INTRODUCTION
Barrett’s esophagus (BE) is characterized by a change of the normal stratified squamous epithelium lining the esophagus to a metaplastic columnar epithelium with goblet cells. The prevalence of BE is estimated to be 1.5% in the general population [1, 2] and as high as 15% in those with gastroesophageal reflux disease (GERD) [3, 4]. Other risk factors associated with BE are older age, male sex, smoking, central obesity, and white race [5–10]. There also appears to be an increased genetic pre-disposition among those with first-degree relatives with BE [11].
BE is a known precursor to esophageal adenocarcinoma (EAC), and oncogenesis is thought to occur through a sequential progression from metaplasia to dysplasia to carcinoma. The risk of developing EAC is as high as 7% per year in those with high-grade dysplasia (HGD) [12] and 0.7% per year in low-grade dysplasia (LGD). However, reports of EAC risk in LGD are highly disparate, ranging from risks approximating that of non-dysplastic BE (NDBE), to risks of progression to HGD or EAC of 10% per year or more [8, 13–16]. EAC is associated with high mortality and is increasing in incidence in the western world [17–19]. Risk factors for progression of BE to EAC include increasing degree of dysplasia, increasing age, increasing BE segment length, male sex, and smoking, among others [20]. Therefore, there is a need to optimize screening, surveillance, and treatment of high-risk BE with the ultimate goal of decreasing the disease burden and mortality associated with EAC.
In this review article, we will briefly discuss the diagnostic criteria and endoscopic screening for BE. We will then review the indications and performance of endoscopic surveillance, with an emphasis on possible new directions to improve the performance of surveillance. We will conclude with a discussion of the management of BE, with an emphasis on the indications, technique, and outcomes of endoscopic therapy for BE.
DIAGNOSIS
Diagnostic Criteria
Current guidelines recommend that the diagnosis of BE should be based on the presence of columnar epithelium ≥1 cm proximal to the gastroesophageal (GE) junction with biopsies consistent with intestinal metaplasia (IM) [8]. This is in contrast to British diagnostic criteria, where confirmation of IM is not required for diagnosis [21]. The relationship between the presence of IM and progression to EAC has been conflicting [22–24], and complicated both by sampling error [25] and interobserver variability among pathologists [26]. Studies have shown that there is a significant increase in the likelihood of finding IM with increasing number of biopsy samples taken during endoscopy [27].
As a result, the recommended number of random biopsy samples are 4 samples for every 2 cm of BE segment length or 8 for segment length <2 cm in those with suspected BE [28]. In addition, a normal or mildly irregular Z-line should not be routinely biopsied because IM of the cardia is common in chronic GERD patients [29] and has not been definitively demonstrated to imply an increased risk of EAC [30, 31]. In terms of BE classification, a segment >3 cm is defined as long-segment BE and segment <3 cm as short-segment BE. The Prague classification [32] describing the circumferential and maximum extent of BE is used for standardized reporting, in addition to endoscopic landmarks such as the diaphragmatic hiatus, gastroesophageal junction, and the squamocolumnar junction [8].
Screening
The primary goal of screening is to identify patients with BE. However, the question of who to screen is complex, because >90% of patients who develop EAC have no prior history of BE, and the traditional practice of screening GERD patients misses a substantial group destined to develop EAC, because approximately 40% of EAC patients do not have a history of chronic GERD [33–35]. Despite these shortcomings, screening guidelines have traditionally focused on a sub-set that is at higher risk for BE and EAC, which include men with chronic GERD symptoms and 2 additional risk factors including age >50, white race, central obesity, smoking history, and family history [8]. Although risk-stratification models [36–38] have been developed to aid in determining who to screen for BE, these models need further validation and their role in clinical practice is currently limited.
The most commonly used screening modality for BE is conventional per oral upper endoscopy with biopsy samples from any endoscopically visible columnar mucosa in the tubular esophagus. Limitations of endoscopy for screening are that it is an invasive procedure requiring a specialist and that it is costly [39]. Brush cytology sampling might reduce cost, increase the surface area that can be analyzed, and be used in combination with molecular markers to aid in risk-stratification. Wide-area transepithelial sampling (WATS) uses computer-assisted analysis of an abrasive transepithelial brush biopsy to sample a larger surface area, to help overcome the issue of sampling error. When WATS is used in conjunction with 4-quadrant biopsies, there is on average a 40% incremental yield of dysplasia and metaplasia detection in 2 prospective trials [40, 41]. In addition, there is high interobserver agreement [42] for detection of not only BE (κ=0.88) and HGD/EAC (κ=0.95), but also for LGD (κ=0.74), in contrast to the low inter-observer agreement with traditional 4-quadrant biopsies [43]. However, this technology is currently used as an adjunct to per oral endoscopy, meaning that costs associated with endoscopy are not avoided.
Alternative endoscopic techniques for screening include transnasal endoscopy (TNE), and single-fiber endoscopy. TNE uses a smaller-caliber scope and is inserted into the esophagus orally or nasally without the need for sedation [44]. TNE has been shown to be comparable to standard endoscopy for detection of BE and for the quality of biopsy specimens [45–47]. In addition, TNE is well tolerated and has demonstrated efficacy in a community setting [44, 48, 49]. However, most gastroenterologists have limited experience with transnasal approaches, which require good nasopharyngeal anesthesia, and knowledge of pertinent landmarks. Endoscopes with a disposable sheath (EndoSheath; Vision Sciences, Orangeburg, NY) and disposable esophagoscopes (EG scan; IntroMedic, Seoul, South Korea) may be limited by the quality of images generated, a problem likely to be addressed by continuing technological advances. Single-fiber endoscopy is smaller in diameter (1.6 mm) compared with TNE and allows for NBI imaging but does not provide operator control or the ability to collect biopsy samples [50].
There are also non-endoscopic screening devices for BE that are designed to obtain tissue for histologic evaluation. The Cytosponge is a gelatin-coated sponge attached to a string and collects cytologic specimens from the esophageal mucosa when withdrawn and may have the potential to replace traditional endoscopic screening in a cost-effective manner [51]. Preliminary data showed a sensitivity of 73% to 90% for identifying BE when used in combination with immunohistochemistry staining for trefoil factor 3 (TFF3) [4], but the diagnostic accuracy is still being validated.
Esophageal capsule endoscopy (ECE), another non-invasive capsule device, has shown conflicting data as to effectiveness in BE diagnosis [52–54] without being more cost-effective [55] and, as a result, is not commonly used for screening. Tethered capsule endomicroscopy (TCE) can provide additional information regarding the microscopic features and architecture of the esophageal wall, and is currently being investigated [50].
Surveillance
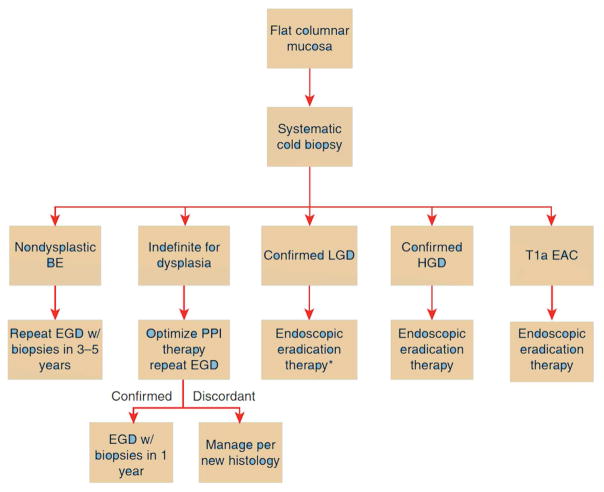
Surveillance in BE is aimed at early detection of dysplasia. Dysplasia is categorized as NDBE, indeterminate, LGD, HGD, or carcinoma [56]. The presence of dysplasia should be confirmed by a second pathologist expert in GI histopathology, due to a high degree of inter-observer variability [56]. The degree of dysplasia dictates recommended surveillance intervals. Patients with NDBE are recommended to have a repeat endoscopy in 3 to 5 years, and those with indeterminate dysplasia are recommended to undergo a repeat examination in 3 to 6 months after optimization of proton pump inhibitor therapy [8]. Patients with LGD can undergo eradication therapy, although ongoing endoscopic surveillance is an acceptable alternative for LGD. Those with a higher degree of dysplasia should be considered for endoscopic eradication therapy (Figure 1).
Figure 1.
Schematic for management of non-nodular Barrett’s esophagus (BE) [8]. Surveillance upper endoscopy at 1-year intervals is an acceptable alternative to endoscopic eradication therapy. T1a esophageal adenocarcinoma (EAC) is amenable for endoscopic therapy. (Image reproduced with permission).
Careful endoscopic examination of esophageal mucosa and obtaining an adequate number of biopsy samples is vital for effective surveillance [57, 58]. Longer mucosal inspection time has been associated with increased detection of HGD/EAC [59]. In addition, highly dysplastic lesions in BE are more often found in the right side of the esophagus, so particular attention to this area maybe beneficial [60–63]. A standardized biopsy protocol for surveillance includes random 4-quadrant biopsies every 2 cm in NDBE and every 1 cm in dysplastic BE [64], in addition to targeted sampling of focal mucosal abnormalities. Any mucosal abnormalities noted on surveillance should be sampled; among those with a history of dysplasia, endoscopic mucosal resection (EMR) is recommended for optimal disease staging [65]. Empiric data demonstrate that in current practice, a majority of patients often do not undergo adequate biopsies when surveillance is performed leading to decreased dysplasia detection [66].
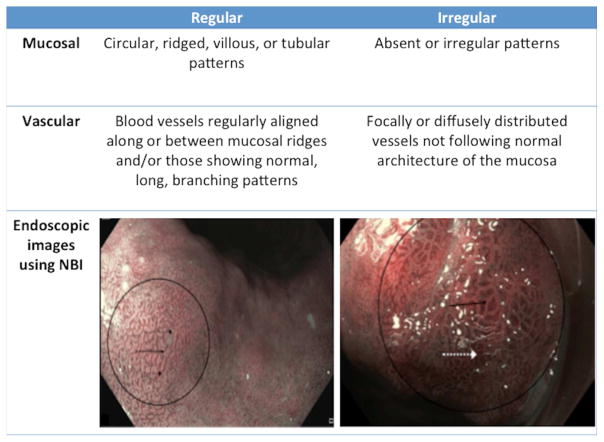
A variety of endoscopic imaging techniques have been developed to improve visualization of the esophageal mucosa for detection of dysplasia and neoplasia, although none has been adopted for wide-scale routine use presently (Table 1). The current criterion standard for both screening and surveillance is use of high-resolution white-light endoscopy (HD-WLE). NBI increases detection of dysplasia when compared with HD-WLE and also requires a lower number of biopsies [67, 68]. In addition, the type and regularity of mucosal and vascular patterns using narrow-band imaging (NBI) have recently been shown to identify dysplasia in BE patients with 80% sensitivity and 88% specificity using a new validated NBI classification system [69] (Figure 2). Autofluorescence imaging (AFI) can detect mucosal abnormalities with high sensitivity but poor specificity compared with HD-WLE [70]. In one prospective study, despite using tri-modal imaging with HD-WLE, NBI, and AFI, 10% of patients had advanced lesions that were not visibly apparent and were only detected on random biopsies [71]. Magnifying endoscopy with chromoendoscopy has been shown to improve detection of both IM and dysplasia by enhancing mucosal visibility [72–76]. Confocal laser endomicroscopy (CLE) can increase the yield of dysplasia detection [77–79] with good accuracy [80, 81] compared with random biopsies but is limited by longer procedure times, cost, and the restricted time for mucosal inspection before the injected fluorescein dye obscures visualization. Unlike CLE, modalities such as VLE can image a larger surface area in a short period of time and can identify sub-squamous BE [82], making it a potentially useful tool for surveillance. However, other than case reports and series [83, 84], VLE’s role and efficacy in BE surveillance is not completely elucidated.
Table 1.
Endoscopic imaging modalities for detection of dysplasia in Barrett’s esophagus
| Imaging modality | Description | Sensitivity | Specificity | Image | References |
|---|---|---|---|---|---|
| High-definition white light (HD-WLE) | HD-WLE is the current criterion standard for both screening and surveillance of BE. | 40–64% | 98%–100% |
 Endoscopic image of BE with areas of early neoplasia. |
[67, 79, 150] |
| Narrow-band imaging (NBI) | NBI visually emphasizes vascular patterns allowing for better differentiation between columnar and squamous tissue in the esophagus. | 94%* | 94%* |
 Nodular lesions visualized with NBI. |
[85, 150] |
| Chromoendoscopy methylene blue | Endoscopic evaluation of mucosa after application of dyes or contrast agents. | 64%* | 96%* |
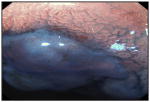 Focal staining of BE mucosa with methylene. blue |
[85, 150] |
| Acetic acid | 97%* | 85%* |
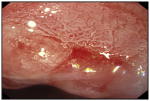 An irregular white area on Barrett’s mucosa after treated with acetic acid. |
[85, 150] | |
| Autofluorescence imaging (AFI) | Relies on spectroscopic characteristics of light to induce fluorescence of biomolecules that can be used to detect mucosal abnormalities. | 37%–50% | 61%–92% |
 AFI image of a neoplastic lesion in BE. |
[150–153] |
| Confocal laser endomicroscopy (CLE) | Provides up to a 1000-fold magnification of the esophageal mucosa and allows for real-time histologic evaluation of the esophagus via endoscope and probe-based methods. | 90%* | 90%* |
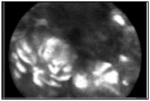 Dysplastic BE with intense intracellular fluorescence, heterogeneous cellular sizes and disorganized architecture. |
[85, 154] |
| Optical coherence tomography (OCT) | Uses light waves to generate cross sectional images of the esophageal epithelial and sub-epithelial tissue atchitecture. | 68%–83% | 75%–82% |
 OCT image of IMC/HGD showing disorganized architecture and increased surface |
[155, 156] |
| Volumetric laser endomicroscopy (VLE) | Uses OCT to produce fast, high-resolution images to a depth of 3 mm and can scan larger surface areas compared with OCT. | 86%* | 88%* |
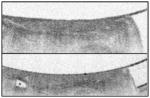 VLE images irregular glandular architecture and increased surface reflectivity. |
[85, 154] |
| Spectroscopy | Include several types such as light-scattering, reflectance and Raman spectroscopy that use scattered light to differentiate abnormal vascular, nuclear, and tissue patterns. | 86–90% | 85%–90% |
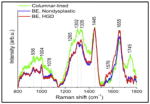 Mean confocal spectra of columnar epithelium, NDBE, and BE with HGD. |
[157–159] |
BE, Barrett’s esophagus.
Pooled estimate generated from ASGE PIVI analysis [85].
Figure 2.
Classification of regular and irregular mucosal and vascular patterns using the validated Barrett’s International NBI Group (BING) criteria for detection of high-grade dysplasia and esophageal adenocarcinoma using narrow-band imaging (NBI) in Barrett’s esophagus [69]. (Image reproduced with permission).
Whether these advanced imaging techniques may obviate the need for random esophageal biopsies is a matter of great interest. The “Imaging in Barrett’s Esophagus Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI)” initiative [85] by ASGE, established minimum performance thresholds for an imaging modality with targeted biopsies to replace the need for random biopsies in BE. To meet PIVI performance thresholds, an imaging technology in combination with targeted biopsies should have a sensitivity of ≥90% and negative predictive value (NPV) of ≥98% for detection of HGD/EAC, and a specificity of 80% to replace random biopsy protocol. Acetic acid chromoendoscopy, NBI, and endoscope-based CLE currently meet these criteria for BE surveillance and are endorsed by ASGE for use in surveillance of NDBE by experienced operators to obtain targeted biopsies [86].
Surveillance has been shown to be beneficial in identifying EAC at earlier stages and in improving mortality in several retrospective studies [33, 87–89]. However, these studies are susceptible to lead-time and length-time biases. Given differences in growth rates between tumors, it is quite possible that only the most indolent disease is detected by surveillance endoscopy. Consistent with concerns about over-estimation of the effectiveness of surveillance, no survival benefit from surveillance was noted in a case–control study from the Northern California Kaiser Permanente population [90] or in the US Veterans Health Administration [91]. Even if surveillance is effective, the cost-effectiveness of this intervention has been questioned [92, 93]. Of note, all these studies focus on survival benefit after EAC diagnosis in those undergoing surveillance and do not assess the benefit of preventing EAC from endoscopic eradication of dysplasia. Given that recent guidelines suggest endoscopic therapy before the development of EAC [8, 94, 95], the impact of intervention in dysplastic disease may be under-appreciated. Therefore, despite conflicting evidence on the effectiveness of surveillance, it is a recommended practice in the management of BE [8].
Current surveillance strategies based on histologic tissue analysis are not without limitations. There is evidence that a meaningful proportion of BE patients in a surveillance program can progress to EAC despite having no history of dysplasia [87, 96], highlighting the limitations of current surveillance in accurately risk-stratifying individuals. Several potential sources of error include lack of adherence to recommended biopsy protocol, sampling error, and interobserver variability between pathologists of degree of dysplasia [25, 27, 43, 97]. As a result, adjunct techniques to improve risk-stratification, such as WATS [41] with computer-aided analysis to overcome limitations with sampling error, as well as biomarkers, have been explored. Although early data on this technology suggest that its use adjunctive to standard biopsy protocols might increase detection of dysplasia, its operating characteristics are not completely defined, and its application adds time and expense to the procedure.
Molecular biomarkers have been investigated to identify individuals with BE who are at an increased risk of progressing to EAC (Table 2). Because BE is thought to develop from a dysplasia to carcinoma sequence, the degree of genetic aberration can be used to predict disease progression [98]. Molecular abnormalities such as chromosomal aneuploidy or tetraploidy, hypermethylation of p16, loss of heterozygosity of p53, and microRNA expression, among others, have been associated with progression to HGD or EAC [99–104]. Panels of markers have also been tested to increase the predictive ability carcinogenesis [105]. All these markers are based on histologic tissue and thus cannot overcome the limitation of sampling error. Therefore, attempts have been made to develop serum biomarkers, including interleukins, EAC-specific proteins, and serum microRNAs with mixed results [38, 106, 107]. To date, none of these markers are routinely used in BE risk-stratification with the exception of immunohistochemical testing of p53, which is recommended by the British Society of Gastroenterology Guidelines (BSG) as an adjunct to analysis of biopsy samples during BE surveillance [21].
Table 2.
Biomarkers for Risk Stratification in Barrett’s Esophagus
| Biomarker | Baseline to Outcome Histology | Mean or Median Follow-up (Years) | Results | References |
|---|---|---|---|---|
| Protein Markers | ||||
|
| ||||
| p53 | NDBE to HGD/EAC | 6.6 years | Overexpression - RR: 5.6 (95% CI, 3.1–10.3) Loss – RR: 14.0 (95% CI, 5.3–37.2) |
[160] |
|
| ||||
| Gene and DNA content abnormalities | ||||
|
| ||||
| LOH of 17p+9p, DNA abnormalities | NDBE to EAC | 6.7 years | RR: 38.7 (95% CI, 10.8–138.5) | [105] |
| 9p LOH | NDBE to EAC | 5.0 years | RR: 2.6 (95% CI, 1.1–6.0) | [161] |
| 17p LOH | NDBE/LGD to EAC | 3.0 years | RR: 16 (95% CI, 6.2–39) | [100] |
| Aneuploidy/Tetraploidy | NDBE/LGD to EAC | 5.0 years | RR: 11 (95% CI, 5.8–21) | [161] |
| Panel of LGD, abnormal DNA ploidy, and Aspergillus oryzae lectin | NDBE or LGD to EAC | 6.7 years | Baseline LGD – OR: 3.9 (95% CI, 2.4–6.4) Baseline NDBE – OR: 3.3 (95% CI, 1.8–6.00) |
[162] |
|
| ||||
| FISH | ||||
|
| ||||
| FISH panel: P16, P53, Her-2/neu, 20q, and MYC, and chromosomal centromeric probes 7 and 17 to detect aneuploidy | NDBE to EAC | 3.8 years | p16 loss or aneuploidy - RR: 3.2 (95% CI, 1.3–7.9) | [163] |
|
| ||||
| DNA Methylation | ||||
|
| ||||
| Promoter methylation of p16, RUNX3, HPP1 | NDBE to HGD/EAC | 6.3 years | p16 – OR: 1.7 (95% CI, 1.33–2.20), RUNX3 – OR: 1.80 (95% CI, 1.1–2.8) HPP1 – OR: 1.8 (95% CI, 1.1–2.8) |
[104] |
| 8-gene methylation panel (p16, RUNX3, HPP1, NELL1, TAC1, SST, AKAP12, and CDH13) | NDBE to HGD/EAC | 2.0 or 4.0 years | AUC: 0.84, 80% sensitivity, 70% specificity | [164] |
| 4-gene methylation panel (SLC22A18, PIGR, GJA12, and RIN2) | NDBE to EAC | n/a | AUC: 0.99, 94 % sensitivity, 97 % specificity, | [165] |
|
| ||||
| Gene Expression and MicroRNA (miRNA) | ||||
|
| ||||
| miRNAs −192, −194, −196a, and −196b | NDBE to EAC | 4.6 years | 71%–85% sensitivity, 50%–71% specificity | [103] |
|
| ||||
| Genetic and Clonal Diversity | ||||
|
| ||||
| Shannon LOH diversity index | NDBE to EAC | 4.5 years | RR: 11.0 (95% CI, 5.8–21.0) | [166, 167] |
| Mean pairwise divergence by LOH | 5.0 years | RR: 2.15 (95% CI, 1.67–2.77) | ||
| Number of LOH clones | 5.0 years | RR: 1.99 (95% CI, 1.71–2.32) | ||
|
| ||||
| Proliferation and Cell Cycle Markers | ||||
|
| ||||
| Cyclin D | NDBE to EAC | 4.3 years | OR: 6.9 (95% CI, 1.6–29.9) | [168] |
| Mcm2 | NDBE/LGD to HGD/EAC | 6.0 years | OR: 136 (95% CI, 7.5–2464) | [169] |
NDBE, non-dysplastic Barrett’s esophagus; LGD, low-grade dysplasia; HGD, high-grade dysplasia; EAC esophageal adenocarcinoma; LOH loss of heterozygosity; FISH fluorescent in-situ hybridization; RR relative risk; CI confidence interval; AUC area under curve; OR odds ratio.
MANAGEMENT
Chemoprevention
The utility of chemopreventive agents in BE is unclear. Because those with baseline dysplasia are often treated with endoscopic ablation and those with NDBE have a very low risk of progression, the safety and cost-effectiveness of long-term use of any agent for chemoprevention needs to be justified. Currently, it is recommended that all patients with BE, regardless of the presence of GERD symptoms, be treated with once daily PPI based on evidence [108] that progression to neoplasia is reduced compared with no PPI therapy or with the use of H2 receptor blockers. Although the use of nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with a diminished incidence of EAC [109], and a reduced the risk of progression to EAC in BE patients by up to 30% [110], the bleeding risk associated with NSAIDs may outweigh these benefits and, therefore, they are not currently recommended as a chemopreventive strategy in BE [8].
Endoscopic Therapy
Once the diagnosis of BE is confirmed, further management is dictated by the degree of dysplasia (Figure 2). In HGD, endoscopic eradication therapy appears to be associated with a decreased risk of subsequent adenocarcinoma compared with surveillance endoscopy, and may have a similar all-cause mortality rate when compared with esophagectomy [111, 112]. There is also evidence [16] that treating LGD [14] with endoscopic eradication therapy results in a lower rate of progression to HGD or EAC during a 3-year follow-up period. Currently there are differences among professional society guidelines regarding management of BE with LGD. Although the ACG and AGA recommend consideration of endoscopic eradication therapy for LGD confirmed by a second pathologist, the BSG suggests endoscopic surveillance [8, 21, 95]. At this time, routine endoscopic eradication therapy is not recommended for NDBE, given the low risk of progression to neoplasia [113], the small but real risk of procedure-related adverse events, and the costs inherent in the procedures [114, 115]. In general, before initiating treatment, overall patient health, including other comorbidities, need to be considered, particularly in those with LGD, where the rates of progression to EAC are low.
There are multiple endoscopic therapies available for BE eradication, including resection and ablation modalities. The presence of irregular, raised or nodular esophageal mucosa within BE is associated with higher rates of malignancy [116], so initial resection of these areas with either EMR or endoscopic submucosal dissection (ESD) [117, 118] is necessary to determine depth of invasion for staging and selection of further therapy [119]. EMR can be performed by band ligation technique or with an endoscopic resection cap and the use of a snare for resection. Both techniques are comparable, but in a head to head comparison, band ligation was found to be less costly, more time effective, and with fewer adverse events [120]. Compared with EMR, ESD offers a more controlled and precise resection of the target area and, for larger lesions, determination of adequacy of resection at the lateral margins. In general, because the deep margin of the resection is the most important clinically actionable data from mucosal resection, and because most centers see small volumes of subjects needing ESD, for most Western endoscopists, the focus should be on performing quality EMR.
If the resected nodular area shows LGD, HGD, or T1a EAC without lymphatic or vascular involvement, then subsequent endoscopic ablative therapy is recommended [8] for complete eradication of IM (CEIM). Although stepwise, radical complete EMR of the entire BE segment demonstrates high rates of eradication and remission over a 2-year follow-up period [121, 122], stricture rates are demonstrably higher than focal EMR followed by radiofrequency ablation (RFA) [123]. Therefore, combination therapy with EMR followed by ablation is the recommended approach for most patients, and has been shown to eradicate HGD in 86% to 92% of the cases and IM in 62% to 87% of cases [124, 125]. Adverse events of endoscopic resection techniques include bleeding, perforation, and a dose-dependent risk of stricture formation [121, 122].
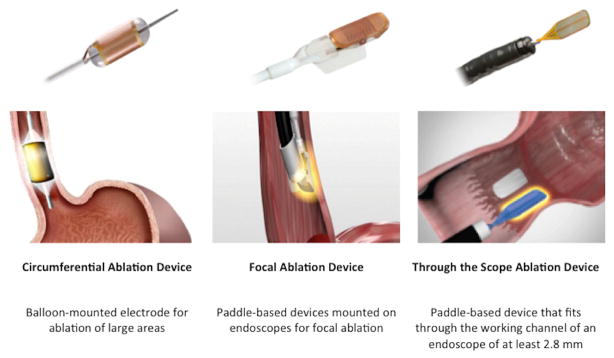
For non-nodular BE, several ablative options are available, but RFA is the ablative treatment of choice based on efficacy, safety, and availability of a large amount of high-quality data. Radiofrequency energy can be delivered either circumferentially through balloon-based devices or focally through devices attached to the end of the endoscope (Figure 3). RFA is highly effective in eradicating dysplasia in BE patients with 81% of HGD and 91% of LGD achieving complete eradication of dysplasia (CED) at 12 months in a multicenter US randomized controlled trial [111]. The most common adverse events of RFA is post-ablation stricture with a pooled estimate of 5.6% (95% CI, 4.2%–7.4%) [126]. Other rare adverse events include bleeding, perforation and post-procedure chest pain requiring hospital admission for control.
Figure 3.
Available radiofrequency ablation devices (RFA) include a circumferential device that can be used for ablation of large areas and focal devices comprising a paddle that can either be attached to the tip or placed through the working channel of an upper endoscope. (All rights reserved. Used with the Permission of Medtronic).
Cryoablation is another effective ablative modality for management of BE, which delivers either liquid nitrogen or carbon dioxide to the intended tissue via a spray catheter inserted though the upper endoscope. A newer device is also available to deliver cryotherapy using nitrous oxide via a self-contained, balloon based system [127]. Cryoablation can achieve complete eradication of HGD in a high proportion of patients with BE in retrospective studies, with good durability during a 24-month follow-up period [128, 129]. Similar efficacy was seen in prospective data with 81% to 94% eradication of HGD [130, 131]. A more recent prospective study showed that cryoablation with pressurized CO2, when combined with EMR for treatment of BE with nodular neoplasia provided CED in only 44% of the patients [132]. There is a paucity of randomized trials comparing mucosal ablation modalities to each other.
Although photodynamic therapy (PDT) is rarely used currently due to cost and side effects, level 1 evidence exists for the efficacy of PDT in preventing cancer in BE with HGD [133]. In a multicenter study, PDT resulted in eradication of HGD in 77% of the cases with maintenance of remission in 85% during a 5-year follow-up period [133, 134]. Despite this efficacy, use of PDT is limited by high rates of post-ablation strictures with reports as high as 36% and higher procedural cost compared with RFA [134, 135]. Other ablative modalities that are available are argon plasma coagulation (APC) and multipolar electrocoagulation, which have been shown to have similar rates of CEIM [136]. The most frequent use of APC is to treat residual disease after ablation as this can promote sustained remission for a greater duration after CEIM [137]. Although no head to head comparisons exist, APC has been shown to have similar cost efficacy to RFA [93].
After CEIM, the risk of recurrence of intestinal metaplasia is significant, especially with risk factors such as increasing age, BE segment length, and baseline dysplasia [138] (Figure 4). A recent meta-analysis [139] that assessed all types of endoscopic eradication modalities showed that the annual incidence of recurrent IM was 7.1%, of dysplastic BE was 1.3%, and of HGD/EAC was 0.8%, over more than 10000 patient years of follow-up time. When the analysis was restricted to only treatments with RFA, the annual incidence of recurrent IM was 9.5%, of dysplastic BE was 2%, and of HGD/EAC was 1.2%. There was a similar risk of recurrence in those treated with combination therapy with EMR followed by ablation [140]. Although the recurrence of dysplasia is low, it is not insignificant, and thus it is important for patients who have achieved CEIM and CED to undergo periodic post-ablation surveillance with careful mucosal inspection and both targeted and random biopsies.
Figure 4.
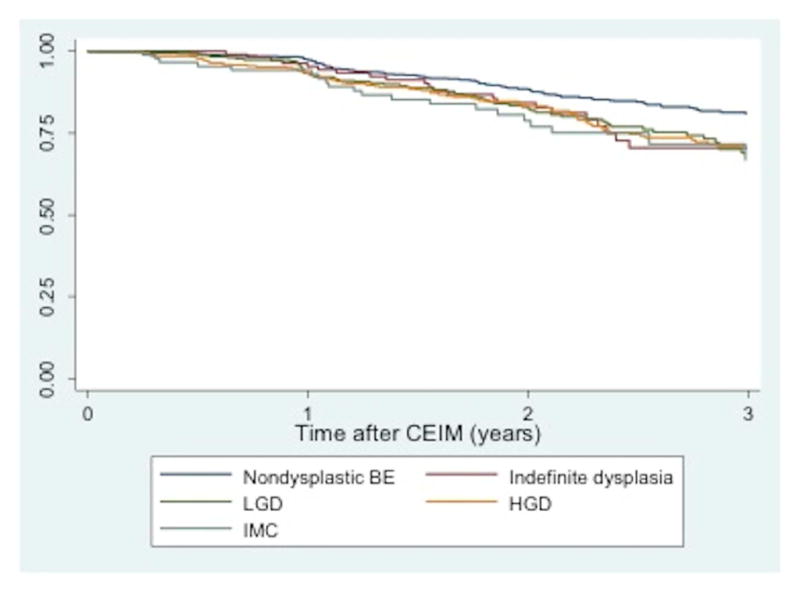
Kaplan-Meier plot of intestinal metaplasia (IM) recurrence among patients (n=1613) who achieved complete eradication of intestinal metaplasia (CEIM) after RFA, with pretreatment histology non-dysplastic BE (NDBE), low-grade dysplasia (LGD), and high-grade dysplasia (HGD) [138]. (Image reproduced with permission).
Recent work suggests that the highest yield for random biopsies is at the squamocolumnar junction, and that strategies that heavily sample that area might improve the yield of dysplasia without incurring more biopsies or extra costs [141]. The frequency of current surveillance examinations is based on baseline pathology and expert opinion [8]. For patients treated for baseline LGD, one commonly used strategy is to perform surveillance every 6 months for the first year after CEIM, then annually after. For patients treated with baseline HGD, surveillance can be performed every 3 months in the first year after CEIM, every 6 months in the second year after CEIM, and then annually after. Recurrent disease is treated in a similar manner as before initial endoscopic therapy, and success rates of a second CEIM after recurrence of BE are high [138, 142].
Surgical Therapy
Anti-reflux surgery has not been shown to be superior to medical therapy in preventing EAC incidence in BE patients based on 2 meta-analyses [143, 144] and is not recommended for prevention of neoplasia in BE. There is some evidence that post-ablation the neosquamous epithelium is potentially more prone to reflux injury [145], possibly increasing the risk of BE recurrence. A recent study found decreased BE recurrence after RFA with Nissen fundoplication versus PPI therapy, in the subgroup of patients with long-segment BE and a hiatal hernia >3 cm [146]. However, current data as to any incremental benefit of a surgical anti-reflux procedure compared with medical therapy after successful RFA remains inconclusive [147, 148], and in general the indications for consideration of fundoplication after RFA remain similar to those in the general GERD population.
The utility of surgery, specifically esophagectomy, is more evident in BE patients with advanced neoplasia. In patients with T1a esophageal adenocarcinoma, esophagectomy may be indicated in cases with poorly differentiated tumors, lymphovascular invasion, or cases in which ablation is technically difficult or failed [149]. Traditionally, esophagectomy has been viewed as the standard of care for all patients with T1b esophageal adenocarcinoma. However, the relative merits of esophagectomy and endoscopic therapy in tumors only superficially invasive into the submucosa (T1b sm1) have recently come into question. Although any submucosal invasion has traditionally been considered to be associated with prohibitively high rates of lymph node involvement to consider endoscopic therapy, recent data suggest that at least a subgroup of such patients may be effectively treated endoscopically [117]. Currently, the precise degree of tumor invasion to preclude endoscopic therapy in a good surgical candidate is unsettled, and data to support endoscopic management of patients with tumors showing superficial submucosal invasion is not yet robust enough to allow definitive conclusions to be drawn.
CONCLUSION
Although only a small proportion of patients with BE develop EAC, the high mortality and cost associated with this outcome drives screening, surveillance, and treatment practices of BE, with the ultimate goal of preventing advanced neoplasia. Despite the technical advances in detection of metaplasia and neoplasia, the incidence of EAC is rising, highlighting inadequacies in current screening and surveillance practices. Because the risk factors of BE and EAC extend beyond a history of GERD symptoms, developing cost-effective screening tools to identify those at risk is imperative. Once individuals with BE are identified, the goal is to prevent progression to EAC through early dysplasia detection and treatment. This must involve better risk stratification in the large pool of BE patients to understand who is at increased risk of progression. The wide range of evolving imaging and therapeutic modalities will likely enhance our ability to detect mucosal abnormalities, but detection of dysplasia is currently based on histology, which has its limitations. The use of biomarkers and risk-stratification models will have utility to identify individuals with BE who are at highest risk of progressing to EAC, and this improved risk stratification can help guide targeted interventions. In terms of treatment, endoscopic ablation or endoscopic resection is efficacious and has an acceptable safety profile for treating BE with early neoplasia. Esophagectomy is generally reserved for advanced cases of EAC, and those failing endoscopic eradication therapy.
Acknowledgments
Grant Support: Funding for this analysis was supported in part by NIH award number T32 DK07634 (SE), K24DK100548 (NJS)
Acronyms
- BE
Barrett’s esophagus
- GERD
gastroesophageal reflux disease
- EAC
esophageal adenocarcinoma
- HGD
high-grade dysplasia
- LGD
low-grade dysplasia
- NDBE
non-dysplastic Barrett’s esophagus
- GE
gastroesophageal
- IM
intestinal metaplasia
- NBI
narrow-band imaging
- WATS
Wide-area transepithelial sampling
- TNE
transnasal endoscopy
- TFF3
trefoil factor 3
- ECE
Esophageal capsule endoscopy
- TCE
Tethered capsule endomicroscopy
- EMR
endoscopic mucosal resection
- HD-WLE
high-resolution white-light endoscopy
- AFI
autofluorescence imaging
- CLE
confocal laser endomicroscopy
- PIVI
Preservation and Incorporation of Valuable Endoscopic Innovations
- NPV
negative predictive value
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- BSG
British Society of Gastroenterology Guidelines
- ESD
endoscopic submucosal dissection
- RFA
radiofrequency ablation
- CEIM
complete eradication of intestinal metaplasia
- CED
complete eradication of dysplasia
- PDT
photodynamic therapy
- APC
argon plasma coagulation
- LOH
loss of heterozygosity
- FISH
fluorescent in-situ hybridization
- RR
relative risk
- CI
confidence interval
- AUC
area under curve
- OR
odds ratio
- BING
Barrett’s International NBI Group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–31. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 2.Zagari RM, Fuccio L, Wallander MA, Johansson S, Fiocca R, Casanova S, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354–9. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 3.Connor MJ, Weston AP, Mayo MS, Sharma P. The prevalence of Barrett’s esophagus and erosive esophagitis in patients undergoing upper endoscopy for dyspepsia in a VA population. Dig Dis Sci. 2004;49:920–4. doi: 10.1023/b:ddas.0000034549.55326.67. [DOI] [PubMed] [Google Scholar]
- 4.Kadri SR, Lao-Sirieix P, O’Donovan M, Debiram I, Das M, Blazeby JM, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ (Clinical research ed) 2010;341:c4372. doi: 10.1136/bmj.c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2013;28:1258–73. doi: 10.1111/jgh.12230. [DOI] [PubMed] [Google Scholar]
- 6.Kubo A, Cook MB, Shaheen NJ, Vaughan TL, Whiteman DC, Murray L, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s oesophagus: a pooled analysis from the international BEACON consortium. Gut. 2013;62:1684–91. doi: 10.1136/gutjnl-2012-303753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointestinal endoscopy. 2010;71:21–7. doi: 10.1016/j.gie.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. The American journal of gastroenterology. 2016;111:30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corley DA, Kubo A, Levin TR, Block G, Habel L, Rumore G, et al. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community-based study, 1994–2006. Gut. 2009;58:182–8. doi: 10.1136/gut.2008.163360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnamoorthi R, Borah B, Heien H, Das A, Chak A, Iyer PG. Rates and predictors of progression to esophageal carcinoma in a large population-based Barrett’s esophagus cohort. Gastrointestinal endoscopy. 2016 doi: 10.1016/j.gie.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chak A, Lee T, Kinnard MF, Brock W, Faulx A, Willis J, et al. Familial aggregation of Barrett’s oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut. 2002;51:323–8. doi: 10.1136/gut.51.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rastogi A, Puli S, El-Serag HB, Bansal A, Wani S, Sharma P. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointestinal endoscopy. 2008;67:394–8. doi: 10.1016/j.gie.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Manickam P, Amin AV, Samala N, Schouten LJ, Iyer PG, et al. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointestinal endoscopy. 2014;79:897–909. e4. doi: 10.1016/j.gie.2014.01.009. quiz 83.e1, 83.e3. [DOI] [PubMed] [Google Scholar]
- 14.Curvers WL, ten Kate FJ, Krishnadath KK, Visser M, Elzer B, Baak LC, et al. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. The American journal of gastroenterology. 2010;105:1523–30. doi: 10.1038/ajg.2010.171. [DOI] [PubMed] [Google Scholar]
- 15.Duits LC, Phoa KN, Curvers WL, Ten Kate FJ, Meijer GA, Seldenrijk CA, et al. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut. 2014 doi: 10.1136/gutjnl-2014-307278. [DOI] [PubMed] [Google Scholar]
- 16.Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2014;311:1209–17. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 17.Eloubeidi MA, Mason AC, Desmond RA, El-Serag HB. Temporal trends (1973–1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope? The American journal of gastroenterology. 2003;98:1627–33. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 18.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. Journal of the National Cancer Institute. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 19.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:3155–62. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 20.Gopal DV, Lieberman DA, Magaret N, Fennerty MB, Sampliner RE, Garewal HS, et al. Risk factors for dysplasia in patients with Barrett’s esophagus (BE): results from a multicenter consortium. Dig Dis Sci. 2003;48:1537–41. doi: 10.1023/a:1024715824149. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 22.Kelty CJ, Gough MD, Van Wyk Q, Stephenson TJ, Ackroyd R. Barrett’s oesophagus: intestinal metaplasia is not essential for cancer risk. Scand J Gastroenterol. 2007;42:1271–4. doi: 10.1080/00365520701420735. [DOI] [PubMed] [Google Scholar]
- 23.Dias Pereira A, Chaves P. Columnar-lined oesophagus without intestinal metaplasia: results from a cohort with a mean follow-up of 7 years. Aliment Pharmacol Ther. 2012;36:282–9. doi: 10.1111/j.1365-2036.2012.05170.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Hahn H, Odze RD, Goyal RK. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell-containing epithelium. The American journal of gastroenterology. 2009;104:816–24. doi: 10.1038/ajg.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. The New England journal of medicine. 2011;365:1375–83. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 26.Faller G, Borchard F, Ell C, Seitz G, Stolte M, Walch A, et al. Histopathological diagnosis of Barrett’s mucosa and associated neoplasias: results of a consensus conference of the Working Group for Gastroenterological Pathology of the German Society for Pathology on 22 September 2001 in Erlangen. Virchows Archiv : an international journal of pathology. 2003;443:597–601. doi: 10.1007/s00428-003-0894-z. [DOI] [PubMed] [Google Scholar]
- 27.Harrison R, Perry I, Haddadin W, McDonald S, Bryan R, Abrams K, et al. Detection of intestinal metaplasia in Barrett’s esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. The American journal of gastroenterology. 2007;102:1154–61. doi: 10.1111/j.1572-0241.2007.01230.x. [DOI] [PubMed] [Google Scholar]
- 28.Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus--the need for standardization of the definition and of endoscopic criteria. The American journal of gastroenterology. 1998;93:1033–6. doi: 10.1111/j.1572-0241.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 29.Spechler SJ, Zeroogian JM, Antonioli DA, Wang HH, Goyal RK. Prevalence of metaplasia at the gastro-oesophageal junction. Lancet. 1994;344:1533–6. doi: 10.1016/s0140-6736(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 30.Weston AP, Krmpotich PT, Cherian R, Dixon A, Topalovski M. Prospective evaluation of intestinal metaplasia and dysplasia within the cardia of patients with Barrett’s esophagus. Dig Dis Sci. 1997;42:597–602. doi: 10.1023/a:1018811512939. [DOI] [PubMed] [Google Scholar]
- 31.Zaninotto G, Avellini C, Barbazza R, Baruchello G, Battaglia G, Benedetti E, et al. Prevalence of intestinal metaplasia in the distal oesophagus, oesophagogastric junction and gastric cardia in symptomatic patients in north-east Italy: a prospective, descriptive survey. The Italian Ulcer Study Group “GISU”. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2001;33:316–21. doi: 10.1016/s1590-8658(01)80084-0. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez Herrero L, Curvers WL, van Vilsteren FG, Wolfsen H, Ragunath K, Wong Kee Song LM, et al. Validation of the Prague C&M classification of Barrett’s esophagus in clinical practice. Endoscopy. 2013;45:876–82. doi: 10.1055/s-0033-1344952. [DOI] [PubMed] [Google Scholar]
- 33.Bhat SK, McManus DT, Coleman HG, Johnston BT, Cardwell CR, McMenamin U, et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett’s oesophagus: a population-based study. Gut. 2015;64:20–5. doi: 10.1136/gutjnl-2013-305506. [DOI] [PubMed] [Google Scholar]
- 34.Chak A, Faulx A, Eng C, Grady W, Kinnard M, Ochs-Balcom H, et al. Gastroesophageal reflux symptoms in patients with adenocarcinoma of the esophagus or cardia. Cancer. 2006;107:2160–6. doi: 10.1002/cncr.22245. [DOI] [PubMed] [Google Scholar]
- 35.Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 36.Thrift AP, Kendall BJ, Pandeya N, Vaughan TL, Whiteman DC. A clinical risk prediction model for Barrett esophagus. Cancer prevention research (Philadelphia, Pa) 2012;5:1115–23. doi: 10.1158/1940-6207.CAPR-12-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubenstein JH, Morgenstern H, Appelman H, Scheiman J, Schoenfeld P, McMahon LF, Jr, et al. Prediction of Barrett’s esophagus among men. The American journal of gastroenterology. 2013;108:353–62. doi: 10.1038/ajg.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thrift AP, Garcia JM, El-Serag HB. A multibiomarker risk score helps predict risk for Barrett’s esophagus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:1267–71. doi: 10.1016/j.cgh.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbiere JM, Lyratzopoulos G. Cost-effectiveness of endoscopic screening followed by surveillance for Barrett’s esophagus: a review. Gastroenterology. 2009;137:1869–76. doi: 10.1053/j.gastro.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Anandasabapathy S, Sontag S, Graham DY, Frist S, Bratton J, Harpaz N, et al. Computer-assisted brush-biopsy analysis for the detection of dysplasia in a high-risk Barrett’s esophagus surveillance population. Dig Dis Sci. 2011;56:761–6. doi: 10.1007/s10620-010-1459-z. [DOI] [PubMed] [Google Scholar]
- 41.Johanson JF, Frakes J, Eisen D. Computer-assisted analysis of abrasive transepithelial brush biopsies increases the effectiveness of esophageal screening: a multicenter prospective clinical trial by the EndoCDx Collaborative Group. Dig Dis Sci. 2011;56:767–72. doi: 10.1007/s10620-010-1497-6. [DOI] [PubMed] [Google Scholar]
- 42.Vennalaganti PR, Naag Kanakadandi V, Gross SA, Parasa S, Wang KK, Gupta N, et al. Inter-Observer Agreement among Pathologists Using Wide-Area Transepithelial Sampling With Computer-Assisted Analysis in Patients With Barrett’s Esophagus. The American journal of gastroenterology. 2015;110:1257–60. doi: 10.1038/ajg.2015.116. [DOI] [PubMed] [Google Scholar]
- 43.Downs-Kelly E, Mendelin JE, Bennett AE, Castilla E, Henricks WH, Schoenfield L, et al. Poor Interobserver Agreement in the Distinction of High-Grade Dysplasia and Adenocarcinoma in Pretreatment Barrett’s Esophagus Biopsies. The American journal of gastroenterology. 2008;103:2333–40. doi: 10.1111/j.1572-0241.2008.02020.x. [DOI] [PubMed] [Google Scholar]
- 44.Peery AF, Hoppo T, Garman KS, Dellon ES, Daugherty N, Bream S, et al. Feasibility, safety, acceptability, and yield of office-based, screening transnasal esophagoscopy (with video) Gastrointestinal endoscopy. 2012;75:945–53. e2. doi: 10.1016/j.gie.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jobe BA, Hunter JG, Chang EY, Kim CY, Eisen GM, Robinson JD, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. The American journal of gastroenterology. 2006;101:2693–703. doi: 10.1111/j.1572-0241.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 46.Saeian K, Staff DM, Vasilopoulos S, Townsend WF, Almagro UA, Komorowski RA, et al. Unsedated transnasal endoscopy accurately detects Barrett’s metaplasia and dysplasia. Gastrointestinal endoscopy. 2002;56:472–8. doi: 10.1067/mge.2002.128131. [DOI] [PubMed] [Google Scholar]
- 47.Shariff MK, Bird-Lieberman EL, O’Donovan M, Abdullahi Z, Liu X, Blazeby J, et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett’s esophagus. Gastrointestinal endoscopy. 2012;75:954–61. doi: 10.1016/j.gie.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 48.Sami SS, Dunagan KT, Johnson ML, Schleck CD, Shah ND, Zinsmeister AR, et al. A randomized comparative effectiveness trial of novel endoscopic techniques and approaches for Barrett’s esophagus screening in the community. The American journal of gastroenterology. 2015;110:148–58. doi: 10.1038/ajg.2014.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alashkar B, Faulx AL, Hepner A, Pulice R, Vemana S, Greer KB, et al. Development of a program to train physician extenders to perform transnasal esophagoscopy and screen for Barrett’s esophagus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:785–92. doi: 10.1016/j.cgh.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seibel EJ, Carroll RE, Dominitz JA, Johnston RS, Melville CD, Lee CM, et al. Tethered capsule endoscopy, a low-cost and high-performance alternative technology for the screening of esophageal cancer and Barrett’s esophagus. IEEE transactions on bio-medical engineering. 2008;55:1032–42. doi: 10.1109/TBME.2008.915680. [DOI] [PubMed] [Google Scholar]
- 51.Benaglia T, Sharples LD, Fitzgerald RC, Lyratzopoulos G. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett’s esophagus. Gastroenterology. 2013;144:62–73. e6. doi: 10.1053/j.gastro.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 52.Eliakim R, Sharma VK, Yassin K, Adler SN, Jacob H, Cave DR, et al. A prospective study of the diagnostic accuracy of PillCam ESO esophageal capsule endoscopy versus conventional upper endoscopy in patients with chronic gastroesophageal reflux diseases. J Clin Gastroenterol. 2005;39:572–8. doi: 10.1097/01.mcg.0000170764.29202.24. [DOI] [PubMed] [Google Scholar]
- 53.Galmiche JP, Sacher-Huvelin S, Coron E, Cholet F, Soussan EB, Sebille V, et al. Screening for esophagitis and Barrett’s esophagus with wireless esophageal capsule endoscopy: a multicenter prospective trial in patients with reflux symptoms. The American journal of gastroenterology. 2008;103:538–45. doi: 10.1111/j.1572-0241.2007.01731.x. [DOI] [PubMed] [Google Scholar]
- 54.Lin OS, Schembre DB, Mergener K, Spaulding W, Lomah N, Ayub K, et al. Blinded comparison of esophageal capsule endoscopy versus conventional endoscopy for a diagnosis of Barrett’s esophagus in patients with chronic gastroesophageal reflux. Gastrointestinal endoscopy. 2007;65:577–83. doi: 10.1016/j.gie.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 55.Gerson L, Lin OS. Cost-benefit analysis of capsule endoscopy compared with standard upper endoscopy for the detection of Barrett’s esophagus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5:319–25. doi: 10.1016/j.cgh.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 56.Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Human pathology. 2001;32:368–78. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 57.Abela JE, Going JJ, Mackenzie JF, McKernan M, O’Mahoney S, Stuart RC. Systematic four-quadrant biopsy detects Barrett’s dysplasia in more patients than nonsystematic biopsy. The American journal of gastroenterology. 2008;103:850–5. doi: 10.1111/j.1572-0241.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 58.Fitzgerald RC, Saeed IT, Khoo D, Farthing MJ, Burnham WR. Rigorous surveillance protocol increases detection of curable cancers associated with Barrett’s esophagus. Dig Dis Sci. 2001;46:1892–8. doi: 10.1023/a:1010678913481. [DOI] [PubMed] [Google Scholar]
- 59.Gupta N, Gaddam S, Wani SB, Bansal A, Rastogi A, Sharma P. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointestinal endoscopy. 2012;76:531–8. doi: 10.1016/j.gie.2012.04.470. [DOI] [PubMed] [Google Scholar]
- 60.Enestvedt BK, Lugo R, Guarner-Argente C, Shah P, Falk GW, Furth E, et al. Location, location, location: does early cancer in Barrett’s esophagus have a preference? Gastrointestinal endoscopy. 2013;78:462–7. doi: 10.1016/j.gie.2013.03.167. [DOI] [PubMed] [Google Scholar]
- 61.Cassani L, Sumner E, Slaughter JC, Yachimski P. Directional distribution of neoplasia in Barrett’s esophagus is not influenced by distance from the gastroesophageal junction. Gastrointestinal endoscopy. 2013;77:877–82. doi: 10.1016/j.gie.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 62.Kariyawasam VC, Bourke MJ, Hourigan LF, Lim G, Moss A, Williams SJ, et al. Circumferential location predicts the risk of high-grade dysplasia and early adenocarcinoma in short-segment Barrett’s esophagus. Gastrointestinal endoscopy. 2012;75:938–44. doi: 10.1016/j.gie.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 63.Pech O, Gossner L, Manner H, May A, Rabenstein T, Behrens A, et al. Prospective evaluation of the macroscopic types and location of early Barrett’s neoplasia in 380 lesions. Endoscopy. 2007;39:588–93. doi: 10.1055/s-2007-966363. [DOI] [PubMed] [Google Scholar]
- 64.Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus. Gastroenterology. 1993;105:40–50. doi: 10.1016/0016-5085(93)90008-z. [DOI] [PubMed] [Google Scholar]
- 65.Peters FP, Brakenhoff KP, Curvers WL, Rosmolen WD, Fockens P, ten Kate FJ, et al. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett’s esophagus. Gastrointestinal endoscopy. 2008;67:604–9. doi: 10.1016/j.gie.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 66.Abrams JA, Kapel RC, Lindberg GM, Saboorian MH, Genta RM, Neugut AI, et al. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7:736–42. doi: 10.1016/j.cgh.2008.12.027. quiz 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma P, Hawes RH, Bansal A, Gupta N, Curvers W, Rastogi A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: a prospective, international, randomised controlled trial. Gut. 2013;62:15–21. doi: 10.1136/gutjnl-2011-300962. [DOI] [PubMed] [Google Scholar]
- 68.Wolfsen HC, Crook JE, Krishna M, Achem SR, Devault KR, Bouras EP, et al. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s Esophagus. Gastroenterology. 2008;135:24–31. doi: 10.1053/j.gastro.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 69.Sharma P, Bergman JJ, Goda K, Kato M, Messmann H, Alsop BR, et al. Development and Validation of a Classification System to Identify High-Grade Dysplasia and Esophageal Adenocarcinoma in Barrett’s Esophagus Using Narrow-Band Imaging. Gastroenterology. 2016;150:591–8. doi: 10.1053/j.gastro.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 70.Kara MA, Peters FP, Ten Kate FJ, Van Deventer SJ, Fockens P, Bergman JJ. Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett’s esophagus. Gastrointestinal endoscopy. 2005;61:679–85. doi: 10.1016/s0016-5107(04)02577-5. [DOI] [PubMed] [Google Scholar]
- 71.Curvers WL, Singh R, Song LM, Wolfsen HC, Ragunath K, Wang K, et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus: a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2008;57:167–72. doi: 10.1136/gut.2007.134213. [DOI] [PubMed] [Google Scholar]
- 72.Guelrud M, Herrera I, Essenfeld H, Castro J. Enhanced magnification endoscopy: a new technique to identify specialized intestinal metaplasia in Barrett’s esophagus. Gastrointestinal endoscopy. 2001;53:559–65. doi: 10.1067/mge.2001.114059. [DOI] [PubMed] [Google Scholar]
- 73.Sharma P, Weston AP, Topalovski M, Cherian R, Bhattacharyya A, Sampliner RE. Magnification chromoendoscopy for the detection of intestinal metaplasia and dysplasia in Barrett’s oesophagus. Gut. 2003;52:24–7. doi: 10.1136/gut.52.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Endo T, Awakawa T, Takahashi H, Arimura Y, Itoh F, Yamashita K, et al. Classification of Barrett’s epithelium by magnifying endoscopy. Gastrointestinal endoscopy. 2002;55:641–7. doi: 10.1067/mge.2002.123420. [DOI] [PubMed] [Google Scholar]
- 75.Tholoor S, Bhattacharyya R, Tsagkournis O, Longcroft-Wheaton G, Bhandari P. Acetic acid chromoendoscopy in Barrett’s esophagus surveillance is superior to the standardized random biopsy protocol: results from a large cohort study (with video) Gastrointestinal endoscopy. 2014;80:417–24. doi: 10.1016/j.gie.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 76.Longcroft-Wheaton G, Duku M, Mead R, Poller D, Bhandari P. Acetic acid spray is an effective tool for the endoscopic detection of neoplasia in patients with Barrett’s esophagus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8:843–7. doi: 10.1016/j.cgh.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 77.Bertani H, Frazzoni M, Dabizzi E, Pigo F, Losi L, Manno M, et al. Improved detection of incident dysplasia by probe-based confocal laser endomicroscopy in a Barrett’s esophagus surveillance program. Dig Dis Sci. 2013;58:188–93. doi: 10.1007/s10620-012-2332-z. [DOI] [PubMed] [Google Scholar]
- 78.Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, et al. Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointestinal endoscopy. 2011;74:465–72. doi: 10.1016/j.gie.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Canto MI, Anandasabapathy S, Brugge W, Falk GW, Dunbar KB, Zhang Z, et al. In vivo endomicroscopy improves detection of Barrett’s esophagus-related neoplasia: a multicenter international randomized controlled trial (with video) Gastrointestinal endoscopy. 2014;79:211–21. doi: 10.1016/j.gie.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–13. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 81.Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:979–87. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 82.Swager AF, Boerwinkel DF, de Bruin DM, Faber DJ, van Leeuwen TG, Weusten BL, et al. Detection of buried Barrett’s glands after radiofrequency ablation with volumetric laser endomicroscopy. Gastrointestinal endoscopy. 2016;83:80–8. doi: 10.1016/j.gie.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 83.Leggett CL, Gorospe E, Owens VL, Anderson M, Lutzke L, Wang KK. Volumetric laser endomicroscopy detects subsquamous Barrett’s adenocarcinoma. The American journal of gastroenterology. 2014;109:298–9. doi: 10.1038/ajg.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trindade AJ, Vamadevan AS, Sejpal DV. Finding a needle in a haystack: use of volumetric laser endomicroscopy in targeting focal dysplasia in long-segment Barrett’s esophagus. Gastrointestinal endoscopy. 2015;82:756. doi: 10.1016/j.gie.2015.03.1984. discussion 7. [DOI] [PubMed] [Google Scholar]
- 85.Sharma P, Savides TJ, Canto MI, Corley DA, Falk GW, Goldblum JR, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on imaging in Barrett’s Esophagus. Gastrointestinal endoscopy. 2012;76:252–4. doi: 10.1016/j.gie.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 86.Thosani N, Abu Dayyeh BK, Sharma P, Aslanian HR, Enestvedt BK, Komanduri S, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointestinal endoscopy. 2016;83:684–98. e7. doi: 10.1016/j.gie.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 87.Verbeek RE, Leenders M, Ten Kate FJ, van Hillegersberg R, Vleggaar FP, van Baal JW, et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. The American journal of gastroenterology. 2014;109:1215–22. doi: 10.1038/ajg.2014.156. [DOI] [PubMed] [Google Scholar]
- 88.Cooper GS, Kou TD, Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. The American journal of gastroenterology. 2009;104:1356–62. doi: 10.1038/ajg.2009.159. [DOI] [PubMed] [Google Scholar]
- 89.Cooper GS, Yuan Z, Chak A, Rimm AA. Association of prediagnosis endoscopy with stage and survival in adenocarcinoma of the esophagus and gastric cardia. Cancer. 2002;95:32–8. doi: 10.1002/cncr.10646. [DOI] [PubMed] [Google Scholar]
- 90.Corley DA, Mehtani K, Quesenberry C, Zhao W, de Boer J, Weiss NS. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology. 2013;145:312–9. e1. doi: 10.1053/j.gastro.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kearney DJ, Crump C, Maynard C, Boyko EJ. A case-control study of endoscopy and mortality from adenocarcinoma of the esophagus or gastric cardia in persons with GERD. Gastrointestinal endoscopy. 2003;57:823–9. doi: 10.1016/s0016-5107(03)70015-7. [DOI] [PubMed] [Google Scholar]
- 92.Gordon LG, Mayne GC, Hirst NG, Bright T, Whiteman DC, Watson DI. Cost-effectiveness of endoscopic surveillance of non-dysplastic Barrett’s esophagus. Gastrointestinal endoscopy. 2014;79:242–56. e6. doi: 10.1016/j.gie.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 93.Inadomi JM, Somsouk M, Madanick RD, Thomas JP, Shaheen NJ. A cost-utility analysis of ablative therapy for Barrett’s esophagus. Gastroenterology. 2009;136:2101–14. e1–6. doi: 10.1053/j.gastro.2009.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Playford RJ. New British Society of Gastroenterology (BSG) guidelines for the diagnosis and management of Barrett’s oesophagus. Gut. 2006;55:442. doi: 10.1136/gut.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084–91. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 96.Rubenstein JH, Sonnenberg A, Davis J, McMahon L, Inadomi JM. Effect of a prior endoscopy on outcomes of esophageal adenocarcinoma among United States veterans. Gastrointestinal endoscopy. 2008;68:849–55. doi: 10.1016/j.gie.2008.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ormsby A, Petras R, Henricks W, Rice T, Rybicki L, Richter J, et al. Observer variation in the diagnosis of superficial oesophageal adenocarcinoma. Gut. 2002;51:671–6. doi: 10.1136/gut.51.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Selaru FM, Zou T, Xu Y, Shustova V, Yin J, Mori Y, et al. Global gene expression profiling in Barrett’s esophagus and esophageal cancer: a comparative analysis using cDNA microarrays. Oncogene. 2002;21:475–8. doi: 10.1038/sj.onc.1205111. [DOI] [PubMed] [Google Scholar]
- 99.Rabinovitch PS, Longton G, Blount PL, Levine DS, Reid BJ. Predictors of progression in Barrett’s esophagus III: baseline flow cytometric variables. The American journal of gastroenterology. 2001;96:3071–83. doi: 10.1111/j.1572-0241.2001.05261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reid BJ, Prevo LJ, Galipeau PC, Sanchez CA, Longton G, Levine DS, et al. Predictors of progression in Barrett’s esophagus II: baseline 17p (p53) loss of heterozygosity identifies a patient subset at increased risk for neoplastic progression. The American journal of gastroenterology. 2001;96:2839–48. doi: 10.1111/j.1572-0241.2001.04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maley CC, Galipeau PC, Li X, Sanchez CA, Paulson TG, Blount PL, et al. The combination of genetic instability and clonal expansion predicts progression to esophageal adenocarcinoma. Cancer research. 2004;64:7629–33. doi: 10.1158/0008-5472.CAN-04-1738. [DOI] [PubMed] [Google Scholar]
- 102.Bansal A, Lee IH, Hong X, Anand V, Mathur SC, Gaddam S, et al. Feasibility of mcroRNAs as biomarkers for Barrett’s Esophagus progression: a pilot cross-sectional, phase 2 biomarker study. The American journal of gastroenterology. 2011;106:1055–63. doi: 10.1038/ajg.2011.37. [DOI] [PubMed] [Google Scholar]
- 103.Revilla-Nuin B, Parrilla P, Lozano JJ, de Haro LF, Ortiz A, Martinez C, et al. Predictive value of MicroRNAs in the progression of barrett esophagus to adenocarcinoma in a long-term follow-up study. Annals of surgery. 2013;257:886–93. doi: 10.1097/SLA.0b013e31826ddba6. [DOI] [PubMed] [Google Scholar]
- 104.Schulmann K, Sterian A, Berki A, Yin J, Sato F, Xu Y, et al. Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett’s-associated neoplastic progression and predicts progression risk. Oncogene. 2005;24:4138–48. doi: 10.1038/sj.onc.1208598. [DOI] [PubMed] [Google Scholar]
- 105.Galipeau PC, Li X, Blount PL, Maley CC, Sanchez CA, Odze RD, et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS medicine. 2007;4:e67. doi: 10.1371/journal.pmed.0040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zaidi AH, Gopalakrishnan V, Kasi PM, Zeng X, Malhotra U, Balasubramanian J, et al. Evaluation of a 4-protein serum biomarker panel-biglycan, annexin-A6, myeloperoxidase, and protein S100-A9 (B-AMP)-for the detection of esophageal adenocarcinoma. Cancer. 2014;120:3902–13. doi: 10.1002/cncr.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Baal JW, Bus P, Kestens C, Ten Kate FT, Peters WH, Drenth JP, et al. 544 Comprehensive Profiling of Plasma MicroRNAs Reveals Potential Biomarkers for Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastroenterology. 2014;146:S-97. [Google Scholar]
- 108.Kastelein F, Spaander MC, Steyerberg EW, Biermann K, Valkhoff VE, Kuipers EJ, et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:382–8. doi: 10.1016/j.cgh.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 109.Liao LM, Vaughan TL, Corley DA, Cook MB, Casson AG, Kamangar F, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012;142:442–52. e5. doi: 10.1053/j.gastro.2011.11.019. quiz e22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang S, Zhang XQ, Ding XW, Yang RK, Huang SL, Kastelein F, et al. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a meta-analysis. British journal of cancer. 2014;110:2378–88. doi: 10.1038/bjc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. The New England journal of medicine. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 112.Prasad GA, Wang KK, Buttar NS, Wongkeesong LM, Krishnadath KK, Nichols FC, 3rd, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2007;132:1226–33. doi: 10.1053/j.gastro.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wani S, Falk G, Hall M, Gaddam S, Wang A, Gupta N, et al. Patients with nondysplastic Barrett’s esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:220–7. doi: 10.1016/j.cgh.2010.11.008. quiz e26. [DOI] [PubMed] [Google Scholar]
- 114.Bulsiewicz WJ, Kim HP, Dellon ES, Cotton CC, Pasricha S, Madanick RD, et al. Safety and efficacy of endoscopic mucosal therapy with radiofrequency ablation for patients with neoplastic Barrett’s esophagus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:636–42. doi: 10.1016/j.cgh.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hur C, Choi SE, Rubenstein JH, Kong CY, Nishioka NS, Provenzale DT, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology. 2012;143:567–75. doi: 10.1053/j.gastro.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buttar NS, Wang KK, Sebo TJ, Riehle DM, Krishnadath KK, Lutzke LS, et al. Extent of high-grade dysplasia in Barrett’s esophagus correlates with risk of adenocarcinoma. Gastroenterology. 2001;120:1630–9. doi: 10.1053/gast.2001.25111. [DOI] [PubMed] [Google Scholar]
- 117.Manner H, Pech O, Heldmann Y, May A, Pohl J, Behrens A, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:630–5. doi: 10.1016/j.cgh.2012.12.040. quiz e45. [DOI] [PubMed] [Google Scholar]
- 118.Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–6. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 119.Wani S, Abrams J, Edmundowicz SA, Gaddam S, Hovis CE, Green D, et al. Endoscopic mucosal resection results in change of histologic diagnosis in Barrett’s esophagus patients with visible and flat neoplasia: a multicenter cohort study. Dig Dis Sci. 2013;58:1703–9. doi: 10.1007/s10620-013-2689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pouw RE, van Vilsteren FG, Peters FP, Alvarez Herrero L, Ten Kate FJ, Visser M, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett’s neoplasia. Gastrointestinal endoscopy. 2011;74:35–43. doi: 10.1016/j.gie.2011.03.1243. [DOI] [PubMed] [Google Scholar]
- 121.Chennat J, Konda VJ, Ross AS, de Tejada AH, Noffsinger A, Hart J, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. The American journal of gastroenterology. 2009;104:2684–92. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 122.Larghi A, Lightdale CJ, Ross AS, Fedi P, Hart J, Rotterdam H, et al. Long-term follow-up of complete Barrett’s eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy. 2007;39:1086–91. doi: 10.1055/s-2007-966788. [DOI] [PubMed] [Google Scholar]
- 123.Phoa KN, Pouw RE, Bisschops R, Pech O, Ragunath K, Weusten BL, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of an European multicentre study (EURO-II) Gut. 2016;65:555–62. doi: 10.1136/gutjnl-2015-309298. [DOI] [PubMed] [Google Scholar]
- 124.Haidry RJ, Dunn JM, Butt MA, Burnell MG, Gupta A, Green S, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology. 2013;145:87–95. doi: 10.1053/j.gastro.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 125.Phoa KN, Pouw RE, van Vilsteren FG, Sondermeijer CM, Ten Kate FJ, Visser M, et al. Remission of Barrett’s esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology. 2013;145:96–104. doi: 10.1053/j.gastro.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 126.Qumseya BJ, Wani S, Desai M, Qumseya A, Bain P, Sharma P, et al. Adverse Events After Radiofrequency Ablation in Patients With Barrett’s Esophagus: A Systematic Review and Meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016;14:1086–95. e6. doi: 10.1016/j.cgh.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 127.Scholvinck DW, Kunzli HT, Kestens C, Siersema PD, Vleggaar FP, Canto MI, et al. Treatment of Barrett’s esophagus with a novel focal cryoablation device: a safety and feasibility study. Endoscopy. 2015;47:1106–12. doi: 10.1055/s-0034-1392417. [DOI] [PubMed] [Google Scholar]
- 128.Ghorbani S, Tsai FC, Greenwald BD, Jang S, Dumot JA, McKinley MJ, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s dysplasia: results of the National Cryospray Registry. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / ISDE. 2016;29:241–7. doi: 10.1111/dote.12330. [DOI] [PubMed] [Google Scholar]
- 129.Halsey KD, Chang JW, Waldt A, Greenwald BD. Recurrent disease following endoscopic ablation of Barrett’s high-grade dysplasia with spray cryotherapy. Endoscopy. 2011;43:844–8. doi: 10.1055/s-0030-1256649. [DOI] [PubMed] [Google Scholar]
- 130.Gosain S, Mercer K, Twaddell WS, Uradomo L, Greenwald BD. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high-grade dysplasia: long-term results. Gastrointestinal endoscopy. 2013;78:260–5. doi: 10.1016/j.gie.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 131.Greenwald BD, Dumot JA, Horwhat JD, Lightdale CJ, Abrams JA. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / ISDE. 2010;23:13–9. doi: 10.1111/j.1442-2050.2009.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Verbeek RE, Vleggaar FP, Ten Kate FJ, van Baal JW, Siersema PD. Cryospray ablation using pressurized CO2 for ablation of Barrett’s esophagus with early neoplasia: early termination of a prospective series. Endoscopy international open. 2015;3:E107–12. doi: 10.1055/s-0034-1390759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Overholt BF, Lightdale CJ, Wang KK, Canto MI, Burdick S, Haggitt RC, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointestinal endoscopy. 2005;62:488–98. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 134.Overholt BF, Wang KK, Burdick JS, Lightdale CJ, Kimmey M, Nava HR, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointestinal endoscopy. 2007;66:460–8. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 135.Ertan A, Zaheer I, Correa AM, Thosani N, Blackmon SH. Photodynamic therapy vs radiofrequency ablation for Barrett’s dysplasia: efficacy, safety and cost-comparison. World journal of gastroenterology : WJG. 2013;19:7106–13. doi: 10.3748/wjg.v19.i41.7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dulai GS, Jensen DM, Cortina G, Fontana L, Ippoliti A. Randomized trial of argon plasma coagulation vs. multipolar electrocoagulation for ablation of Barrett’s esophagus. Gastrointestinal endoscopy. 2005;61:232–40. doi: 10.1016/s0016-5107(04)02576-3. [DOI] [PubMed] [Google Scholar]
- 137.Manner H, Rabenstein T, Pech O, Braun K, May A, Pohl J, et al. Ablation of residual Barrett’s epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study) Endoscopy. 2014;46:6–12. doi: 10.1055/s-0033-1358813. [DOI] [PubMed] [Google Scholar]
- 138.Pasricha S, Bulsiewicz WJ, Hathorn KE, Komanduri S, Muthusamy VR, Rothstein RI, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:1840–7. e1. doi: 10.1016/j.cgh.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Krishnamoorthi R, Singh S, Ragunathan K, DAK, KKW, PGI Risk of recurrence of Barrett’s esophagus after successful endoscopic therapy. Gastrointestinal endoscopy. 2016 doi: 10.1016/j.gie.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guarner-Argente C, Buoncristiano T, Furth EE, Falk GW, Ginsberg GG. Long-term outcomes of patients with Barrett’s esophagus and high-grade dysplasia or early cancer treated with endoluminal therapies with intention to complete eradication. Gastrointestinal endoscopy. 2013;77:190–9. doi: 10.1016/j.gie.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 141.Cotton CC, Wolf WA, Pasricha S, Li N, Madanick RD, Spacek MB, et al. Recurrent intestinal metaplasia after radiofrequency ablation for Barrett’s esophagus: endoscopic findings and anatomic location. Gastrointestinal endoscopy. 2015;81:1362–9. doi: 10.1016/j.gie.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gupta M, Iyer PG, Lutzke L, Gorospe EC, Abrams JA, Falk GW, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology. 2013;145:79–86. e1. doi: 10.1053/j.gastro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang EY, Morris CD, Seltman AK, O’Rourke RW, Chan BK, Hunter JG, et al. The effect of antireflux surgery on esophageal carcinogenesis in patients with barrett esophagus: a systematic review. Annals of surgery. 2007;246:11–21. doi: 10.1097/01.sla.0000261459.10565.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. The American journal of gastroenterology. 2003;98:2390–4. doi: 10.1111/j.1572-0241.2003.08702.x. [DOI] [PubMed] [Google Scholar]
- 145.Orlando RC. How good is the neosquamous epithelium? Digestive diseases (Basel, Switzerland) 2014;32:164–70. doi: 10.1159/000357185. [DOI] [PubMed] [Google Scholar]
- 146.Skrobic O, Simic A, Radovanovic N, Ivanovic N, Micev M, Pesko P. Significance of Nissen fundoplication after endoscopic radiofrequency ablation of Barrett’s esophagus. Surgical endoscopy. 2015 doi: 10.1007/s00464-015-4677-9. [DOI] [PubMed] [Google Scholar]
- 147.Johnson CS, Louie BE, Wille A, Dunst CM, Worrell SG, DeMeester SR, et al. The Durability of Endoscopic Therapy for Treatment of Barrett’s Metaplasia, Dysplasia, and Mucosal Cancer After Nissen Fundoplication. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2015;19:799–805. doi: 10.1007/s11605-015-2783-6. [DOI] [PubMed] [Google Scholar]
- 148.O’Connell K, Velanovich V. Effects of Nissen fundoplication on endoscopic endoluminal radiofrequency ablation of Barrett’s esophagus. Surgical endoscopy. 2011;25:830–4. doi: 10.1007/s00464-010-1270-0. [DOI] [PubMed] [Google Scholar]
- 149.Peyre CG, Watson TJ. Surgical Management of Barrett’s Esophagus. Gastroenterology clinics of North America. 2015;44:459–71. doi: 10.1016/j.gtc.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 150.Boerwinkel DF, Swager A, Curvers WL, Bergman JJ. The clinical consequences of advanced imaging techniques in Barrett’s esophagus. Gastroenterology. 2014;146:622–9. e4. doi: 10.1053/j.gastro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 151.Borovicka J, Fischer J, Neuweiler J, Netzer P, Gschossmann J, Ehmann T, et al. Autofluorescence endoscopy in surveillance of Barrett’s esophagus: a multicenter randomized trial on diagnostic efficacy. Endoscopy. 2006;38:867–72. doi: 10.1055/s-2006-944726. [DOI] [PubMed] [Google Scholar]
- 152.Egger K, Werner M, Meining A, Ott R, Allescher HD, Hofler H, et al. Biopsy surveillance is still necessary in patients with Barrett’s oesophagus despite new endoscopic imaging techniques. Gut. 2003;52:18–23. doi: 10.1136/gut.52.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Giacchino M, Bansal A, Kim RE, Singh V, Hall SB, Singh M, et al. Clinical utility and interobserver agreement of autofluorescence imaging and magnification narrow-band imaging for the evaluation of Barrett’s esophagus: a prospective tandem study. Gastrointestinal endoscopy. 2013;77:711–8. doi: 10.1016/j.gie.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 154.Leggett CL, Gorospe EC, Chan DK, Muppa P, Owens V, Smyrk TC, et al. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett’s esophagus. Gastrointestinal endoscopy. 2016;83:880–8. e2. doi: 10.1016/j.gie.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Evans JA, Poneros JM, Bouma BE, Bressner J, Halpern EF, Shishkov M, et al. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:38–43. doi: 10.1053/S1542-3565(05)00746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Isenberg G, Sivak MV, Jr, Chak A, Wong RC, Willis JE, Wolf B, et al. Accuracy of endoscopic optical coherence tomography in the detection of dysplasia in Barrett’s esophagus: a prospective, double-blinded study. Gastrointestinal endoscopy. 2005;62:825–31. doi: 10.1016/j.gie.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 157.Almond LM, Hutchings J, Lloyd G, Barr H, Shepherd N, Day J, et al. Endoscopic Raman spectroscopy enables objective diagnosis of dysplasia in Barrett’s esophagus. Gastrointestinal endoscopy. 2014;79:37–45. doi: 10.1016/j.gie.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 158.Bergholt MS, Zheng W, Ho KY, Teh M, Yeoh KG, Yan So JB, et al. Fiberoptic confocal raman spectroscopy for real-time in vivo diagnosis of dysplasia in Barrett’s esophagus. Gastroenterology. 2014;146:27–32. doi: 10.1053/j.gastro.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 159.Wallace MB, Perelman LT, Backman V, Crawford JM, Fitzmaurice M, Seiler M, et al. Endoscopic detection of dysplasia in patients with Barrett’s esophagus using light-scattering spectroscopy. Gastroenterology. 2000;119:677–82. doi: 10.1053/gast.2000.16511. [DOI] [PubMed] [Google Scholar]
- 160.Kastelein F, Biermann K, Steyerberg EW, Verheij J, Kalisvaart M, Looijenga LH, et al. Aberrant p53 protein expression is associated with an increased risk of neoplastic progression in patients with Barrett’s oesophagus. Gut. 2013;62:1676–83. doi: 10.1136/gutjnl-2012-303594. [DOI] [PubMed] [Google Scholar]
- 161.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. The American journal of gastroenterology. 2000;95:1669–76. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bird-Lieberman EL, Dunn JM, Coleman HG, Lao-Sirieix P, Oukrif D, Moore CE, et al. Population-based study reveals new risk-stratification biomarker panel for Barrett’s esophagus. Gastroenterology. 2012;143:927–35. e3. doi: 10.1053/j.gastro.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 163.Timmer MR, Lau CT, Rosmolen W, Meijer SL, Dijkgraaf MG, Mallant-Hent RC, et al. 164 A FISH Biomarker Panel for the Prediction of High-grade Dysplasia and Adenocarcinoma in Non-Dysplastic Barrett’s Esophagus; Results From a Long-Term Prospective Cohort Study. Gastrointestinal endoscopy. 2014;79:AB115–AB6. [Google Scholar]
- 164.Jin Z, Cheng Y, Gu W, Zheng Y, Sato F, Mori Y, et al. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in Barrett’s esophagus. Cancer research. 2009;69:4112–5. doi: 10.1158/0008-5472.CAN-09-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Alvi MA, Liu X, O’Donovan M, Newton R, Wernisch L, Shannon NB, et al. DNA methylation as an adjunct to histopathology to detect prevalent, inconspicuous dysplasia and early-stage neoplasia in Barrett’s esophagus. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:878–88. doi: 10.1158/1078-0432.CCR-12-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maley CC, Galipeau PC, Finley JC, Wongsurawat VJ, Li X, Sanchez CA, et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nature genetics. 2006;38:468–73. doi: 10.1038/ng1768. [DOI] [PubMed] [Google Scholar]
- 167.Pacha A, Graham TA, Maley CC, Krishnadath KK. Su1911 increasing clonal diversity predicts progression in Barrett esophagus patients, results from a phase IV 5 year prospective follow up study. Gastroenterology. 2012;142:S-534. [Google Scholar]
- 168.Bani-Hani K, Martin IG, Hardie LJ, Mapstone N, Briggs JA, Forman D, et al. Prospective study of cyclin D1 overexpression in Barrett’s esophagus: association with increased risk of adenocarcinoma. Journal of the National Cancer Institute. 2000;92:1316–21. doi: 10.1093/jnci/92.16.1316. [DOI] [PubMed] [Google Scholar]
- 169.Sirieix PS, O’Donovan M, Brown J, Save V, Coleman N, Fitzgerald RC. Surface expression of minichromosome maintenance proteins provides a novel method for detecting patients at risk for developing adenocarcinoma in Barrett’s esophagus. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:2560–6. [PubMed] [Google Scholar]