoimmun. 2014;53:85–94. doi: 10.1016/j.jaut.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Y, Jin P, Peng J, Zhang X, Wong FS, Wen L. Different immunological responses to early-life antibiotic exposure affecting autoimmune diabetes development in NOD mice. J Autoimmun. 2016;72:47–56. doi: 10.1016/j.jaut.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson JA, Wong FS, Wen L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun. 2016;66:76–88. doi: 10.1016/j.jaut.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galipeau HJ, McCarville JL, Huebener S, Litwin O, Meisel M, Jabri B, Sanz Y, Murray JA, Jordana M, Alaedini A, Chirdo FG, Verdu EF. Intestinal Microbiota Modulates Gluten-Induced Immunopathology in Humanized Mice. Am J Pathol. 2015;185(11):2969–2982. doi: 10.1016/j.ajpath.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horai R, Zarate-Blades CR, Dillenburg-Pilla P, Chen J, Kielczewski JL, Silver PB, Jittayasothorn Y, Chan CC, Yamane H, Honda K, Caspi RR. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity. 2015;43(2):343–353. doi: 10.1016/j.immuni.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macpherson AJ, Uhr T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann N Y Acad Sci. 2004;1029:36–43. doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- 33.Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011;14(1):82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Natividad JM, Petit V, Huang X, de Palma G, Jury J, Sanz Y, Philpott D, Garcia Rodenas CL, McCoy KD, Verdu EF. Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1−/−; Nod2−/− mice. Inflamm Bowel Dis. 2012;18(8):1434–1446. doi: 10.1002/ibd.22848. [DOI] [PubMed] [Google Scholar]
- 35.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12(5):319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dave M, Higgins PD, Middha S, Rioux KP. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160(4):246–257. doi: 10.1016/j.trsl.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology. 2011;140(6):1713–1719. doi: 10.1053/j.gastro.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2011;108(28):11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau K, Benitez P, Ardissone A, Wilson TD, Collins EL, Lorca G, Li N, Sankar D, Wasserfall C, Neu J, Atkinson MA, Shatz D, Triplett EW, Larkin J., 3rd Inhibition of type 1 diabetes correlated to a Lactobacillus johnsonii N6.2-mediated Th17 bias. J Immunol. 2011;186(6):3538–3546. doi: 10.4049/jimmunol.1001864. [DOI] [PubMed] [Google Scholar]
- 40.Satoh J, Shintani S, Oya K, Tanaka S, Nobunaga T, Toyota T, Goto Y. Treatment with streptococcal preparation (OK-432) suppresses anti-islet autoimmunity and prevents diabetes in BB rats. Diabetes. 1988;37(9):1188–1194. doi: 10.2337/diab.37.9.1188. [DOI] [PubMed] [Google Scholar]
- 41.Sai P, Rivereau AS. Prevention of diabetes in the nonobese diabetic mouse by oral immunological treatments. Comparative efficiency of human insulin and two bacterial antigens, lipopolysacharide from Escherichia coli and glycoprotein extract from Klebsiella pneumoniae. Diabetes Metab. 1996;22(5):341–348. [PubMed] [Google Scholar]
- 42.Alam C, Bittoun E, Bhagwat D, Valkonen S, Saari A, Jaakkola U, Eerola E, Huovinen P, Hanninen A. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia. 2011;54(6):1398–1406. doi: 10.1007/s00125-011-2097-5. [DOI] [PubMed] [Google Scholar]
- 43.King C, Sarvetnick N. The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PLoS One. 2011;6(2):e17049. doi: 10.1371/journal.pone.0017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bach JF, Chatenoud L. The hygiene hypothesis: an explanation for the increased frequency of insulin-dependent diabetes. Cold Spring Harb Perspect Med. 2012;2(2):a007799. doi: 10.1101/cshperspect.a007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hamalainen AM, Peet A, Tillmann V, Uibo R, Mokurov S, Dorshakova N, Ilonen J, Virtanen SM, Szabo SJ, Porter JA, Lahdesmaki H, Huttenhower C, Gevers D, Cullen TW, Knip M, Group DS, Xavier RJ. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell. 2016;165(4):842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai N, Peng J, Liu F, Gulden E, Hu Y, Zhang X, Chen L, Wong FS, Wen L. Microbial antigen mimics activate diabetogenic CD8 T cells in NOD mice. J Exp Med. 2016;213(10):2129–2146. doi: 10.1084/jem.20160526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Atkinson MA. The role for gut permeability in the pathogenesis of type 1 diabetes - a solid or leaky concept? Pediatr Diabetes. 2015;16(7):485–492. doi: 10.1111/pedi.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Kort S, Keszthelyi D, Masclee AA. Leaky gut and diabetes mellitus: what is the link? Obes Rev. 2011;12(6):449–458. doi: 10.1111/j.1467-789X.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu Z, Li N, Neu J. Tight junctions, leaky intestines, and pediatric diseases. Acta Paediatr. 2005;94(4):386–393. doi: 10.1111/j.1651-2227.2005.tb01904.x. [DOI] [PubMed] [Google Scholar]
- 50.Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, Peet A, Tillmann V, Poho P, Mattila I, Lahdesmaki H, Franzosa EA, Vaarala O, de Goffau M, Harmsen H, Ilonen J, Virtanen SM, Clish CB, Oresic M, Huttenhower C, Knip M, Xavier RJ. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17(2):260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kupila A, Muona P, Simell T, Arvilommi P, Savolainen H, Hamalainen AM, Korhonen S, Kimpimaki T, Sjoroos M, Ilonen J, Knip M, Simell O. Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia. 2001;44(3):290–297. doi: 10.1007/s001250051616. [DOI] [PubMed] [Google Scholar]
- 52.Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sorensen SJ, Buschard K, Hansen AK. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55(8):2285–2294. doi: 10.1007/s00125-012-2564-7. [DOI] [PubMed] [Google Scholar]
- 53.Tormo-Badia N, Hakansson A, Vasudevan K, Molin G, Ahrne S, Cilio CM. Antibiotic treatment of pregnant non-obese diabetic mice leads to altered gut microbiota and intestinal immunological changes in the offspring. Scand J Immunol. 2014;80(4):250–260. doi: 10.1111/sji.12205. [DOI] [PubMed] [Google Scholar]
- 54.Lammi N, Karvonen M, Tuomilehto J. Do microbes have a causal role in type 1 diabetes? Med Sci Monit. 2005;11(3):RA63–69. [PubMed] [Google Scholar]
- 55.Alyanakian MA, Grela F, Aumeunier A, Chiavaroli C, Gouarin C, Bardel E, Normier G, Chatenoud L, Thieblemont N, Bach JF. Transforming growth factor-beta and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes. 2006;55(1):179–185. [PubMed] [Google Scholar]
- 56.Korsgren S, Molin Y, Salmela K, Lundgren T, Melhus A, Korsgren O. On the etiology of type 1 diabetes: a new animal model signifying a decisive role for bacteria eliciting an adverse innate immunity response. Am J Pathol. 2012;181(5):1735–1748. doi: 10.1016/j.ajpath.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peraneva L, Fogarty CL, Pussinen PJ, Forsblom C, Groop PH, Lehto M. Systemic exposure to Pseudomonal bacteria: a potential link between type 1 diabetes and chronic inflammation. Acta Diabetol. 2013;50(3):351–361. doi: 10.1007/s00592-012-0421-2. [DOI] [PubMed] [Google Scholar]
- 58.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16(2):228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patrick C, Wang GS, Lefebvre DE, Crookshank JA, Sonier B, Eberhard C, Mojibian M, Kennedy CR, Brooks SP, Kalmokoff ML, Maglio M, Troncone R, Poussier P, Scott FW. Promotion of autoimmune diabetes by cereal diet in the presence or absence of microbes associated with gut immune activation, regulatory imbalance, and altered cathelicidin antimicrobial Peptide. Diabetes. 2013;62(6):2036–2047. doi: 10.2337/db12-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Candon S, Perez-Arroyo A, Marquet C, Valette F, Foray AP, Pelletier B, Milani C, Ventura M, Bach JF, Chatenoud L. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS One. 2015;10(5):e0125448. doi: 10.1371/journal.pone.0125448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown K, Godovannyi A, Ma C, Zhang Y, Ahmadi-Vand Z, Dai C, Gorzelak MA, Chan Y, Chan JM, Lochner A, Dutz JP, Vallance BA, Gibson DL. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. Isme J. 2016;10(2):321–332. doi: 10.1038/ismej.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978–985. doi: 10.1001/archpediatrics.2009.188. [DOI] [PubMed] [Google Scholar]
- 63.Card RM, Mafura M, Hunt T, Kirchner M, Weile J, Rashid MU, Weintraub A, Nord CE, Anjum MF. Impact of Ciprofloxacin and Clindamycin Administration on Gram-Negative Bacteria Isolated from Healthy Volunteers and Characterization of the Resistance Genes They Harbor. Antimicrob Agents Chemother. 2015;59(8):4410–4416. doi: 10.1128/AAC.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brugman S, Klatter FA, Visser JT, Wildeboer-Veloo AC, Harmsen HJ, Rozing J, Bos NA. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49(9):2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 66.Visser JT, Bos NA, Harthoorn LF, Stellaard F, Beijer-Liefers S, Rozing J, van Tol EA. Potential mechanisms explaining why hydrolyzed casein-based diets outclass single amino acid-based diets in the prevention of autoimmune diabetes in diabetes-prone BB rats. Diabetes Metab Res Rev. 2012;28(6):505–513. doi: 10.1002/dmrr.2311. [DOI] [PubMed] [Google Scholar]
- 67.Roesch LF, Lorca GL, Casella G, Giongo A, Naranjo A, Pionzio AM, Li N, Mai V, Wasserfall CH, Schatz D, Atkinson MA, Neu J, Triplett EW. Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. Isme J. 2009;3(5):536–548. doi: 10.1038/ismej.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Goffau MC, Luopajarvi K, Knip M, Ilonen J, Ruohtula T, Harkonen T, Orivuori L, Hakala S, Welling GW, Harmsen HJ, Vaarala O. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes. 2013;62(4):1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F, Soriguer F, Queipo-Ortuno MI. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46. doi: 10.1186/1741-7015-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson CL, Vier R, Mikaelyan A, Wienemann T, Brune A. ‘Candidatus Arthromitus’ revised: segmented filamentous bacteria in arthropod guts are members of Lachnospiraceae. Environ Microbiol. 2012;14(6):1454–1465. doi: 10.1111/j.1462-2920.2012.02731.x. [DOI] [PubMed] [Google Scholar]
- 71.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bolotin A, de Wouters T, Schnupf P, Bouchier C, Loux V, Rhimi M, Jamet A, Dervyn R, Boudebbouze S, Blottiere HM, Sorokin A, Snel J, Cerf-Bensussan N, Gaboriau-Routhiau V, van de Guchte M, Maguin E. Genome Sequence of “Candidatus Arthromitus” sp. Strain SFB-Mouse-NL, a Commensal Bacterium with a Key Role in Postnatal Maturation of Gut Immune Functions. Genome Announc. 2014;2(4) doi: 10.1128/genomeA.00705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caselli M, Cassol F, Gentili V, Di Luca D. Genome sequences of segmented filamentous bacteria in animals: implications for human research. Gut Microbes. 2012;3(5):401–405. doi: 10.4161/gmic.20736. [DOI] [PubMed] [Google Scholar]
- 75.Pamp SJ, Harrington ED, Quake SR, Relman DA, Blainey PC. Single-cell sequencing provides clues about the host interactions of segmented filamentous bacteria (SFB) Genome Res. 2012;22(6):1107–1119. doi: 10.1101/gr.131482.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prakash T, Oshima K, Morita H, Fukuda S, Imaoka A, Kumar N, Sharma VK, Kim SW, Takahashi M, Saitou N, Taylor TD, Ohno H, Umesaki Y, Hattori M. Complete genome sequences of rat and mouse segmented filamentous bacteria, a potent inducer of th17 cell differentiation. Cell Host Microbe. 2011;10(3):273–284. doi: 10.1016/j.chom.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 77.Flannigan KL, Ngo VL, Geem D, Harusato A, Hirota SA, Parkos CA, Lukacs NW, Nusrat A, Gaboriau-Routhiau V, Cerf-Bensussan N, Gewirtz AT, Denning TL. IL-17A-mediated neutrophil recruitment limits expansion of segmented filamentous bacteria. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paun A, Yau C, Danska JS. Immune recognition and response to the intestinal microbiome in type 1 diabetes. J Autoimmun. 2016;71:10–18. doi: 10.1016/j.jaut.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 79.Scott FW, Pound LD, Patrick C, Eberhard CE, Crookshank JA. Where genes meet environment-integrating the role of gut luminal contents, immunity and pancreas in type 1 diabetes. Transl Res. 2017;179:183–198. doi: 10.1016/j.trsl.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Umenai T, Hirai H, Shime N, Nakaya T, Asahara T, Nomoto K, Kita M, Tanaka Y, Imanishi J. Eradication of the commensal intestinal microflora by oral antimicrobials interferes with the host response to lipopolysaccharide. Eur J Clin Microbiol Infect Dis. 2010;29(6):633–641. doi: 10.1007/s10096-010-0905-3. [DOI] [PubMed] [Google Scholar]
- 81.Haileselassie Y, Navis M, Vu N, Qazi KR, Rethi B, Sverremark-Ekstrom E. Lactobacillus reuteri and Staphylococcus aureus differentially influence the generation of monocyte-derived dendritic cells and subsequent autologous T cell responses. Immun Inflamm Dis. 2016;4(3):315–326. doi: 10.1002/iid3.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dolpady J, Sorini C, Di Pietro C, Cosorich I, Ferrarese R, Saita D, Clementi M, Canducci F, Falcone M. Oral Probiotic VSL#3 Prevents Autoimmune Diabetes by Modulating Microbiota and Promoting Indoleamine 2,3-Dioxygenase-Enriched Tolerogenic Intestinal Environment. J Diabetes Res. 2016;2016:7569431. doi: 10.1155/2016/7569431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Livanos AE, Greiner TU, Vangay P, Pathmasiri W, Stewart D, McRitchie S, Li H, Chung J, Sohn J, Kim S, Gao Z, Barber C, Kim J, Ng S, Rogers AB, Sumner S, Zhang XS, Cadwell K, Knights D, Alekseyenko A, Backhed F, Blaser MJ. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol. 2016;1(11):16140. doi: 10.1038/nmicrobiol.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Costa FR, Francozo MC, de Oliveira GG, Ignacio A, Castoldi A, Zamboni DS, Ramos SG, Camara NO, de Zoete MR, Palm NW, Flavell RA, Silva JS, Carlos D. Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J Exp Med. 2016;213(7):1223–1239. doi: 10.1084/jem.20150744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hansen CH, Krych L, Buschard K, Metzdorff SB, Nellemann C, Hansen LH, Nielsen DS, Frokiaer H, Skov S, Hansen AK. A maternal gluten-free diet reduces inflammation and diabetes incidence in the offspring of NOD mice. Diabetes. 2014;63(8):2821–2832. doi: 10.2337/db13-1612. [DOI] [PubMed] [Google Scholar]
- 86.Marietta EV, Gomez AM, Yeoman C, Tilahun AY, Clark CR, Luckey DH, Murray JA, White BA, Kudva YC, Rajagopalan G. Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome. PLoS One. 2013;8(11):e78687. doi: 10.1371/journal.pone.0078687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greer RL, Dong X, Moraes AC, Zielke RA, Fernandes GR, Peremyslova E, Vasquez-Perez S, Schoenborn AA, Gomes EP, Pereira AC, Ferreira SR, Yao M, Fuss IJ, Strober W, Sikora AE, Taylor GA, Gulati AS, Morgun A, Shulzhenko N. Akkermansia muciniphila mediates negative effects of IFNgamma on glucose metabolism. Nat Commun. 2016;7:13329. doi: 10.1038/ncomms13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mikkelsen KH, Frost M, Bahl MI, Licht TR, Jensen US, Rosenberg J, Pedersen O, Hansen T, Rehfeld JF, Holst JJ, Vilsboll T, Knop FK. Effect of Antibiotics on Gut Microbiota, Gut Hormones and Glucose Metabolism. PLoS One. 2015;10(11):e0142352. doi: 10.1371/journal.pone.0142352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amarri S, Benatti F, Callegari ML, Shahkhalili Y, Chauffard F, Rochat F, Acheson KJ, Hager C, Benyacoub J, Galli E, Rebecchi A, Morelli L. Changes of gut microbiota and immune markers during the complementary feeding period in healthy breast-fed infants. J Pediatr Gastroenterol Nutr. 2006;42(5):488–495. doi: 10.1097/01.mpg.0000221907.14523.6d. [DOI] [PubMed] [Google Scholar]
- 90.Endesfelder D, Engel M, Davis-Richardson AG, Ardissone AN, Achenbach P, Hummel S, Winkler C, Atkinson M, Schatz D, Triplett E, Ziegler AG, zu Castell W. Towards a functional hypothesis relating anti-islet cell autoimmunity to the dietary impact on microbial communities and butyrate production. Microbiome. 2016;4:17. doi: 10.1186/s40168-016-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clausen TD, Bergholt T, Bouaziz O, Arpi M, Eriksson F, Rasmussen S, Keiding N, Lokkegaard EC. Broad-Spectrum Antibiotic Treatment and Subsequent Childhood Type 1 Diabetes: A Nationwide Danish Cohort Study. PLoS One. 2016;11(8):e0161654. doi: 10.1371/journal.pone.0161654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, Morgan XC, Kostic AD, Luo C, Gonzalez A, McDonald D, Haberman Y, Walters T, Baker S, Rosh J, Stephens M, Heyman M, Markowitz J, Baldassano R, Griffiths A, Sylvester F, Mack D, Kim S, Crandall W, Hyams J, Huttenhower C, Knight R, Xavier RJ. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80(1):62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 95.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 96.Vaughn BP, Vatanen T, Allegretti JR, Bai A, Xavier RJ, Korzenik J, Gevers D, Ting A, Robson SC, Moss AC. Increased Intestinal Microbial Diversity Following Fecal Microbiota Transplant for Active Crohn’s Disease. Inflamm Bowel Dis. 2016;22(9):2182–2190. doi: 10.1097/MIB.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lopez J, Grinspan A. Fecal Microbiota Transplantation for Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 2016;12(6):374–379. [PMC free article] [PubMed] [Google Scholar]
- 98.Ishikawa D, Sasaki T, Osada T, Kuwahara-Arai K, Haga K, Shibuya T, Hiramatsu K, Watanabe S. Changes in Intestinal Microbiota Following Combination Therapy with Fecal Microbial Transplantation and Antibiotics for Ulcerative Colitis. Inflamm Bowel Dis. 2017;23(1):116–125. doi: 10.1097/MIB.0000000000000975. [DOI] [PubMed] [Google Scholar]
- 99.Mittal C, Miller N, Meighani A, Hart BR, John A, Ramesh M. Fecal microbiota transplant for recurrent Clostridium difficile infection after peripheral autologous stem cell transplant for diffuse large B-cell lymphoma. Bone Marrow Transplant. 2015;50(7):1010. doi: 10.1038/bmt.2015.85. [DOI] [PubMed] [Google Scholar]
- 100.Blackburn LM, Bales A, Caldwell M, Cordell L, Hamilton S, Kreider H. Fecal microbiota transplantation in patients with cancer undergoing treatment. Clin J Oncol Nurs. 2015;19(1):111–114. doi: 10.1188/15.CJON.111-114. [DOI] [PubMed] [Google Scholar]
- 101.Kulecka M, Paziewska A, Zeber-Lubecka N, Ambrozkiewicz F, Kopczynski M, Kuklinska U, Pysniak K, Gajewska M, Mikula M, Ostrowski J. Prolonged transfer of feces from the lean mice modulates gut microbiota in obese mice. Nutr Metab (Lond) 2016;13(1):57. doi: 10.1186/s12986-016-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916. e917. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 103.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 104.Girotra M, Garg S, Anand R, Song Y, Dutta SK. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection in the Elderly: Long-Term Outcomes and Microbiota Changes. Dig Dis Sci. 2016;61(10):3007–3015. doi: 10.1007/s10620-016-4229-8. [DOI] [PubMed] [Google Scholar]
- 105.Jalanka J, Mattila E, Jouhten H, Hartman J, de Vos WM, Arkkila P, Satokari R. Long-term effects on luminal and mucosal microbiota and commonly acquired taxa in faecal microbiota transplantation for recurrent Clostridium difficile infection. BMC Med. 2016;14(1):155. doi: 10.1186/s12916-016-0698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fischer M, Sipe B, Cheng YW, Phelps E, Rogers N, Sagi S, Bohm M, Xu H, Kassam Z. Fecal Microbiota Transplant in Severe and Severe-Complicated Clostridium difficile: A Promising Treatment Approach. Gut Microbes. 2016:0. doi: 10.1080/19490976.2016.1273998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hirsch BE, Saraiya N, Poeth K, Schwartz RM, Epstein ME, Honig G. Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect Dis. 2015;15:191. doi: 10.1186/s12879-015-0930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W, Lee CH. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149(1):102–109. e106. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 109.Stallmach A, Lange K, Buening J, Sina C, Vital M, Pieper DH. Fecal Microbiota Transfer in Patients With Chronic Antibiotic-Refractory Pouchitis. Am J Gastroenterol. 2016;111(3):441–443. doi: 10.1038/ajg.2015.436. [DOI] [PubMed] [Google Scholar]
- 110.Ott SJ, Waetzig GH, Rehman A, Moltzau-Anderson J, Bharti R, Grasis JA, Cassidy L, Tholey A, Fickenscher H, Seegert D, Rosenstiel P, Schreiber S. Efficacy of Sterile Fecal Filtrate Transfer for Treating Patients With Clostridium difficile Infection. Gastroenterology. 2016;151 doi: 10.1053/j.gastro.2016.11.010. (epub before print) [DOI] [PubMed] [Google Scholar]
- 111.Rautava S. Early microbial contact, the breast milk microbiome and child health. J Dev Orig Health Dis. 2015:1–10. doi: 10.1017/S2040174415001233. [DOI] [PubMed] [Google Scholar]
- 112.Goedert JJ, Hua X, Yu G, Shi J. Diversity and composition of the adult fecal microbiome associated with history of cesarean birth or appendectomy: Analysis of the American Gut Project. EBioMedicine. 2014;1(2–3):167–172. doi: 10.1016/j.ebiom.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cacho N, Neu J. Manipulation of the intestinal microbiome in newborn infants. Adv Nutr. 2014;5(1):114–118. doi: 10.3945/an.113.004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA, Johansen FE. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One. 2011;6(3):e17996. doi: 10.1371/journal.pone.0017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tietze K, Dalpke A, Morath S, Mutters R, Heeg K, Nonnenmacher C. Differences in innate immune responses upon stimulation with gram-positive and gram-negative bacteria. J Periodontal Res. 2006;41(5):447–454. doi: 10.1111/j.1600-0765.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 116.Dogi CA, Galdeano CM, Perdigon G. Gut immune stimulation by non pathogenic Gram(+) and Gram(−) bacteria. Comparison with a probiotic strain. Cytokine. 2008;41(3):223–231. doi: 10.1016/j.cyto.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 117.Skovbjerg S, Martner A, Hynsjo L, Hessle C, Olsen I, Dewhirst FE, Tham W, Wold AE. Gram-positive and gram-negative bacteria induce different patterns of cytokine production in human mononuclear cells irrespective of taxonomic relatedness. J Interferon Cytokine Res. 2010;30(1):23–32. doi: 10.1089/jir.2009.0033. [DOI] [PubMed] [Google Scholar]
- 118.De Palma G, Cinova J, Stepankova R, Tuckova L, Sanz Y. Pivotal Advance: Bifidobacteria and Gram-negative bacteria differentially influence immune responses in the proinflammatory milieu of celiac disease. J Leukoc Biol. 2010;87(5):765–778. doi: 10.1189/jlb.0709471. [DOI] [PubMed] [Google Scholar]
- 119.Jin Y, Chen X, Podolsky R, Hopkins D, Makala LH, Muir A, She JX. APC dysfunction is correlated with defective suppression of T cell proliferation in human type 1 diabetes. Clin Immunol. 2009;130(3):272–279. doi: 10.1016/j.clim.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Manirarora JN, Kosiewicz MM, Parnell SA, Alard P. APC activation restores functional CD4(+)CD25(+) regulatory T cells in NOD mice that can prevent diabetes development. PLoS One. 2008;3(11):e3739. doi: 10.1371/journal.pone.0003739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patel R, DuPont HL. New approaches for bacteriotherapy: prebiotics, new-generation probiotics, and synbiotics. Clin Infect Dis. 2015;60(Suppl 2):S108–121. doi: 10.1093/cid/civ177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol. 2016;12(3):154–167. doi: 10.1038/nrendo.2015.218. [DOI] [PubMed] [Google Scholar]
- 123.Kolb H, von Herrath M. Immunotherapy for Type 1 Diabetes: Why Do Current Protocols Not Halt the Underlying Disease Process? Cell Metab. 2016 doi: 10.1016/j.cmet.2016.10.009. [DOI] [PubMed] [Google Scholar]