Abstract
Humans cannot synthesize fat-soluble vitamins such as vitamin E and vitamin K. For this reason, they must be obtained from the diet via intestinal absorption. As the deficiency or excess of these vitamins has been reported to cause several types of diseases and disorders in humans, the intestinal absorption of these nutrients must be properly regulated to ensure good health. However, the mechanism of their intestinal absorption remains poorly understood. Recent studies on cholesterol using genome-edited mice, genome-wide association approaches, gene mutation analyses, and the development of cholesterol absorption inhibitors have revealed that several membrane proteins play crucial roles in the intestinal absorption of cholesterol. Surprisingly, detailed analyses of these cholesterol transporters have revealed that they can also transport vitamin E and vitamin K, providing clues to uncover the molecular mechanisms underlying the intestinal absorption of these fat-soluble vitamins. In this review, we focus on the membrane proteins (Niemann-Pick C1 like 1, scavenger receptor class B type I, cluster of differentiation 36, and ATP-binding cassette transporter A1) that are (potentially) involved in the intestinal absorption of cholesterol, vitamin E, and vitamin K and discuss their physiological and pharmacological importance. We also discuss the related uncertainties that need to be explored in future studies.
Keywords: ABCA1, CD36, Ezetimibe, NPC1L1, SR-BI
Introduction
The excess intake of cholesterol (Fig. 1A) due to the westernization of eating habits has led to an increased number of patients worldwide with lifestyle-related diseases such as dyslipidemia and obesity. Meanwhile, the moderate intake of antioxidant and/or anticalcification lipids such as vitamin E (Fig. 1B) and vitamin K (Fig. 1C) is reported to reduce the risk of atherosclerosis and cardiovascular events1, 2). The appropriate regulation of intestinal lipid absorption is therefore important for a healthy life and is also considered an attractive strategy to prevent lifestyle-related diseases.
Fig. 1.
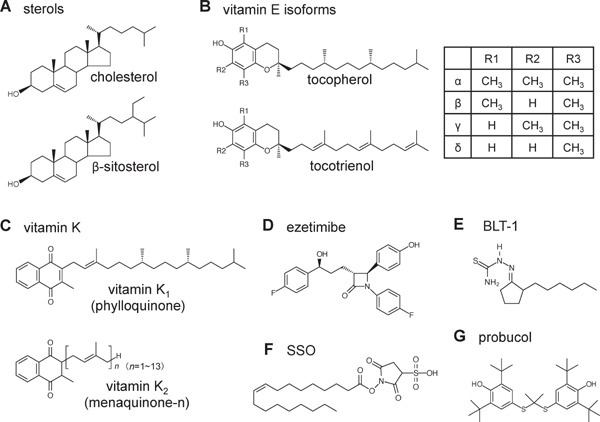
Chemical structures of (A) sterols, (B) vitamin E isoforms, (C) vitamin K, (D) ezetimibe, (E) BLT-1, (F) SSO, and (G) probucol.
It was long assumed that dietary cholesterol and fat-soluble vitamins, including vitamin E and vitamin K, were absorbed via a passive diffusion process across the plasma membrane of enterocytes. However, recent advances in genomic analyses3–8), studies with genome-edited mice9–14), and the development of ezetimibe (Fig. 1D), a cholesterol absorption inhibitor clinically used for dyslipidemia15, 16), have revealed that several membrane transporters play key roles in intestinal cholesterol absorption. Interestingly, the identification of such cholesterol transporters has also provided novel insights into the molecular mechanism underlying the absorption of fat-soluble vitamins because most of these transporters can transport vitamin E and vitamin K in addition to cholesterol.
In this review, we provide an overview of the recent findings on intestinal cholesterol transporters that are also (potentially) involved in the absorption of vitamin E and vitamin K and discuss clinical topics related to the pharmacological effects of ezetimibe on the absorption of these fat-soluble vitamins (Fig. 2).
Fig. 2.
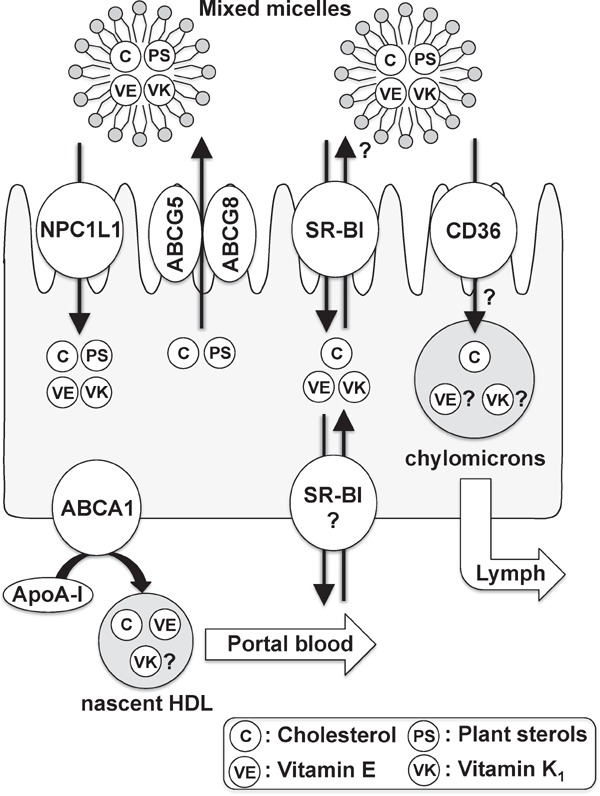
Membrane proteins mediating the intestinal absorption of cholesterol, plant sterols, vitamin E, and vitamin K1.
Recent research has revealed that several transmembrane proteins such as NPC1L1, ABCG5/G8, SR-B1, CD36, and ABCA1 play crucial roles in the intestinal absorption of cholesterol, plant sterols, vitamin E, and vitamin K1.
Niemann-Pick C1 Like 1
Niemann-Pick C1 like 1 (NPC1L1) is recognized as a key player in intestinal cholesterol absorption and a molecular target of ezetimibe. NPC1L1 was originally identified as a homologue of the Niemann-Pick C1 (NPC1) protein17), which is involved in intracellular cholesterol trafficking from lysosomes to other organelles such as the endoplasmic reticulum and plasma membrane18). Deficiency of the NPC1 protein causes Niemann Pick disease type C, a genetic disorder characterized by lysosomal cholesterol accumulation resulting in neurodegeneration and premature death19, 20). Unlike NPC1 knockout (KO) mice, NPC1L1 KO mice appear healthy without severe phenotypes. However, in 2004, Altmann et al. found that intestinal cholesterol absorption in NPC1L1 KO mice was reduced to about 30% of that in wild-type (WT) mice, and the degree of this reduction was almost the same as that observed in ezetimibe-treated WT mice9). In addition, ezetimibe had little effect on the remaining level of intestinal cholesterol absorption in NPC1L1 KO mice. Based on these results and the fact that NPC1L1 is highly expressed on the brush border membrane of enterocytes in the proximal intestine, where cholesterol absorption primarily occurs9), as well as in vitro observations that ezetimibe binds to the NPC1L1 protein21, 22), NPC1L1 is now thought to be a central player in intestinal cholesterol absorption and a molecular target of ezetimibe. In this section, we summarize our recent findings on NPC1L1 function in addition to the drug-drug interaction between ezetimibe and warfarin.
NPC1L1-Mediated Sterol Absorption
Detailed analyses of NPC1L1 function were performed in vitro using NPC1L1-overexpressing Caco-2 (colorectal adenocarcinoma) cells23). Consistent with the physiological localization of NPC1L1, the introduced NPC1L1 protein was expressed on the apical membrane in Caco-2 cells. In addition, the cellular uptake of cholesterol dissolved in mixed micelles containing taurocholate and phosphatidylcholine was increased by NPC1L1 overexpression in Caco-2 cells. Moreover, this increase was inhibited by ezetimibe in a concentration-dependent manner. These in vitro results clearly indicate that NPC1L1 has micellar cholesterol uptake activity, which is sensitive to ezetimibe.
Interestingly, in vitro studies using NPC1L1-overexpressing Caco-2 cells also showed that micellar taurocholate could increase NPC1L1-mediated cholesterol uptake in a concentration-dependent manner23). By contrast, micellar phosphatidylcholine showed a negative correlation with cholesterol uptake by NPC1L1. These results are consistent with in vivo observations that patients with a hereditary defect in bile acid synthesis exhibited a reduction in cholesterol absorption24) and that cholesterol absorption was suppressed by phosphatidylcholine supplementation in humans25). Based on these findings, the effects of the micellar composition on NPC1L1 activity would be an important factor to control the efficiency of intestinal cholesterol absorption.
It has been reported that the absorption of plant sterols in NPC1L1 KO mice is lower than that in WT mice26). Consistently, our in vitro study demonstrated that β-sitosterol, a major dietary plant sterol (Fig. 1A), was taken up more efficiently in NPC1L1-overexpressing Caco-2 cells than in control cells, indicating that NPC1L1 has the ability to transport β-sitosterol in addition to cholesterol23). However, it should be noted that the NPC1L1-mediated uptake of β-sitosterol was approximately 30% of that of cholesterol. It has been reported that ∼50% of dietary cholesterol is absorbed in the intestine compared to only 5%–15% of dietary plant sterols27). Our in vitro results indicate that the lower level of intestinal absorption of plant sterols might be due to the lower level of uptake of plant sterols by NPC1L1, in addition to the contribution of the well-known luminal backflux of sterols by the heterodimer of ATP-binding cassette transporter G5 and G8 (ABCG5/G8)6, 8) (Fig. 2).
NPC1L1-Mediated Vitamin E Absorption
Given that fat-soluble vitamins, similar to cholesterol, are solubilized in mixed micelles and then absorbed in the small intestine, we assumed that some of these vitamins might be taken up by enterocytes via a shared pathway with cholesterol. Based on this hypothesis, α-tocopherol (a major isoform of vitamin E) (Fig. 1B) and retinol (vitamin A) uptake were first examined using NPC1L1-overexpressing Caco-2 cells28). Although ezetimibe-sensitive retinol uptake was not observed, α-tocopherol uptake was increased by the overexpression of NPC1L1 and this increase was significantly blocked by ezetimibe in a concentration-dependent manner. In addition, nonsynonymous variants of NPC1L1 identified in cholesterol low absorbers (A395V, G402S, R417W and G434R)7) showed decreased transport activities for α-tocopherol and cholesterol compared to the WT, suggesting that the recognition and transport mechanisms for α-tocopherol are similar to those for cholesterol among the four variants and WT NPC1L129). In accordance with in vitro observations, ezetimibe administration significantly inhibited the intestinal absorption of not only cholesterol but also α-tocopherol in Wistar rats, although the inhibitory effect on α-tocopherol absorption (approximately 35% reduction by 0.3 mg/kg ezetimibe) was less than that on cholesterol absorption (60%–80% reduction)28, 30). Together with observations that other isoforms of vitamin E such as γ-tocopherol, δ-tocopherol, and tocotrienols (Fig. 1B) could also be taken up via the ezetimibe-sensitive NPC1L1-mediated pathway31–33), these results suggest that vitamin E is absorbed, at least partly, via the NPC1L1-dependent pathway.
Although ezetimibe inhibits vitamin E absorption in vivo, a clinical study has reported that serum concentrations of vitamin E were not significantly reduced after a 12-week administration of ezetimibe15). Based on the well-known fact that α-tocopherol transfer protein (α-TTP), a cytosolic protein expressed in the liver, plays a key role in maintaining vitamin E homeostasis by regulating hepatic storage and trafficking of α-tocopherol34, 35), the partial inhibition of vitamin E absorption by ezetimibe may not immediately cause vitamin E deficiency in humans. However, considering that humans cannot synthesize vitamin E and that it is therefore necessary to ingest this nutrient as part of the diet, the ezetimibe-mediated malabsorption of vitamin E should be taken into consideration as a potential risk for vitamin E deficiency.
NPC1L1-Mediated Vitamin K1 Absorption
Vitamin K is an essential nutrient that facilitates blood coagulation by activating clotting factors such as prothrombin and factors II, VII, IX and X in the liver36, 37). Vitamin K antagonists such as warfarin are therefore clinically used to prevent thromboembolism. Dietary vitamin K is generally categorized into two forms: phylloquinone (vitamin K1) and menaquinones (collectively referred to as vitamin K2), which comprise 13 compounds classified based on the length of their side chain (menaquinone-1 to menaquinone-13) (Fig. 1C). Vitamin K1 is enriched in green leafy vegetables, beans, and certain plant oils. On the other hand, most vitamin K2 is produced by bacteria in the intestine. It has been reported that vitamin K1 accounts for approximately 90% of dietary vitamin K38).
Despite the importance of vitamin K, there is little information on the molecular mechanism of intestinal vitamin K1 absorption. Based on the following three reasons, we hypothesized that NPC1L1 is a physiological vitamin K1 importer in the small intestine. First, the intestinal absorption of vitamin K1 depends on bile39), similar to that of cholesterol and vitamin E. Second, vitamin K1 is mainly absorbed in the upper intestine, where NPC1L1 is highly expressed9, 40). Third, the package insert for ezetimibe indicates a potential drug interaction between ezetimibe and warfarin that may enhance the anticoagulant effect of warfarin41) (please see the next subsection for details). To determine whether vitamin K1 is taken up via the NPC1L1-mediated pathway, in vitro vitamin K1 uptake assays using NPC1L1-overexpressing Caco-2 cells were conducted42). The results showed that the cellular uptake of vitamin K1 was significantly increased by NPC1L1 overexpression and that NPC1L1-mediated vitamin K1 uptake was inhibited by ezetimibe in a concentration-dependent manner. In addition, in vivo acute vitamin K1 absorption studies revealed that the intestinal absorption of vitamin K1 in NPC1L1 KO mice was dramatically reduced to less than 30% of that in WT mice, which was similar to the intestinal cholesterol absorption results (Fig. 3A). Moreover, ezetimibe administration significantly inhibited vitamin K1 absorption in Wistar rats and WT mice, whereas that in NPC1L1 KO mice was hardly affected by ezetimibe treatment (Fig. 3B). These results clearly indicate that the ezetimibe-sensitive NPC1L1-dependent pathway is primarily involved in intestinal vitamin K1 absorption as well as cholesterol absorption.
Fig. 3.
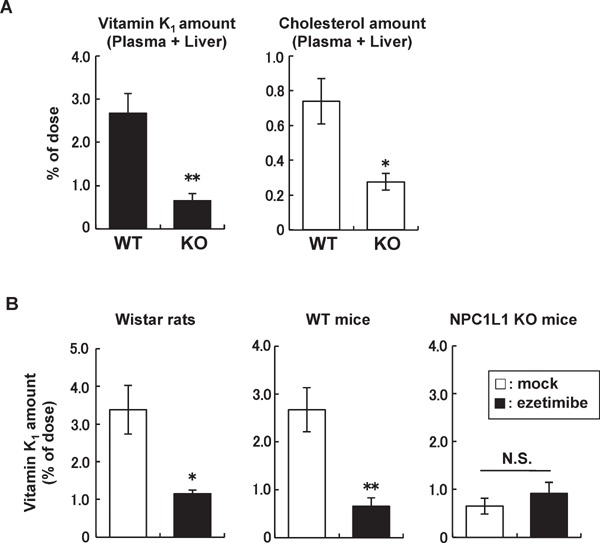
Intestinal vitamin K1 absorption in rodents.
(A) Intestinal absorption of vitamin K1 and cholesterol was examined in wild-type (WT) mice and NPC1L1 knockout (KO) mice. Vitamin K1 and [3H]cholesterol concentrations in the plasma and liver were examined 2 h after the intraduodenal administration of a vitamin K1- or [3H]cholesterol-containing emulsion. (B) Vitamin K1 absorption was examined in Wistar rats, WT mice, and NPC1L1 KO mice treated with or without ezetimibe (0.3 mg/kg for rats or 0.45 mg/kg for mice). Vitamin K1 concentrations in the plasma and liver were examined 3 h (for rats) or 2 h (for mice) after the intraduodenal administration of a vitamin K1-containing emulsion. Data are shown as the mean ± SEM (n = 4–8). **Significantly different by Student's t test (p < 0.01). *Significantly different by Student's t test (p < 0.05). N.S., not significantly different. (Modified and cited from reference 42).
Drug Interaction between Ezetimibe and Warfarin
Based on reports showing that anticoagulant activity is enhanced in patients taking warfarin in combination with ezetimibe43), the ezetimibe package insert warns that caution should be exercised when ezetimibe is co-administered with warfarin41). However, the mechanism of the warfarin-ezetimibe interaction was not clear. The results of vitamin K1 absorption studies (Fig. 3), together with the fact that clotting factors are activated in the liver through the cyclic conversion of hepatic vitamin K (vitamin K cycle)44), led to the hypothesis that the inhibition of vitamin K absorption by ezetimibe would apparently enhance the inhibitory effect of warfarin on the activation of clotting factors. To test this hypothesis, the effect of ezetimibe on anticoagulant activities and on hepatic vitamin K levels in Wistar rats treated with (or without) warfarin was examined42). The anticoagulant effect of warfarin was reflected in the extension of prothrombin time. Consistent with a previous case report in humans43), prothrombin time in rats co-treated with warfarin and ezetimibe (co-treatment group) was significantly longer than that in rats treated with warfarin alone (warfarin alone group) (Fig. 4A). Analysis of the hepatic vitamin K1 level in these rats showed that the liver vitamin K1 concentration in the co-treatment group was significantly lower than that in the warfarin alone group (Fig. 4B). In addition, vitamin K1 rescue experiments revealed that restoration of the hepatic vitamin K1 level by oral vitamin K1 supplementation canceled the extension of prothrombin time observed in the co-treatment group (Fig. 4). Taken together with the observation that the co-administration of ezetimibe did not result in significant changes in the hepatic warfarin concentration in rats, these data suggest that the effect of the drug interaction between ezetimibe and warfarin is mediated by the decrease in the hepatic vitamin K level, which is caused by ezetimibe-mediated vitamin K1 malabsorption (Fig. 5).
Fig. 4.
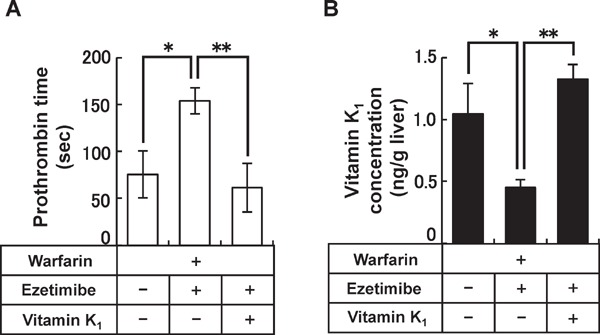
Drug interaction between ezetimibe and warfarin in Wistar rats.
(A) Prothrombin time and (B) hepatic vitamin K1 concentrations of Wistar rats after 1 week of daily oral co-administration of warfarin (0.2 mg/kg per day), ezetimibe (1 mg/kg per day), and vitamin K1 (1 µg/200 g body weight per day). Data are shown as the mean ± SEM (n = 6–13). **Significantly different by Mann-Whitney test with Bonferroni-Holm correction (p < 0.01). *Significantly different by Mann-Whitney test with Bonferroni-Holm correction (p < 0.05). (Modified and cited from reference 42).
Fig. 5.
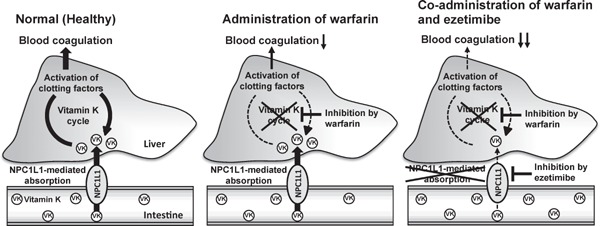
Proposed mechanism of the drug interaction between ezetimibe and warfarin.
Vitamin K (VK) is absorbed by NPC1L1 in the intestine and then circulates through the vitamin K cycle in the liver. In this cycle, vitamin K activates clotting factors and regulates blood coagulation. When warfarin, an anticoagulant drug that inhibits the vitamin K cycle, and ezetimibe, an NPC1L1 inhibitor clinically used for dyslipidemia, are administered together, the anticoagulant effect of warfarin is apparently enhanced by the ezetimibe-related reduction in vitamin K absorption.
This drug-drug interaction mechanism was supported by our retrospective survey of clinical records at the University of Tokyo Hospital, showing that in more than 85% of warfarin-treated patients, the prothrombin time international normalized ratio (PT-INR) increased after starting the co-treatment with ezetimibe42). This result indicates that the ezetimibe-warfarin interaction occurs with high frequency in humans as a non-idiosyncratic reaction via the inhibition of vitamin K absorption. Hashikata et al. conducted further studies on this drug-drug interaction by retrospectively evaluating outpatients at the Kitasato University Hospital45). Interestingly, they revealed that the ezetimibe-warfarin interaction more frequently appeared in patients taking statins. It has been reported that the inhibition of cholesterol synthesis by statins increases intestinal cholesterol absorption via a physiological compensation process to maintain cholesterol homeostasis46). Indeed, Tremblay et al. showed that a 12-week atorvastatin treatment increased both the mRNA and protein levels of NPC1L1 in intestinal biopsy samples obtained from hyperlipidemic patients47). Based on these observations and our finding that NPC1L1 is a major vitamin K1 importer in the intestine, it is likely that statin therapy increases ezetimibe-sensitive NPC1L1-mediated vitamin K1 absorption and, therefore, that the drug interaction between ezetimibe and warfarin is strengthened in patients taking statins.
Class B Scavenger Receptors
Scavenger Receptor Class B Type I
Scavenger receptor class B type I (SR-BI) is a membrane protein and was initially identified as an oxidized low-density lipoprotein (LDL) receptor48), although it is currently known to be a high-density lipoprotein (HDL) receptor expressed on the basolateral membrane in the liver49). Given that SR-BI is expressed in several tissues and its cellular localization varies depending on cell type and cell culture conditions50, 51), SR-BI is believed to have many physiological functions in addition to its well-characterized role in HDL metabolism.
In the small intestine, SR-BI is predominantly expressed on the brush border membrane of enterocytes11, 52). Based on in vitro results demonstrating that the uptake of micellar cholesterol by Caco-2 cells and that by brush border membrane vesicles prepared from rabbit enterocytes were effectively inhibited by SR-BI antibodies, SR-BI was assumed to be a receptor (or an acceptor) for cholesterol uptake52). Consistent with these in vitro results, Bietrix et al. reported that transgenic mice overexpressing SR-BI in the intestine showed significantly higher cholesterol absorption than WT mice, suggesting that SR-BI can take up cholesterol in the small intestine11). However, the physiological importance of SR-BI in cholesterol absorption is controversial because it has been reported that there is no significant difference in cholesterol absorption between WT and SR-BI KO mice10). Taken together with the fact that NPC1L1 mediates more than 70% of the cholesterol absorption in the small intestine9), it is currently thought that SR-BI may be involved to some extent11, 53) but is not physiologically essential for cholesterol absorption.
Similar to NPC1L1, SR-BI has been proposed to be involved in the intestinal absorption of fat-soluble vitamins. Reboul et al. demonstrated that vitamin E uptake in Caco-2 TC-7 cells was inhibited by both SR-BI antibodies and BLT-1 (Fig. 1E), a chemical inhibitor of lipid transport that acts via SR-BI54). Consistent with the in vitro results, the bioavailability of γ-tocopherol is significantly (2.7-fold) higher in SR-BI transgenic mice than in WT mice. These results suggest that SR-BI has vitamin E uptake activity both in vitro and in vivo. Additionally, it has been demonstrated that SR-BI has vitamin K1 uptake activity55). In vitro vitamin K1 uptake studies using Caco-2 TC-7 cells demonstrated that the apical uptake of vitamin K1 was significantly blocked by both anti-SR-BI antibodies and BLT-1. In addition, SR-BI-overexpressing HEK293-T cells exhibited significantly enhanced vitamin K1 uptake, which was sensitive to BLT-1 treatment. Consistent with these in vitro observations, SR-BI transgenic mice were found to have a higher content of vitamin K1 in the proximal intestine mucosa 4 h after gavage with a vitamin K1-enriched emulsion compared with WT mice. Moreover, the postprandial plasma vitamin K1 concentration was also increased in SR-BI transgenic mice compared with WT mice. Together, these results indicate that SR-BI has the ability to import vitamin K1. However, based on the result showing that BLT-1 treatment reduced only about 20% of vitamin K1 uptake by intestinal grafts collected from WT mice55) and the finding that more than 70% of vitamin K1 absorbed in the intestine was through NPC1L1-mediated pathway42), the physiological contribution of SR-BI to vitamin K1 absorption might not be very high. Additional studies with SR-BI KO mice are needed to better understand the physiological role of SR-BI in the absorption of vitamin E and vitamin K1.
Although the significance of SR-BI in intestinal lipid absorption is still controversial, considering that SR-BI has bidirectional transport activity (not only uptake but also efflux) for lipids including cholesterol, vitamin E, and vitamin K154–56), and that its cellular localization is dramatically altered by experimental conditions50, 51), it is possible that the function of SR-BI may be dynamically altered in response to changes in physiological and pathological conditions (Fig. 2). Indeed, SR-BI occasionally expressed on the bile canalicular membrane has been reported to be involved in the biliary secretion of cholesterol57) and α-tocopherol58), rather than the (re)uptake of these lipids from bile. Further studies with animal models under several experimental conditions such as hyperlipidemic and lipid-deficient conditions may provide a new perspective on the physiological functions of SR-BI in the small intestine.
Cluster of Differentiation 36
Cluster of differentiation 36 (CD36), a membrane protein belonging to the scavenger receptor class B family, is expressed in various tissues, including in adipose tissue, the heart, and the small intestine59). To date, several studies have indicated that CD36 binds a number of lipids such as lipoproteins, cholesterol, and long-chain fatty acids and that CD36 can facilitate the cellular uptake of these lipids in vitro60–63).
Given that CD36 is predominantly expressed on the brush border membrane of the proximal intestine64), it has been assumed that CD36 might be involved in the apical uptake of dietary lipids in the small intestine. However, fecal lipid analyses showed that there were no significant differences in the intestinal absorption of fatty acids and cholesterol between WT and CD36 KO mice12, 13). This result suggests that CD36 in the small intestine is unlikely to be involved in the apical uptake of these lipids. However, based on the observation that the protein expression of NPC1L1 in the small intestine was significantly higher in CD36 KO mice compared with WT mice14), it is also possible that CD36 deficiency was compensated by the up-regulation of NPC1L1, therefore leaving overall cholesterol absorption unchanged in CD36 KO mice.
Regarding the intestinal function of CD36, it has been also reported that CD36 KO mice exhibit deficiencies in the formation and secretion of chylomicrons, as evidenced by significantly less apolipoprotein B-48, a protein marker of chylomicrons, and smaller lipoprotein particles secreted into the lymph in CD36 KO mice compared with WT mice13). In accordance with this defect, CD36 KO mice exhibited a significant reduction in cholesterol and fatty acid (triglyceride) secretion into the lymph after a 6-h intraduodenal infusion with a lipid emulsion13). These findings indicate that CD36 in the small intestine is involved in the lymphatic secretion of lipids with chylomicrons. Although cholesterol secretion into the lymph decreased, there was no significant accumulation of cholesterol in the small intestine in CD36 KO mice13). This observation, together with the fact that overall cholesterol absorption was not reduced by CD36 deficiency12, 13), implies that other pathways for cellular cholesterol secretion in the small intestine, such as ATP-binding cassette transporter A1 (ABCA1)-mediated cholesterol efflux into the portal blood with HDL65) (Fig. 2), might be enhanced in CD36 KO mice to compensate for the disrupted lymphatic secretion of cholesterol with chylomicrons.
Similar to SR-BI, CD36 has been reported to have the ability to import vitamin E and vitamin K1. Recent in vitro studies showed that the cellular uptakes of γ-tocopherol and vitamin K1 were significantly increased by CD36 overexpression in HEK293-T cells, and these increases were blocked by treatment with sulfo-N-succinimidyl oleate (SSO) (Fig. 1F), a chemical inhibitor of CD3655, 66). However, inconsistent with these in vitro results, in vivo acute absorption studies demonstrated that the plasma levels of γ-tocopherol and vitamin K1 after force-feeding with an emulsion containing these vitamins did not decrease (but rather increase) in CD36 KO mice compared with WT mice55, 66). In addition, the contents of these vitamins in the intestinal mucosa after the gavage were similar between the two groups of mice. The increase in intestinal NPC1L1 expression14) and the disrupted secretion of chylomicrons in CD36 KO mice13) seem to be key factors to clarify the discrepancy between the in vitro and in vivo observations. Therefore, further studies considering the associations of CD36 with NPC1L1 and/or chylomicrons are needed to clarify the physiological role of CD36 in the absorption of fat-soluble vitamins as well as cholesterol.
ATP-Binding Cassette Transporter A1
ABCA1 is a key player in the biogenesis of HDL, which is responsible for reverse cholesterol transport, a physiological process for eliminating excess cholesterol from peripheral tissues by returning it to the liver67). The loss of ABCA1 function causes Tangier disease, which is characterized by the accumulation of cholesterol (esters) throughout the body due to HDL deficiency, resulting in an increased risk of atherosclerosis and cardiovascular events in patients with Tangier disease3–5). ABCA1 is expressed on the basolateral membrane in several tissues, including in the liver and small intestine, and mediates the ATP-dependent efflux of cellular cholesterol and phospholipids to apolipoprotein A-I (apoA-I), which functions as a lipid acceptor to form nascent HDL67).
Recent studies with ABCA1 KO mice revealed that ABCA1 is involved in the regulation of the plasma and/or tissue levels of fat-soluble vitamins in addition to cholesterol and phospholipids. Indeed, plasma concentrations of vitamin E are extremely low in ABCA1 KO mice (undetectable levels) compared with WT mice (3.4 mg/dL)68). In addition, the hepatic vitamin K1 concentration in ABCA1 KO mice is also reduced to approximately 20% of that in WT mice, which causes a bleeding tendency in ABCA1 KO mice68). These observations raise the possibility that ABCA1 can transport the fat-soluble vitamins as well as cholesterol and phospholipids. Indeed, Oram et al. demonstrated that apoA-I-dependent α-tocopherol efflux was absent in fibroblasts derived from patients with Tangier disease and that ABCA1 overexpression in baby hamster kidney cells (BHK cells) significantly increased α-tocopherol efflux to apoA-I69). Given that the conditioned media collected from ABCA1-overexpressing BHK cells did not promote α-tocopherol efflux from control (empty vector-transfected) BHK cells, the increase in α-tocopherol efflux in ABCA1-overexpressing cells was likely due to the direct transport of α-tocopherol by ABCA1 rather than being secondary to the increase in the amount of HDL particles caused by ABCA1 overexpression69). In addition, it has been reported in vitro that ABCA1 in hepatocyte is involved in the efflux of α-tocopherol to apoA-I, and this efflux is inhibited by probucol (Fig. 1G), which inactivates ABCA1 by inhibiting binding to apoA-I70). Given that the dietary administration of probucol reduced the plasma concentrations of α-tocopherol as well as HDL cholesterol in mice70), ABCA1-mediated α-tocopherol secretion from peripheral tissues (especially from the liver) does occur in vivo. These data, together with the recent finding that vitamin E can be exported to not only apoA-I (nascent HDL) but also more mature HDL via the ATP-binding cassette transporter G1-dependent pathway71), indicate that transporter-mediated vitamin E efflux to HDL particles plays important roles in the whole-body regulation of vitamin E.
In addition to hepatic α-tocopherol secretion, the intestinal absorption of dietary vitamin E can influence the plasma concentrations of this nutrient. It has been reported that the intestinal absorption of vitamin E (γ-tocopherol) was significantly lower in ABCA1 KO mice than in WT mice72). Taken together with the fact that ABCA1 is expressed on the basolateral membrane in the small intestine73), these results suggest that ABCA1 is responsible for the secretion of vitamin E into portal blood with intestinal HDL, and this pathway, as well as the well-known pathway of vitamin E secretion into lymph with chylomicrons, is significantly involved in vitamin E absorption74) (Fig. 2).
As for vitamin K, there is no direct evidence whether ABCA1 mediates vitamin K transport. In vitro studies will be needed to reveal whether ABCA1 has vitamin K efflux activity. In addition, to reveal the mechanism(s) behind the observed reduction in the hepatic vitamin K1 concentration in ABCA1 KO mice68), further studies to analyze vitamin K1 concentrations in other tissues and the efficiency of intestinal vitamin K1 absorption in ABCA1 KO mice are necessary and important.
Conclusions and Future Perspectives
In this review, membrane proteins that have the potential to transport dietary lipids such as cholesterol, vitamin E, and vitamin K1 were discussed. Intestinal lipid absorption is regulated by multiple processes, including apical uptake, apical backflux, basolateral efflux with HDL, and lymphatic secretion with chylomicrons (Fig. 2). Interestingly, despite the differences in chemical structure among cholesterol, vitamin E, and vitamin K1, the membrane transport processes of these lipids are very similar. Indeed, as descried in this review, most of the intestinal cholesterol transporters also have the ability to transport vitamin E and vitamin K1. Vitamin E acts as an antioxidant, potentially preventing the occurrence of cardiovascular events2). Vitamin K inhibits the development of arteriosclerosis by activating the matrix Gla protein, which suppresses vascular calcification1, 75). Based on this data, it seems reasonable that cholesterol transporters are also involved in the intestinal absorption of these vitamins in order to prevent the harmful effects of excess cholesterol on the body. In addition, these findings provide new insights into the development and/or the clinical use of drugs for controlling plasma cholesterol levels. To date, several types of drugs whose molecular targets are cholesterol transporters have been developed or are under development for the treatment of dyslipidemia76, 77). It is important to consider that treatment with these drugs may also affect the behavior of fat-soluble nutrients such as vitamin E and vitamin K, which may cause unexpected adverse effects or unpredictable drug-drug interactions. Further analyses of the association between the intestinal absorption of cholesterol and that of fat-soluble nutrients are necessary for the development of safer drugs and for appropriate pharmacotherapy.
Discrepancies between in vitro and in vivo data have been frequently observed in studies on intestinal lipid transporters, and such discrepancies have made it difficult to uncover the physiological functions of these proteins. One of the main reasons for these discrepancies is that compensation systems are working in vivo to maintain lipid homeostasis. In order to understand the complicated but well-balanced systems for intestinal lipid absorption, it is important to clarify not only the individual functions (abilities) of lipid transporters but also the associations among the systems' components. From this point of view, further studies using in silico methods such as a systems biological approach78) will be helpful to elucidate the tissue-level networks of lipid transporters and related molecules and will contribute to a better understanding of the molecular mechanisms involved in the regulation of intestinal lipid absorption.
Acknowledgement
This work was supported by Grants-in-Aid for Challenging Exploratory Research (15K14996 to YY and 16K15155 to TT) and a Grant-in-Aid for Young Scientists (A) (16H06219 to YY) from the Japan Society for the Promotion of Science and a Grant-in-Aid for Scientific Research on Innovative Areas HD-physiology (22136015) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Conflict of Interest
YY has received GSK Japan Reserch Grant and a grant from the Takeda Science Foundation. TT has received grants from the LOTTE Foundation, and Kobayashi International Scholarship Foundation. HS has received grants from Astellas Research Support, and Pfizer Academic Contributions.
References
- 1). Shea MK, Holden RM: Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr, 2012; 3: 158-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Bielli A, Scioli MG, Mazzaglia D, Doldo E, Orlandi A: Antioxidants and vascular health. Life Sci, 2015; 143: 209-216 [DOI] [PubMed] [Google Scholar]
- 3). Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G: The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet, 1999; 22: 347-351 [DOI] [PubMed] [Google Scholar]
- 4). Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr., Hayden MR: Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet, 1999; 22: 336-345 [DOI] [PubMed] [Google Scholar]
- 5). Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G: Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet, 1999; 22: 352-355 [DOI] [PubMed] [Google Scholar]
- 6). Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH: Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science, 2000; 290: 1771-1775 [DOI] [PubMed] [Google Scholar]
- 7). Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, Grundy SM, Hobbs HH: Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc Natl Acad Sci U S A, 2006; 103: 1810-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Sehayek E: Genetic regulation of cholesterol absorption and plasma plant sterol levels: commonalities and differences. J Lipid Res, 2003; 44: 2030-2038 [DOI] [PubMed] [Google Scholar]
- 9). Altmann SW, Davis HR, Jr., Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP: Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science, 2004; 303: 1201-1204 [DOI] [PubMed] [Google Scholar]
- 10). Mardones P, Quinones V, Amigo L, Moreno M, Miquel JF, Schwarz M, Miettinen HE, Trigatti B, Krieger M, VanPatten S, Cohen DE, Rigotti A: Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J Lipid Res, 2001; 42: 170-180 [PubMed] [Google Scholar]
- 11). Bietrix F, Yan D, Nauze M, Rolland C, Bertrand-Michel J, Comera C, Schaak S, Barbaras R, Groen AK, Perret B, Terce F, Collet X: Accelerated lipid absorption in mice overexpressing intestinal SR-BI. J Biol Chem, 2006; 281: 7214-7219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Nguyen DV, Drover VA, Knopfel M, Dhanasekaran P, Hauser H, Phillips MC: Influence of class B scavenger receptors on cholesterol flux across the brush border membrane and intestinal absorption. J Lipid Res, 2009; 50: 2235-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P: CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology, 2006; 131: 1197-1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Nassir F, Wilson B, Han X, Gross RW, Abumrad NA: CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem, 2007; 282: 19493-19501 [DOI] [PubMed] [Google Scholar]
- 15). Knopp RH, Gitter H, Truitt T, Bays H, Manion CV, Lipka LJ, LeBeaut AP, Suresh R, Yang B, Veltri EP, Ezetimibe Study G : Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J, 2003; 24: 729-741 [DOI] [PubMed] [Google Scholar]
- 16). Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM, Investigators I-I : Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med, 2015; 372: 2387-2397 [DOI] [PubMed] [Google Scholar]
- 17). Davies JP, Levy B, Ioannou YA: Evidence for a Niemann-pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics, 2000; 65: 137-145 [DOI] [PubMed] [Google Scholar]
- 18). Liscum L, Sturley SL: Intracellular trafficking of Niemann-Pick C proteins 1 and 2: obligate components of subcellular lipid transport. Biochim Biophys Acta, 2004; 1685: 22-27 [DOI] [PubMed] [Google Scholar]
- 19). Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ: Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science, 1997; 277: 232-235 [DOI] [PubMed] [Google Scholar]
- 20). Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O'Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA: Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science, 1997; 277: 228-231 [DOI] [PubMed] [Google Scholar]
- 21). Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr., Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O'Neill K A, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA: The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci U S A, 2005; 102: 8132-8137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Weinglass AB, Kohler M, Schulte U, Liu J, Nketiah EO, Thomas A, Schmalhofer W, Williams B, Bildl W, McMasters DR, Dai K, Beers L, McCann ME, Kaczorowski GJ, Garcia ML: Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc Natl Acad Sci U S A, 2008; 105: 11140-11145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Yamanashi Y, Takada T, Suzuki H: Niemann-Pick C1-like 1 overexpression facilitates ezetimibe-sensitive cholesterol and beta-sitosterol uptake in CaCo-2 cells. J Pharmacol Exp Ther, 2007; 320: 559-564 [DOI] [PubMed] [Google Scholar]
- 24). Woollett LA, Wang Y, Buckley DD, Yao L, Chin S, Granholm N, Jones PJ, Setchell KD, Tso P, Heubi JE: Micellar solubilisation of cholesterol is essential for absorption in humans. Gut, 2006; 55: 197-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Cohn JS, Kamili A, Wat E, Chung RW, Tandy S: Dietary phospholipids and intestinal cholesterol absorption. Nutrients, 2010; 2: 116-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Davis HR, Jr., Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW: Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem, 2004; 279: 33586-33592 [DOI] [PubMed] [Google Scholar]
- 27). von Bergmann K, Sudhop T, Lutjohann D: Cholesterol and plant sterol absorption: recent insights. Am J Cardiol, 2005; 96: 10D-14D [DOI] [PubMed] [Google Scholar]
- 28). Narushima K, Takada T, Yamanashi Y, Suzuki H: Niemann-pick C1-like 1 mediates alpha-tocopherol transport. Mol Pharmacol, 2008; 74: 42-49 [DOI] [PubMed] [Google Scholar]
- 29). Yamanashi Y, Takada T, Suzuki H: In-vitro characterization of the six clustered variants of NPC1L1 observed in cholesterol low absorbers. Pharmacogenet Genomics, 2009; 19: 884-892 [DOI] [PubMed] [Google Scholar]
- 30). Yamamoto T, Ito K, Honma M, Takada T, Suzuki H: Cholesterol-lowering effect of ezetimibe in uridine diphosphate glucuronosyltransferase 1A-deficient (Gunn) rats. Drug Metab Dispos, 2007; 35: 1455-1458 [DOI] [PubMed] [Google Scholar]
- 31). Takada T, Suzuki H: Molecular mechanisms of membrane transport of vitamin E. Mol Nutr Food Res, 2010; 54: 616-622 [DOI] [PubMed] [Google Scholar]
- 32). Abuasal BS, Sylvester PW, Kaddoumi A: Intestinal absorption of gamma-tocotrienol is mediated by Niemann-Pick C1-like 1: in situ rat intestinal perfusion studies. Drug Metab Dispos, 2010; 38: 939-945 [DOI] [PubMed] [Google Scholar]
- 33). Abuasal BS, Qosa H, Sylvester PW, Kaddoumi A: Comparison of the intestinal absorption and bioavailability of gamma-tocotrienol and alpha-tocopherol: in vitro, in situ and in vivo studies. Biopharm Drug Dispos, 2012; 33: 246-256 [DOI] [PubMed] [Google Scholar]
- 34). Kaempf-Rotzoll DE, Traber MG, Arai H: Vitamin E and transfer proteins. Curr Opin Lipidol, 2003; 14: 249-254 [DOI] [PubMed] [Google Scholar]
- 35). Kono N, Ohto U, Hiramatsu T, Urabe M, Uchida Y, Satow Y, Arai H: Impaired alpha-TTP-PIPs interaction underlies familial vitamin E deficiency. Science, 2013; 340: 1106-1110 [DOI] [PubMed] [Google Scholar]
- 36). Alexander B, Goldstein R, Landwehr G, Cook CD: Congenital SPCA deficiency: a hitherto unrecognized coagulation defect with hemorrhage rectified by serum and serum fractions. J Clin Invest, 1951; 30: 596-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Hougie C, Barrow EM, Graham JB: Stuart clotting defect. I. Segregation of an hereditary hemorrhagic state from the heterogeneous group heretofore called stable factor (SPCA, proconvertin, factor VII) deficiency. J Clin Invest, 1957; 36: 485-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Booth SL, Suttie JW: Dietary intake and adequacy of vitamin K. J Nutr, 1998; 128: 785-788 [DOI] [PubMed] [Google Scholar]
- 39). Shearer MJ, McBurney A, Barkhan P: Studies on the absorption and metabolism of phylloquinone (vitamin K1) in man. Vitam Horm, 1974; 32: 513-542 [DOI] [PubMed] [Google Scholar]
- 40). Hollander D, Rim E, Muralidhara KS: Vitamin K1 intestinal absorption in vivo: influence of luminal contents on transport. Am J Physiol, 1977; 232: E69-74 [DOI] [PubMed] [Google Scholar]
- 41). Merck/Schering-Plough Pharmaceutics: Zetia (ezetimibe) tablets prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021445s014lbl.pdf
- 42). Takada T, Yamanashi Y, Konishi K, Yamamoto T, Toyoda Y, Masuo Y, Yamamoto H, Suzuki H: NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy. Sci Transl Med, 2015; 7: 275ra223. [DOI] [PubMed] [Google Scholar]
- 43). Ritchie SR, Orr DW, Black PN: Severe jaundice following treatment with ezetimibe. Eur J Gastroenterol Hepatol, 2008; 20: 572-573 [DOI] [PubMed] [Google Scholar]
- 44). Oldenburg J, Marinova M, Muller-Reible C, Watzka M: The vitamin K cycle. Vitam Horm, 2008; 78: 35-62 [DOI] [PubMed] [Google Scholar]
- 45). Hashikata T, Yamaoka-Tojo M, Kakizaki R, Nemoto T, Fujiyoshi K, Namba S, Kitasato L, Hashimoto T, Ishii S, Kameda R, Shimohama T, Tojo T, Ako J: Ezetimibe enhances and stabilizes anticoagulant effect of warfarin. Heart Vessels, 2016; [DOI] [PubMed] [Google Scholar]
- 46). Miettinen TA, Gylling H: Cholesterol absorption efficiency and sterol metabolism in obesity. Atherosclerosis, 2000; 153: 241-248 [DOI] [PubMed] [Google Scholar]
- 47). Tremblay AJ, Lamarche B, Lemelin V, Hoos L, Benjannet S, Seidah NG, Davis HR, Jr., Couture P: Atorvastatin increases intestinal expression of NPC1L1 in hyperlipidemic men. J Lipid Res, 2011; 52: 558-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Acton SL, Scherer PE, Lodish HF, Krieger M: Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem, 1994; 269: 21003-21009 [PubMed] [Google Scholar]
- 49). Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M: Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science, 1996; 271: 518-520 [DOI] [PubMed] [Google Scholar]
- 50). Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M: Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature, 1997; 387: 414-417 [DOI] [PubMed] [Google Scholar]
- 51). Cai SF, Kirby RJ, Howles PN, Hui DY: Differentiation-dependent expression and localization of the class B type I scavenger receptor in intestine. J Lipid Res, 2001; 42: 902-909 [PubMed] [Google Scholar]
- 52). Hauser H, Dyer JH, Nandy A, Vega MA, Werder M, Bieliauskaite E, Weber FE, Compassi S, Gemperli A, Boffelli D, Wehrli E, Schulthess G, Phillips MC: Identification of a receptor mediating absorption of dietary cholesterol in the intestine. Biochemistry, 1998; 37: 17843-17850 [DOI] [PubMed] [Google Scholar]
- 53). Reboul E, Soayfane Z, Goncalves A, Cantiello M, Bott R, Nauze M, Terce F, Collet X, Comera C: Respective contributions of intestinal Niemann-Pick C1-like 1 and scavenger receptor class B type I to cholesterol and tocopherol uptake: in vivo v. in vitro studies. Br J Nutr, 2012; 107: 1296-1304 [DOI] [PubMed] [Google Scholar]
- 54). Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, Margotat A, Lagrost L, Collet X, Borel P: Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J Biol Chem, 2006; 281: 4739-4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Goncalves A, Margier M, Roi S, Collet X, Niot I, Goupy P, Caris-Veyrat C, Reboul E: Intestinal scavenger receptors are involved in vitamin K1 absorption. J Biol Chem, 2014; 289: 30743-30752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Sehayek E, Ono JG, Shefer S, Nguyen LB, Wang N, Batta AK, Salen G, Smith JD, Tall AR, Breslow JL: Biliary cholesterol excretion: a novel mechanism that regulates dietary cholesterol absorption. Proc Natl Acad Sci U S A, 1998; 95: 10194-10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Wiersma H, Gatti A, Nijstad N, Oude Elferink RP, Kuipers F, Tietge UJ: Scavenger receptor class B type I mediates biliary cholesterol secretion independent of ATP-binding cassette transporter g5/g8 in mice. Hepatology, 2009; 50: 1263-1272 [DOI] [PubMed] [Google Scholar]
- 58). Mardones P, Strobel P, Miranda S, Leighton F, Quinones V, Amigo L, Rozowski J, Krieger M, Rigotti A: Alphatocopherol metabolism is abnormal in scavenger receptor class B type I (SR-BI)-deficient mice. J Nutr, 2002; 132: 443-449 [DOI] [PubMed] [Google Scholar]
- 59). Febbraio M, Hajjar DP, Silverstein RL: CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest, 2001; 108: 785-791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA: CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem, 1993; 268: 11811-11816 [PubMed] [Google Scholar]
- 61). Calvo D, Gomez-Coronado D, Suarez Y, Lasuncion MA, Vega MA: Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J Lipid Res, 1998; 39: 777-788 [PubMed] [Google Scholar]
- 62). Werder M, Han CH, Wehrli E, Bimmler D, Schulthess G, Hauser H: Role of scavenger receptors SR-BI and CD36 in selective sterol uptake in the small intestine. Biochemistry, 2001; 40: 11643-11650 [DOI] [PubMed] [Google Scholar]
- 63). Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA: Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem, 1993; 268: 17665-17668 [PubMed] [Google Scholar]
- 64). Poirier H, Degrace P, Niot I, Bernard A, Besnard P: Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP). Eur J Biochem, 1996; 238: 368-373 [DOI] [PubMed] [Google Scholar]
- 65). Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR: Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest, 2006; 116: 1052-1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Goncalves A, Roi S, Nowicki M, Niot I, Reboul E: Cluster-determinant 36 (CD36) impacts on vitamin E postprandial response. Mol Nutr Food Res, 2014; 58: 2297-2306 [DOI] [PubMed] [Google Scholar]
- 67). Nagao K, Tomioka M, Ueda K: Function and regulation of ABCA1--membrane meso-domain organization and reorganization. FEBS J, 2011; 278: 3190-3203 [DOI] [PubMed] [Google Scholar]
- 68). Orso E, Broccardo C, Kaminski WE, Bottcher A, Liebisch G, Drobnik W, Gotz A, Chambenoit O, Diederich W, Langmann T, Spruss T, Luciani MF, Rothe G, Lackner KJ, Chimini G, Schmitz G: Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat Genet, 2000; 24: 192-196 [DOI] [PubMed] [Google Scholar]
- 69). Oram JF, Vaughan AM, Stocker R: ATP-binding cassette transporter A1 mediates cellular secretion of alpha-tocopherol. J Biol Chem, 2001; 276: 39898-39902 [DOI] [PubMed] [Google Scholar]
- 70). Shichiri M, Takanezawa Y, Rotzoll DE, Yoshida Y, Kokubu T, Ueda K, Tamai H, Arai H: ATP-binding cassette transporter A1 is involved in hepatic alphatocopherol secretion. J Nutr Biochem, 2010; 21: 451-456 [DOI] [PubMed] [Google Scholar]
- 71). Olivier M, Bott GR, Frisdal E, Nowick M, Plengpanich W, Desmarchelier C, Roi S, Quinn CM, Gelissen I, Jessup W, Van Eck M, Guerin M, Le Goff W, Reboul E: ABCG1 is involved in vitamin E efflux. Biochim Biophys Acta, 2014; 1841: 1741-1751 [DOI] [PubMed] [Google Scholar]
- 72). Reboul E, Trompier D, Moussa M, Klein A, Landrier JF, Chimini G, Borel P: ATP-binding cassette transporter A1 is significantly involved in the intestinal absorption of alpha- and gamma-tocopherol but not in that of retinyl palmitate in mice. Am J Clin Nutr, 2009; 89: 177-184 [DOI] [PubMed] [Google Scholar]
- 73). Mulligan JD, Flowers MT, Tebon A, Bitgood JJ, Wellington C, Hayden MR, Attie AD: ABCA1 is essential for efficient basolateral cholesterol efflux during the absorp tion of dietary cholesterol in chickens. J Biol Chem, 2003; 278: 13356-13366 [DOI] [PubMed] [Google Scholar]
- 74). Hussain MM: Intestinal lipid absorption and lipoprotein formation. Curr Opin Lipidol, 2014; 25: 200-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75). Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G: Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature, 1997; 386: 78-81 [DOI] [PubMed] [Google Scholar]
- 76). Tomkin GH, Owens D: Investigational therapies for the treatment of atherosclerosis. Expert Opin Investig Drugs, 2014; 23: 1411-1421 [DOI] [PubMed] [Google Scholar]
- 77). Ajufo E, Rader DJ: Recent advances in the pharmacological management of hypercholesterolaemia. Lancet Diabetes Endocrinol, 2016; 4: 436-446 [DOI] [PubMed] [Google Scholar]
- 78). Kariya Y, Honma M, Suzuki H: Systems-based understanding of pharmacological responses with combinations of multidisciplinary methodologies. Biopharm Drug Dispos, 2013; 34: 489-507 [DOI] [PubMed] [Google Scholar]


