Abstract
Carotid artery stenosis is responsible for between 10–20% of all ischaemic strokes. Interventions, such as carotid end-arterectomy and carotid stenting, effectively reduce the risk of stroke in selected individuals. This review describes the history of carotid interventions, and summarises reliable evidence on the safety and efficacy of these interventions gained from large randomised clinical trials.
Early trials comparing carotid endarterectomy to medical therapy alone in symptomatic patients, and asymptomatic patients, demonstrated that endarterectomy halved the risk of stroke and perioperative death in these two unique populations. The absolute risk reduction was smaller in the asymptomatic carotid trials, consistent with their lower absolute stroke risk. More recent trials in symptomatic patients, suggest that carotid stenting has similar long term durability to carotid endarterectomy, but possibly has higher procedural hazards dominated by non-disabling strokes. The Asymptomatic Carotid Surgery Trial-2, along with individual patient data meta-analysis of all asymptomatic trials, will provide reliable evidence for the choice of intervention in asymptomatic patients in whom a decision has been made for carotid revascularisation. Given improvements in effective cardiovascular medical therapy, in particular lipid-lowering medications, there is renewed uncertainty as to whether carotid interventions still provide meaningful net reductions in stroke risk in asymptomatic populations. Four large trials in Europe and the US are currently underway, and are expected to report longterm results in the next decade.
It is essential that surgeons, interventionalists, and physicians continue to randomise large numbers of patients from around the world to clarify current uncertainty around the management of asymptomatic carotid stenosis.
Keywords: Carotid artery stenosis, Stroke prevention, Interventions
Introduction
Carotid artery stenosis is an atheromatous narrowing of the common carotid artery or internal carotid artery and is responsible for 10–20% of all strokes.1). Rupture of unstable carotid plaque may precipitate thrombus formation, resulting in embolization and cerebral infarction.2, 3). The risk of stroke among affected people is determined by their history of focal neurological symptoms, their absolute cardiovascular risk, and use of effective cardiovascular medical therapy.4, 5). In the large international REACH registry of outpatients with established cardiovascular disease (or ≥ 3 traditional risk factors), individuals with asymptomatic carotid stenosis had a 1-year absolute stroke risk of over 3%, and a > 50% relative risk of stroke compared to those without carotid stenosis.6). Those with symptomatic disease, defined by an ipsilateral neurological event within the last 6 months thought to be related to the carotid artery, have a higher absolute stroke risk, whereas those with asymptomatic disease have a lower, yet still clinically important absolute stroke risk. Patients with asymptomatic carotid stenosis can be further classified into two distinct subgroups according to their absolute stroke risk: patients with prior symptoms (previous contralateral neurological symptoms, or ipsilateral symptoms > 6 months ago) or evidence of silent brain infarction on cross-sectional imaging; and individuals with no clinical or imaging evidence of previous neurological events (i.e. truly asymptomatic).5).
Carotid artery interventions, such as carotid end-arterectomy (CEA) and, more recently carotid artery stenting (CAS), have been used for over half a century to reduce the risk of stroke in individuals with carotid stenosis. Since the initial development of these interventions, there has been considerable debate around whether CEA, CAS, or medical therapy alone is best in patients with symptomatic and asymptomatic carotid stenosis. This review summarises the historical development of carotid interventions, evidence gained from large randomised clinical trials, and current guidelines and future directions for the interventional management of carotid stenosis.
History of Carotid Interventions
Carotid stenosis was first recognised as a cause of stroke in the early twentieth century by Hans Chiari and James Ramsay Hunt. In 1905, Chiari discovered carotid artery thrombosis on several post-mortem examinations, and hypothesised that emboli could dislodge and cause apoplexy.7). Hunt published a report in 1914 detailing that four out of 20 patients presenting with hemiplegia had absent or diminished carotid pulsations on examination, suggesting that the two pathologies were linked.8). Despite these findings, the clinical importance of carotid atherosclerosis was largely discounted for the next 40 years, until 1951 when Miller Fisher published his landmark case series describing eight patients with carotid artery occlusions and associated hemiplegia.9). Fisher investigated his patients extensively, either through invasive angiography or post-mortem examination, and to his surprise found no intracranial arterial lesions to explain the neurological events. He concluded that, “Hemiplegia of unknown cause in persons in the younger age group is often due to disease of the internal carotid artery”. Fisher went further to suggest that “It is even conceivable that someday vascular surgery will find a way to by-pass the occluded portion of the artery during the period of ominous fleeting symptoms”.
Reconstructive carotid surgery was pioneered by three groups across three different continents. The first successful carotid reconstruction was performed by Carrea, Molins and Murphy in October 1951.10). Carrea operated on a man presenting with acute hemiplegia. He excised the diseased carotid artery segment and performed an end-to-end anastomosis of the internal carotid artery and common carotid artery, with the patient regaining almost all of their function.10). The operation that attracted international acclaim however was that performed by Eastcott, Pickering and Rob in May 1954.11). Eastcott and Rob operated on a 66-year-old woman who had suffered daily transient ischaemic attacks (TIAs) (33 episodes in the preceding months). They performed a similar carotid resection and end-to-end anastomosis with the patient under total-body-immersion hypothermia. The procedure was showcased in front of an international audience of cardiovascular surgeons, and was deemed highly successful, with the patient surviving and having no symptoms for over a decade.11). The first conventional CEA was claimed by Dr Michael DeBakey in 1953, but remained unpublished for another 12 years.12, 13). DeBakey similarly operated on a patient with recurrent TIAs, performing a vertical arteriotomy and removal of endoluminal atherothrombotic material. DeBakey's patient also demonstrated a remarkable recovery, experiencing no further TIAs or strokes for the remaining 19 years of his life.13).
Over the following three decades, several further advances were made in the field of carotid surgery, including the use of temporary intra-operative shunts by Denton Cooley in 1956, patch closure by DeBakey, Crawford and Cooley in 1959, eversion endarterectomy by Etheredge in 1970, and intraoperative electroencephalogram monitoring by Callow in 1980.14–17). Early developments were also made on endovascular devices. Morris, Lechter and Debakey performed an open carotid angioplasty in 1968, and Mathias demonstrated percutaneous carotid angioplasty in 1977.18, 19). The use of carotid stents was not reported until 1994, when Marks et al. deployed Palmaz metal stents in two patients with carotid artery dissections and stenosis.20). This series of landmark studies generated considerable interest around endarterectomy and stenting as a means to treat carotid stenosis. However large-scale randomised evidence was needed to confirm long-term benefit and justify widespread implementation.
Carotid Intervention Trials
The efficacy and peri-procedural risks of carotid interventions have been established over four decades of randomised clinical trials. In the 1980s and 1990s, CEA was compared to medical therapy alone, first in symptomatic patients and subsequently in asymptomatic patients. Then in the 2000s and 2010s, CEA was compared to CAS in symptomatic and asymptomatic patients. We have now turned full circle, with several current clinical trials reassessing the benefits and risks of carotid interventions compared to contemporary medical therapy alone in symptomatic and asymptomatic patients (Fig. 1). While observational studies provide insight into the absolute stroke risk and risk factors for individuals with carotid stenosis, they have limited value in assessing the efficacy and safety of carotid interventions. The inherent bias of observational studies, i.e. through confounding, reverse causation, recall and detection bias, are large in magnitude, and may outweigh the moderate, yet clinically important effects of carotid interventions. Randomised clinical trials on the other hand guarantee strict control of bias, and minimise the influence of random error when adequately powered. Accordingly, any recommendations or guidelines on the use of carotid interventions should be based on reliable evidence from large, well-designed randomised trials, as well as meta-analyses of randomised studies.
Fig. 1.
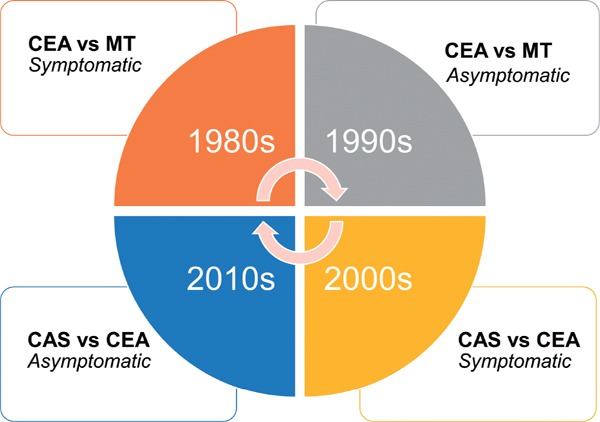
The cycle of large-scale carotid intervention trials.
The long-term efficacy and procedural risks of carotid interventions have been assessed over four decades of large, randomised clinical trials. With improvements in best medical therapy, carotid surgery is again being compared to best medical therapy alone in people with symptomatic and asymptomatic carotid stenosis. CEA, carotid endarterectomy; MT, medical therapy; CAS, carotid artery stenting.
CEA vs Medical Therapy Alone for Symptomatic Carotid Stenosis
Three randomised clinical trials in the 1980s compared CEA to medical therapy alone in patients with symptomatic carotid stenosis. These included the North American Symptomatic Carotid Endarterectomy Trial (NASCET), the European Carotid Surgery Trial (ECST), and the smaller Veterans Affairs 309 (VA309) trial. In NASCET and ECST, men and women with minor stenoses below 50% were included, whereas in VA309 only men with > 50% stenosis were included.21–25). All three trials demonstrated that CEA substantially reduced the risk of stroke in patients with stenosis ≥ 70%. The long-term benefits were partially offset by the procedural risks of surgery, which ranged from 4.5% to 7.0% in the surgical groups compared to 2.4% to 6.1% in the medical therapy groups. In the NASCET trial, a differential effect was demonstrated for CEA according to the degree of stenosis. For participants with ≥ 70% stenosis, 50–69% stenosis and < 50% stenosis, the absolute risk reductions in ipsilateral stroke from carotid surgery were > 15% (p < 0.001), 6.5% (p = 0.045), and 3.8% (p = 0.16) respectively.22). Similarly, ECST demonstrated that patients with severe carotid stenosis ≥ 80% had significantly lower rates of stroke after CEA, however angiographic assessment methods differed from NASCET. On average, a 70% stenosis measured by NASCET criteria was equivalent to 82% stenosis with ECST criteria.25, 26). After three years of follow-up, the rates of ipsilateral stroke and perioperative death were 6.8% in the surgical group vs 20.6% in the medical group (p < 0.001; Table 1).21). No benefits were seen in participants with minor or moderate carotid stenosis. The smaller VA309 trial reported a large reduction in ipsilateral stroke and crescendo TIA in participants randomised to CEA (7.7% vs 19.4%, p = 0.028), which was greatest in participants with carotid stenosis > 70% (7.9% vs 25.6%, p = 0.010).23).
Table 1. Randomised clinical trials comparing carotid endarterectomy to medical therapy alone in patients with carotid artery stenosis.
| Trial | Recruitment | n | Follow-up | Procedural Hazards |
Long-term Stroke Rate |
p-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Definition | CEA | MT | Definition | CEA | MT | |||||
| Symptomatic | ||||||||||
| VA309 | 1988–1991 | 189 | Mean 1.0 year | 30d crescendo TIA, stroke & death | 6.6% | 6.1% | Ipsilateral stroke, crescendo TIA or perioperative death | 7.7% | 19.4% | 0.028 |
| ECST-1 (≥ 80%) | 1981–1994 | 574 (of 3024) | Mean 6.1 years | 30d major stroke & death | 4.5% | 0% | Ipsilateral stroke or perioperative death | 6.8% | 20.6% | < 0.0001 |
| NASCET (≥ 70%) | 1987–1991 | 659 | Mean 1.5 years | 30d stroke & death | 5.8% | 3.3% | Ipsilateral stroke | 9.0% | 26.0% | < 0.001 |
| NASCET (50–69%) | 1987–1996 | 858 | 5 years | 30d stroke & death* | 6.7% | 2.4% | Ipsilateral stroke | 15.7% | 22.2% | 0.045 |
| Asymptomatic | ||||||||||
| VA trial | 1983–1987 | 444 | Mean 4.0 years | 30d permanent stroke & death | 4.7% | 1.3% | Ipsilateral TIA, transient monocular blindness, stroke | 8.0% | 20.6% | < 0.001 |
| ACAS | 1987–1993 | 1662 | Median 2.7 years | 30d stroke & death | 2.3% | 0.4% | Periprocedural stroke or death, and post-operative ipsilateral stroke | 5.1% | 11.0% | 0.004 |
| ACST-1 | 1993–2003 | 3120 | Median 6.1 years | 30d stroke & death | 2.6% | 0.7% | Any stroke or perioperative death | 5y: 6.9% 10y: 13.4% |
5y: 10.9% 10y: 17.9% |
5y: 0.0001 10y: 0.009 |
CEA, carotid endarterectomy; MT, medical therapy; TIA, transient ischaemic attack; NASCET, north american symptomatic carotid endarterectomy trial; ECST, european carotid surgery trial; VA, veterans affairs; ACST, asymptomatic carotid surgery trial; ACAS, asymptomatic carotid atherosclerosis study.
Procedural hazards include an extra 250 patients with < 50% stenosis.
A pooled analysis of individual patient data from NASCET, ECST, and VA309 was conducted by Rothwell et al. to identify the optimal cut-off for performing CEA in patients with symptomatic carotid stenosis (combined sample = 6092 participants).27). These analyses demonstrated that CEA was beneficial in patients with stenosis ≥ 70% (absolute risk reduction 16.0%, p < 0.001) and 50–69% (absolute risk reduction 4.6%, p = 0.04). Intervention was not effective in those with 30–49% stenosis, and was associated with higher risk of stroke in individuals with stenosis < 30% (p = 0.05).27). In addition, Rothwell et al. demonstrated that risk reduction was greatest if surgery was performed early, and recommended that CEA should ideally be performed within 2 weeks of the onset of neurological symptoms.28). Collectively, these trials and accompanying meta-analyses provide high level evidence to justify widespread and expeditious use of CEA in patients with symptomatic carotid stenosis.
CEA vs Medical Therapy Alone for Asymptomatic Carotid Stenosis
Individuals with asymptomatic carotid stenosis have a lower absolute stroke risk. Therefore, any potential absolute risk reduction from carotid intervention is likely to be moderate, but still potentially worth-while. Importantly, this population is defined as individuals with carotid stenosis who have not had focal ipsilateral neurological symptoms within a set time period (usually 6 months), but a considerable proportion of such patients will have had prior neurological events or evidence of silent brain infarcts on cross-sectional imaging. Three randomised clinical trials conducted in the 1990s investigated whether CEA could reduce the risk of stroke in patients with asymptomatic carotid stenosis; namely the Veterans Affairs (VA) Cooperative Study Group, the Asymptomatic Carotid Atherosclerosis Study (ACAS), and the Asymptomatic Carotid Surgery Trial-1 (ACST-1).29–32). These trials predominantly recruited participants with a stenosis ≥ 50%, although ACST-1 had no fixed minimum cut-off. Peri-procedural event rates in the surgical group were much lower than those reported in the symptomatic trials, ranging from 2.3% to 4.7% in patients receiving surgery (Table 1).
The VA Cooperative Study Group reported reductions in their composite endpoint of neurological events (TIA, amaurosis fugax and stroke) in 444 participants who were followed-up for a mean of 4 years. Event rates were 8.0% vs 20.6% in participants randomised to surgery and medical therapy, respectively. However when only stroke events were considered, the difference was no longer statistically significant (8.6% vs 12.4%), possibly explained by the small sample size and low event rates (47 strokes).29). In the ACAS trial of 1662 participants, CEA halved the risk of ipsilateral stroke over a median follow-up of 2.7 years (5.1% CEA vs 11.0% medical therapy; p = 0.004), despite five CEA patients suffering a stroke during preoperative arteriography.30). ACST-1 reported similar absolute and relative reductions in stroke risk. After 5-years follow-up, the risks of stroke in patients allocated surgery and medical therapy were 6.9% and 10.9%, respectively (p = 0.0001), and after 10-years follow-up the risks were 13.4% and 17.9%, respectively (p = 0.009).31, 32). Contrary to the symptomatic carotid surgery trials, no associations were observed between the degree of carotid stenosis and efficacy of CEA in either ACAS (on arteriography) or ACST-1 (on carotid duplex). Importantly, ACST-1 confirmed that CEA was equally effective among males and females, with both subgroups demonstrating similar 10-year absolute risk reductions in any stroke or periprocedural death (female = 5.8%, p = 0.05; males = 5.5%, p = 0.02). One unexpected finding from the ACST-1 subgroup analyses was a reduction in contralateral strokes among patients allocated CEA (rate ratio =0.61 95% confidence interval [CI] 0.37–1.02). Traditionally, it was believed that CEA only reduced the risk of ipsilateral ischaemic strokes, however these findings suggest that CEA may potentially also reduce the risk of contralateral stroke through changes in cerebral blood flow via the Circle of Willis.
A number of limitations have been raised concerning the generalisability of the asymptomatic carotid surgery clinical trials for contemporary real world practice. First, surgeons were screened for low complication rates before they were permitted to recruit patients.33). In ACAS, almost 30% of surgeons who applied to operate in the trial were not allowed to join the trial based on their previous carotid surgery record, however in ACST-1 very few surgeons failed such credentialing.31, 34, 35). Second, there have been major improvements in cardiovascular risk control over time, and annual stroke rates in individuals with asymptomatic carotid stenosis now appear to be lower than in trial reports.36). In ACST-1, less than 10% of participants were taking lipid lowering therapy at baseline, but by the end of follow-up over 80% reported taking lipid lowering therapy.32). It has been suggested that increased uptake of triple medical therapy (i.e. an antiplatelet agent, antihypertensive, and statin) has led to reductions in stroke risk in this population, and that the absolute benefits of surgery may now be lower than expected.37). Subgroup analyses of ACST-1 demonstrate that individuals taking lipid lowering therapy had lower absolute risks of perioperative events (2.5% on lipid-lowering therapy vs 4.2% not on lipid-lowering therapy) and strokes (10.5% on lipid-lowering therapy vs 21% not on lipid-lowering therapy) compared to those not taking a statin, but similar relative risk reductions from carotid surgery (ratio of annual stroke rates =0.52, 95% CI 0.33–0.81).32). Hence allocation to CEA was still associated with a significant absolute reduction in strokes at 5 years (−3.4%, p = 0.0005) and 10 years (−5.8%, p = 0.002) follow-up. It is interesting to note that similar improvements have also been made with regards to the safety of CEA. Between 1991–2010, there has been a 6% proportional annual reduction in procedural events (stroke and death) from CEA, with registry data yielding similar event rates to those seen in recent randomised clinical trials.38)
CEA vs CAS for Symptomatic Carotid Stenosis
Whilst endovascular techniques have revolutionised the management of coronary artery disease and peripheral artery disease, the development of effective endovascular treatments for carotid stenoses has been protracted and less influential, largely due to the risks of cerebral embolization and procedural stroke.39). Early trials evaluating percutaneous carotid interventions reported high procedural stroke rates, and subsequent trials have been complicated by varying levels of technical expertise among interventionalists.40–42).
The first large randomised clinical trial comparing percutaneous carotid intervention to CEA was the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS), which randomised 504 patients with carotid stenosis to endovascular treatment (angioplasty or CAS) vs CEA.41, 43). Early on in the trial, the investigators recognised higher rates of stroke in patients receiving balloon angioplasty compared to primary CAS, and there was a transition from angioplasty at the start of the trial to CAS toward the end of the trial.41). CAVATAS reported periprocedural event rates of 10% in both the endovascular and surgical treatment groups which were notably higher than NASCET and ECST. There was no significant difference in post-procedural ipsilateral strokes between the two treatment groups after a median follow-up of five years (11.3% endovascular vs 8.6% CEA).43).
Three randomised trials were conducted subsequently comparing CEA to CAS in symptomatic patients; The Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE; n = 1214) study; Endarterectomy Versus Angioplasty in patients with Symptomatic Severe carotid Stenosis (EVA-3S; n = 527) trial; and the International Carotid Stenting Study (ICSS; n = 1713).44–49). In addition, subgroup analyses of symptomatic patients from the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial and Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), which included symptomatic and asymptomatic participants, have been reported.50–53). In four of these five trials, CAS was performed by interventionalists (either cardiologists or interventional radiologists) whereas in ICSS CAS was performed by a combination of both interventionalists and vascular surgeons. The credentialing requirements for physicians and surgeons differed across the trials.42). As with many interventional trials, recruiting collaborators with a high level of experience is challenging, particularly when the efficacy and safety of the procedure have not yet been established. The use of embolicprotection devices was low in the SPACE trial, however this increased to between 72–100% in the subsequent four trials.46–48, 52). Data from these trials and other studies demonstrate lower procedural stroke rates when embolic-protection devices are used.54–57).
The 30-day peri-procedural event rates reported in these trials ranged from 3.9% to 9.3% in patients allocated CEA, and 2.1% to 9.6% in patients allocated CAS (Table 2). Three of the five trials reported similar periprocedural event rates between the procedures (SAPPHIRE, SPACE, CREST) whereas two of the trials reported significantly lower event rates for CEA compared to CAS (EVA-3S, ICSS; Table 2). Importantly, EVA-3S and ICSS reported over 70% use of embolic protection devices within stenting procedures. Overall, current evidence suggests that CAS has a higher procedural stroke rate, dominated by non-disabling strokes, which is partially offset by a small yet significantly higher risk of procedural myocardial infarction with CEA.58).
Table 2. Randomised clinical trials comparing carotid endarterectomy to carotid stenting in patients with carotid artery stenosis.
| Trial | Recruitment | n | Follow-up | Procedural Hazards |
Long-Term Stroke Rate |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Definition | CEA | CAS | p-value | Definition | CEA | CAS | p-value | ||||
| Symptomatic | |||||||||||
| CAVATAS | 1992–1997 | 504 | Median 5 years | 30d disabling stroke & death | 5.9% | 6.4% | N.S. | Any stroke & periprocedural death | 23.5% | 29.7% | n.s. |
| SAPPHIRE (subgroup) | 2000–2002 | 96 | 78% at 3 years* | 30d MI, stroke, death | 9.3% | 2.1% | 0.18 | Periprocedural MI, stroke, death & post-procedural ipsilateral stroke & death | 21.7% | 32.0% | N.R. |
| EVA-3S | 2000–2005 | 527 | Median 3.5 years | 30d stroke & death | 3.9% | 9.6% | 0.01 | Periprocedural stroke, death, & post-procedural ipsilateral stroke | 6.2% | 11.1% | 0.03 |
| SPACE-1 | 2001–2006 | 1214 | 2 years | 30d ipsilateral stroke & death | 6.5% | 6.9% | 0.09† | Periprocedural stroke, death, & post-procedural ipsilateral stroke | 8.8% | 9.5% | 0.62 |
| ICSS | 2001–2008 | 1713 | Median 4.2 years | 30d procedural MI, stroke, death | 4.0% | 7.4% | 0.003 | Fatal or disabling stroke | 6.5% | 6.4% | 0.77 |
| CREST-1 (subgroup) | 2000–2008 | 1321 | Median 7.4 years* | 30d MI, stroke, death | 5.4% | 6.7% | n.s. | Periprocedural MI, stroke, death & postprocedural ipsilateral stroke | 5y: 8.7% 10y: 9.8% |
5y: 9.0% 10y: 13.4% |
0.40 |
| Asymptomatic | |||||||||||
| SAPPHIRE (subgroup) | 2000–2002 | 237 | 78% at 3 years* | 30d MI, stroke, death | 10.2% | 5.4% | 0.20 | Periprocedural MI, stroke, death & post-procedural ipsilateral stroke, death | 29.2% | 21.4% | N.R. |
| CREST-1 (subgroup) | 2000–2008 | 1181 | Median 7.4 years* | 30d MI, stroke, death | 3.6% | 3.5% | n.s. | Periprocedural MI, stroke, death & postprocedural ipsilateral stroke | 5y: 5.4% 10y: 10.1% |
5y: 6.1% 10y: 9.6% |
0.95 |
| ACT I | 2005–2013 | 1453 | Up to 5 years | 30d MI, stroke, death | 2.6% | 3.3% | 0.60† | Post-procedural ipsilateral stroke | 2.7% | 2.2% | 0.51† |
| SPACE-2 | 2009–2014 | 513 | Ongoing | 30d stoke, death | 1.97% | 2.54% | N.R. | Periprocedural stroke, death & post-procedural ipsilateral stroke | Pending | ||
CEA, carotid endarterectomy; CAS, carotid artery stenting; MI, myocardial infarction; N.R., not reported; n.s., not significant; CAVATAS, Carotid And Vertebral Artery Transluminal Angioplasty Study; SAPPHIRE, Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy; EVA-3S, Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis; SPACE, Stent-supported Percutaneous Angioplasty of the Carotid artery versus Endarterectomy; ICSS, International Carotid Stenting Study; CREST, Carotid Revascularization Endarterectomy vs Stenting Trial; ACT, Asymptomatic Carotid Trial.
For combined sample of trial participants (symptomatic and asymptomatic)
p-value for non-inferiority
Four of the five trials demonstrated similar longterm stroke rates between CEA and CAS (Table 2). Long-term event rates ranged from 6.2% to 32.0% however were largely influenced by variations in outcome definitions and differences in the length of follow-up. The EVA-3S trial reported lower rates of ipsilateral stroke in patients allocated CEA (6.2% CEA vs 11.1% CAS; p = 0.03), mainly attributable to higher periprocedural events in the stenting group.
A pre-specified individual patient-level data meta-analysis was conducted by the Carotid Stenting Trialists′ Collaboration (CSTC) to clarify the perioperative hazards of CEA compared to CAS, and explore subgroup variations. The CSTC demonstrated that in a combined sample of 3433 randomised participants, individuals allocated to CEA had a significantly lower risk of short-term events (any stroke or death within 120 days) compared to those allocated CAS (risk ratio for CAS 1.53 [95% CI 1.20–1.95], p = 0.0006).59). Importantly, the collaborative group identified that CEA was safe in patients aged over 70 years, whereas the perioperative hazards of CAS increased with age.60).
CEA vs CAS for Asymptomatic Carotid Stenosis
Results have now emerged for the comparison of CEA and CAS in asymptomatic patients. The Asymptomatic Carotid Trial-1 (ACT I) recruited and randomised 1453 asymptomatic patients to CEA and CAS in a 1:3 ratio.61). In addition, subgroup analyses have been reported for the CREST trial (1181 asymptomatic patients) and the SAPPHIRE trial (237 high risk asymptomatic patients).50–53). The larger Asymptomatic Carotid Surgery Trial-2 (ACST-2) is currently recruiting and will randomise 3600 patients by the end of 2019.62).
Neither ACT I or the asymptomatic subgroup of CREST demonstrated a difference in composite periprocedural events between CEA and CAS.53, 61). In ACT I, the rates of stroke, myocardial infarction, or death were 2.6% in the CEA group and 3.3% in the CAS group (p = 0.60), with a non-significantly higher rate of minor periprocedural strokes in the CAS group (2.4% CAS vs 1.1% CEA, p = 0.20). In CREST, the periprocedural hazards were 3.6% in the CEA group and 3.5% in the CAS group. Consideration of all CREST participants (symptomatic & asymptomatic) also suggested that patients allocated CAS had a higher periprocedural stroke rate, but a lower periprocedural myocardial infarction rate compared to those allocated CEA. Whether these two events can be considered comparable has been a topic of ongoing discussion.
One of the main findings of ACT I and CREST was that the long-term stroke rates among asymptomatic patients were much lower than in other carotid surgery trials. The CREST trial demonstrated 10-year stroke rates of 10.1% in asymptomatic patients randomised to CEA compared to 9.6% in those randomised to CAS (p = 0.95). Similarly, ACT I demonstrated 5-year post-procedural stroke rates of 2.7% in the CEA group and 2.2% in the CAS group. The small asymptomatic subgroup in SAPPHIRE demonstrated high 3-year stroke rates of 29.2% and 21.4% in patients allocated CEA and CAS, respectively, consistent with their high risk inclusion criteria. These trials reported relatively wide confidence intervals and may not have been powered to detect moderate but clinically meaningful differences between CEA and CAS.
Given the relatively low background stroke rates in patients with asymptomatic carotid stenosis, large trials are required to minimise the impact of random error and reveal meaningful differences in treatment effects. The ACST-2 trial has currently recruited over 2400 participants and will complete recruitment of 3600 asymptomatic carotid stenosis patients by the end of 2019.62). This trial, along with an individual patient data meta-analysis with ACT I, SPACE-2 and CREST-1, will clarify the best interventional management for patients with asymptomatic carotid stenosis.
Carotid Intervention vs Contemporary Medical Therapy for Carotid Stenosis
Since the early carotid surgery trials comparing CEA to medical therapy alone, there have been major improvements in cardiovascular risk-reduction medications. Most patients with carotid stenosis now receive triple medical therapy, including a statin, antiplatelet agent, and blood pressure-lowering therapy.63). These effective medications, in particular statins, appear to modify carotid plaque composition, with a reduction in features associated with plaque instability.64–66). Large randomised trials and collaborative meta-analyses demonstrate that a 1 mmol/L reduction in LDL-cholesterol from statin therapy reduces the 5-year risk of stroke by about one quarter in patients with vascular disease.67–69). Furthermore, allocation to statin therapy in the Heart Protection Study was associated with a 50% reduction in the rate of CEAs.68). In line with these advances, stroke rates among people with asymptomatic carotid stenosis who are managed conservatively have steadily declined since the original trials in the 1980s and 90s.36). Importantly, the procedural risks of CEA and CAS have also declined, due to improvements in devices, technical experience, and medical therapy.38, 70). This trend was particularly apparent in ACST-1 where statin therapy was associated with a halving in periprocedural stroke risk.31, 32). Given the significant improvements in contemporary medical therapy and safety of carotid procedures, there is renewed uncertainty as to whether intervention plus medical therapy, or medical therapy alone, is best in patients with asymptomatic carotid stenosis. CREST-2 (target = 2480 participants), ECST-2 (target = 2000 participants), and ACTRIS (target = 700 participants) are currently recruiting and randomising patients to clarify this uncertainty, with results expected in the early 2020s. SPACE-2 began recruiting patients in 2009 however closed early after recruiting 513 participants.71). Together these trials will provide reliable evidence from over 5000 randomised patients to better inform the management of carotid stenosis.
Though previous trials have largely been conducted in Europe and North America, it is particularly important that ongoing and future carotid trials recruit patients from other regions of the world such as Asia and Australasia. Recruitment of individuals from all over the world would help trials recruit sufficiently large numbers of participants to look for small treatment effects and subgroup differences. In addition, it would allow assessment of potential regional or ethnic variations, or lack thereof, making the evidence uniquely reliable and generalizable.
Issues Specific for Surgery
When conducting clinical trials of surgical and procedural interventions, there are additional factors to consider such as the performance of the operator, and the quality of anaesthetic and periprocedural care. While CAS can be performed without anaesthesia, CEA requires general or regional anaesthesia which may potentially modify periprocedural stroke risk. The General Anaesthesia versus Local Anaesthesia for carotid surgery (GALA) trial comprising 3526 patients found no significant difference in periprocedural stroke, myocardial infarction, or death among patients randomised to general or regional anaesthesia.72). A recent non-randomised comparison of anaesthesia in the CREST trial showed that the risk of myocardial infarction among those who received general anaesthesia was twice that of those who received regional anaesthesia, although results were limited to a small sample size with potential for residual confounding.73).
In the real-world, the choice of anaesthesia is still decided by surgeon and patient preference. Importantly, the method of CEA and choice of carotid device influence the outcomes of carotid interventions. A meta-analysis of 1967 patients from 10 randomised trials demonstrated that patch angioplasty was associated with lower risk of ipsilateral stroke than primary closure during the periprocedural period (odds ratio [OR] 0.31, 95% CI 0.15–0.63, p = 0.001) and longer term follow-up (OR 0.32, 95% CI 0.16–0.63, p = 0.001).74). Furthermore, a meta-analysis of randomised studies suggested that eversion CEA further reduced the risk of periprocedural stroke compared to patch angioplasty endarterectomy, although the eversion method has been suggested to have a steeper learning curve.75, 76). For CAS, embolic protection devices, carotid flow reversal, and refined stent devices have been suggested to reduce procedural stroke risk, although there is a paucity of head-to-head randomised studies.77, 78). More large-scale, head-to-head randomised comparisons are needed to clarify the optimal method of anaesthesia, surgical technique and carotid stent device system to minimise the procedural hazards of carotid interventions.
Current Guidelines for Carotid Interventions
Many guidelines have been published on the procedural management of symptomatic and asymptomatic car otid stenosis. Here we summarise recommendations from four large international bodies.79–82).
Symptomatic Carotid Stenosis
The American College of Cardiology (ACC)/American Heart Association (AHA), Society for Vascular Surgery (SVS), European Society for Vascular Surgery (ESVS), and European Society of Cardiology (ESC) carotid artery disease guidelines recommend CEA as first line interventional management for patients with symptomatic carotid stenosis (Table 3 and Table 4). All four guidelines support CEA in symptomatic patients with non-occluding carotid stenosis ≥ 70%. ACC/AHA also support CEA in patients with 50–69% stenosis on catheter angiography; ESVS tentatively support CEA in patients with 50–69% stenosis; and ESC state that the decision for CEA in those with 50–69% stenosis should be guided by patient factors. Current guidelines do not recommend intervention in symptomatic patients with stenosis < 50%. ACC/AHA and ESVS guidelines suggest that anticipated perioperative stroke and death rates should be less than 6% according to the operator's experience, and all four guidelines recommend carotid intervention be performed within 2 weeks of the onset of neurological symptoms.
Table 3. Guideline based recommendations for carotid endarterectomy.
| ACC/AHA guidelines (2011)79) | SVS guidelines (2011)82) | ESVS guidelines (2009)81) | ESC guidelines (2011)80) | |
|---|---|---|---|---|
| Symptomatic | Patients at average or low surgical risk who experience nondisabling ischemic stroke or transient cerebral ischemic symptoms, including hemispheric events or amaurosis fugax, within 6 months (symptomatic patients) should undergo CEA if the diameter of the lumen of the ipsilateral internal carotid artery is reduced more than 70% as documented by noninvasive imaging [class I; level of evidence A] or more than 50% as documented by catheter angiography [class I; level of evidence B] and the anticipated rate of perioperative stroke or mortality is less than 6%. | In the majority of patients with carotid stenosis who are candidates for intervention, CEA is preferred to CAS for reduction of all cause stroke and periprocedural mortality. [grade I; level of evidence B] Data from CREST suggest that patients < 70 years of age may be better treated by CAS. These data need further confirmation. | The operative treatment of carotid disease is absolutely indicated in symptomatic patients with > 70% (NASCET) stenosis and probably with > 50% (NASCET) stenosis. The perioperative stroke/death rate should be < 6%. [level of evidence A] | In patients with symptomatic 70–99% stenosis of the internal carotid artery, CEA is recommended for the prevention of recurrent stroke. [class I; level of evidence A] In patients with symptomatic 50–69% stenosis of the internal carotid artery, CEA should be considered for recurrent stroke prevention, depending on patient-specific factors. [class IIa; level of evidence A] |
| Asymptomatic | Selection of asymptomatic patients for carotid revascularization should be guided by an assessment of comorbid conditions, life expectancy, and other individual factors and should include a thorough discussion of the risks and benefits of the procedure with an understanding of patient preferences. [class I; level of evidence C] It is reasonable to perform CEA in asymptomatic patients who have more than 70% stenosis of the internal carotid artery if the risk of perioperative stroke, MI, and death is low. [class IIa; level of evidence A] | Neurologically asymptomatic patients with equal or > 60% diameter stenosis, should be considered for CEA for reduction of long-term risk of stroke provided the patient has a 3- to 5-year life expectancy and perioperative stroke/death rates can be equal to or < 3%. [grade I; level of evidence A]. | CEA can be recommended for asymptomatic men below 75 years with 70–99% stenosis if the risk associated with surgery is less than 3%. [level of evidence A] The benefit from CEA in asymptomatic women with carotid stenosis is significantly less than in men. CEA should therefore be considered only in younger, fit women. [level of evidence A] | In asymptomatic patients with carotid artery stenosis ≥ 60%, CEA should be considered as long as the perioperative stroke and death rate for procedures performed by the surgical team is < 3% and the patient's life expectancy exceeds 5 years. [class IIa; level of evidence A] |
ACC, American College of Cardiology; AHA, American Heart Association; SVS, Society for Vascular Surgery; ESVS, European Society for Vascular Surgery; ESC, European Society for Cardiology; CEA, carotid endarterectomy; CAS, carotid artery stenting; MI, myocardial infarction; CREST, Carotid Revascularization Endarterectomy versus Stenting Trial; NASCET, North American Symptomatic Carotid Endarterectomy Trial. Class and grade indicate the strength of the recommendation.
Table 4. Guideline based recommendations for carotid stenting.
| ACC/AHA guidelines (2011)79) | SVS guidelines (2011)82) | ESVS guidelines (2009)81) | ESC guidelines (2011)80) | |
|---|---|---|---|---|
| Symptomatic | CAS is indicated as an alternative to CEA for symptomatic patients at average or low risk of complications associated with endovascular intervention when the diameter of the lumen of the internal carotid artery is reduced by more than 70% as documented by noninvasive imaging or more than 50% as documented by catheter angiography and the anticipated rate of periprocedural stroke or mortality is less than 6%. [class I; level of evidence B] It is reasonable to choose CAS over CEA when revascularization is indicated in patients with neck anatomy unfavorable for arterial surgery. [class IIa; level of evidence B] | CAS is preferred over CEA in symptomatic patients with ≥ 50% stenosis and prior ipsilateral operation, tracheal stoma, external beam irradiation resulting in fibrosis of the tissues of the ipsilateral neck, or prior cranial nerve injury and lesions that extend proximal to the clavicle or distal to the C2 vertebral body. [grade II; level of evidence B] CAS is preferred over CEA in symptomatic patients with ≥ 50% stenosis and severe uncorrectable CAD, CHF, or COPD. [grade II; level of evidence C] | The available level I evidence suggests that for symptomatic patients, surgery is currently the best option. [level of evidence A] CAS should be offered to symptomatic patients, if they are at high risk for CEA, in high-volume centres with documented low peri-procedural stroke and death rates or inside an RCT. [level of evidence C] | In symptomatic patients at high surgical risk requiring revascularization, CAS should be considered as an alternative to CEA. [class IIa; level of evidence B] In symptomatic patients requiring carotid revascularization, CAS may be considered as an alternative to CEA in high-volume centres with documented death or stroke rate < 6%. [class IIa; level of evidence B] |
| Asymptomatic | Prophylactic CAS might be considered in highly selected patients with asymptomatic carotid stenosis (minimum 60% by angiography, 70% by validated Doppler ultrasound), but its effectiveness compared with medical therapy alone in this situation is not well established. [class IIb; level of evidence B] | There are insufficient data to recommend CAS as primary therapy for neurologically asymptomatic patients with 70% to 99% diameter stenosis. Data from CREST suggest that in properly selected asymptomatic patients, CAS is equivalent to CEA in the hands of experienced interventionalists. Operators and institutions performing CAS must exhibit expertise sufficient to meet the previously established American Heart Association guidelines for treatment of patients with asymptomatic carotid stenosis. Specifically, the combined stroke and death rate must be below 3% to ensure benefit for the patient. [grade II; level of evidence B] | It is advisable to offer CAS in asymptomatic patients only in high-volume centres with documented low peri-procedural stroke and death rates or within well-conducted clinical trials. [level of evidence C] CAS is indicated in case of contralateral laryngeal nerve palsy, previous radical neck dissection, cervical irradiation, with prior CEA (restenosis), with high bifurcation or intracranial extension of a carotid lesion, provided that the peri-interventional stroke or death rate is higher than that accepted for CEA. [level of evidence C] | In asymptomatic patients with an indication for carotid revascularization, CAS may be considered as an alternative to CEA in high-volume centres with documented death or stroke rate < 3%. [class IIb; level of evidence B] |
ACC, American College of Cardiology; AHA, American Heart Association; SVS, Society for Vascular Surgery; ESVS, European Society for Vascular Surgery; ESC, European Society for Cardiology; CAS, carotid artery stenting; CEA, carotid endarterectomy; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CREST, Carotid Revascularization Endarterectomy versus Stenting Trial; RCT, randomised clinical trial. Class and grade indicate the strength of the recommendation.
Current guidelines support a Primary CAS approach in symptomatic patients who are of ‘high surgical risk’ (Table 4). This is defined by ACC/AHA and SVS to include unfavourable neck anatomy, as well as severe medical comorbidities in the SVS guidelines (severe uncorrectable coronary heart disease, heart failure, chronic obstructive pulmonary disease). High surgical risk is not clearly defined in the ESVS and ESC guidelines. Primary CAS is also supported by ESVS and ESC in the setting of a high volume centre with low periprocedural stroke and death rates (< 6%).
Asymptomatic Carotid Stenosis
Current guidelines for asymptomatic carotid interventions are less well defined. ACC/AHA, SVS, ESVS and ESC suggest consideration of CEA in asymptomatic patients with reasonable life expectancy, provided the risks of perioperative events are low (< 3%; Table 3). There is similar disagreement around the minimum carotid artery diameter reduction for which CEA should be considered, with SVS and ESC supporting a threshold of > 60% stenosis, and ACC/AHA and ESVS supporting a threshold of > 70% stenosis.
Recommendations for CAS in asymptomatic patients are also conflicting. ESVS and ESC state that CAS may be considered as an alternative to CEA for asymptomatic patients in high volume centres with low perioperative stroke and death rates (< 3%; Table 4). ACC/AHA and SVS do not provide clear indications for asymptomatic CAS, stating that primary CAS should be used in “highly selected” asymptomatic patients, or that there is insufficient data to recommend it.
Current guidelines do not consider the possibility of regional heterogeneity, and guidelines from Asian countries such as Japan refer largely to evidence from European and American studies.83). Insight may be gained from country-specific studies, for example a Japanese single-centred observational study demonstrated remarkably low periprocedural stroke rates (0.68%) and annual stroke rates (0.64%) for CEA in asymptomatic patients.84). It is hoped that future Japanese studies will reciprocate the benefits and safety of carotid interventions reported in large European and American trials.
Conclusion
There is reliable clinical evidence to support the use of carotid interventions in specific populations with carotid stenosis. These interventions provide a clear benefit in terms of stroke risk reduction for selected individuals with symptomatic and asymptomatic disease. If the current trials comparing a carotid procedure vs no procedure in the current era of good medical therapy with statins, anti-thrombotics and anti-hypertensives confirm the ACST-1 finding of additional benefit from a procedure then, throughout the 2020s and beyond, the key question will be which procedure to recommend. ACST-2 and an IPD meta-analysis including over 6000 patients will provide uniquely reliable results in 2020 to help guide clinical practice for the next decade. It is essential that we continue to recruit large numbers of patients from different geographical regions, to provide reliable evidence that is generalizable to all countries of the world.
Declarations
DM is supported by a General Sir John Monash Scholarship. AH is principal investigator and RB is co-principal investigator of the Asymtpomatic Carotid Surgery Trial-2. AH acknowleded the funding support from the Oxford Biomedical Research Centre. Professor Halliday's research is funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford.
SG discloses the following relationships - Advisory Board: Medscape Cardiology; Data Monitoring Committees: TIMI study group in Harvard University, Population Health Research Institute in McMaster University, Armethron; Honoraria: the American Heart Association (An Associate Editor, Circulation), Thrombosis Research Institute at the University College of London (clinical trial steering committees), CTSU at the University of Oxford (clinical trial steering committees), TIMI study group at Harvard University (clinical trial steering committee), Thrombosis and Haemostasis (Section Editor), Archived of Medicine (Associate Editor), Journal of Cardiology Case (Associate Editor), Other: Paid Lecture for Sanofi; Research Funding: Sanofi, Pfeizer, the Japanese Society of Promotion of Science, and Riken.
References
- 1). Flaherty ML, Kissela B, Khoury JC, Alwell K, Moomaw CJ, Woo D, Khatri P, Ferioli S, Adeoye O, Broderick JP, Kleindorfer D: Carotid artery stenosis as a cause of stroke. Neuroepidemiology, 2013; 40: 36-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Howard DP, van Lammeren GW, Rothwell PM, Redgrave JN, Moll FL, de Vries JP, de Kleijn DP, den Ruijter HM, de Borst GJ, Pasterkamp G: Symptomatic carotid atherosclerotic disease: correlations between plaque composition and ipsilateral stroke risk. Stroke, 2015; 46: 182-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Golledge J, Greenhalgh RM, Davies AH: The symptomatic carotid plaque. Stroke, 2000; 31: 774-781 [DOI] [PubMed] [Google Scholar]
- 4). Paraskevas KI, Hamilton G, Mikhailidis DP: Statins: an essential component in the management of carotid artery disease. J Vasc Surg, 2007; 46: 373-386 [DOI] [PubMed] [Google Scholar]
- 5). Nicolaides AN, Kakkos SK, Griffin M, Sabetai M, Dhanjil S, Tegos T, Thomas DJ, Giannoukas A, Geroulakos G, Georgiou N, Francis S, Ioannidou E, Dore CJ: Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg, 2005; 30: 275-284 [DOI] [PubMed] [Google Scholar]
- 6). Aichner FT, Topakian R, Alberts MJ, Bhatt DL, Haring HP, Hill MD, Montalescot G, Goto S, Touze E, Mas JL, Steg PG, Rother J, Reach Registry Investigators : High cardiovascular event rates in patients with asymptomatic carotid stenosis: the REACH Registry. Eur J Neurol, 2009; 16: 902-908 [DOI] [PubMed] [Google Scholar]
- 7). Chiari H: Ueber das verhalten des teilungswinkels der carotis communis bei der endarteritis chronica deformans. Verh Dtsch Pathol Ges, 1905; 9: 326-330 [Google Scholar]
- 8). Hunt JR: The role of the carotid arteries in the causation of vascular lesions of the brain, with remarks on certain special features of the symptomatology. Am J Med Sci, 1914; 147: 704-713 [DOI] [PubMed] [Google Scholar]
- 9). Fisher M: Occlusion of the internal carotid artery. AMA Archives of Neurology & Psychiatry, 1951; 65: 346-377 [DOI] [PubMed] [Google Scholar]
- 10). Carrea R, Molins M, Murphy G: Surgery of spontaneous thrombosis of the internal carotid in the neck; carotido-carotid anastomosis; case report and analysis of the literature on surgical cases. Medicina (B Aires), 1955; 15: 20-29 [PubMed] [Google Scholar]
- 11). Eastcott HH, Pickering GW, Rob CG: Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet, 1954; 267: 994-996 [DOI] [PubMed] [Google Scholar]
- 12). Debakey ME, Crawford ES, Cooley DA, Morris GC, Garret HE, Fields WS: Cerebral arterial insufficiency: One to 11-year results following arterial reconstructive operation. Ann Surg, 1965; 161: 921-945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). DeBakey ME: Successful carotid endarterectomy for cerebrovascular insufficiency. Nineteen-year follow-up. JAMA, 1975; 233: 1083-1085 [PubMed] [Google Scholar]
- 14). Cooley DA, Al-Naaman YD, Carton CA: Surgical treatment of arteriosclerotic occlusion of common carotid artery. J Neurosurg, 1956; 13: 500-506 [DOI] [PubMed] [Google Scholar]
- 15). De Bakey ME, Crawford ES, Cooley DA, Morris GC: Surgical considerations of occlusive disease of innominate, carotid, subclavian, and vertebral arteries. Ann Surg, 1959; 149: 690-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Etheredge SN: A simple technic for carotid endarterectomy. Am J Surg, 1970; 120: 275-278 [DOI] [PubMed] [Google Scholar]
- 17). Callow AD: David M. Hume Memorial Lecture. An overview of the stroke problem in the carotid territory. Am J Surg, 1980; 140: 181-191 [DOI] [PubMed] [Google Scholar]
- 18). Morris GC, Lechter A, DeBakey ME: Surgical treatment of fibromuscular disease of the carotid arteries. Arch Surg, 1968; 96: 636-643 [DOI] [PubMed] [Google Scholar]
- 19). Mathias K: A new catheter system for percutaneous transluminal angioplasty (PTA) of carotid artery stenoses. Fortschr Med, 1977; 95: 1007-1011 [PubMed] [Google Scholar]
- 20). Marks MP, Dake MD, Steinberg GK, Norbash AM, Lane B: Stent placement for arterial and venous cerebrovascular disease: preliminary experience. Radiology, 1994; 191: 441-446 [DOI] [PubMed] [Google Scholar]
- 21).European Carotid Surgery Trialists' Collaborative Group: Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet, 1998; 351: 1379-1387 [PubMed] [Google Scholar]
- 22). Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD: Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med, 1998; 339: 1415-1425 [DOI] [PubMed] [Google Scholar]
- 23). Mayberg MR, Wilson SE, Yatsu F, Weiss DG, Messina L, Hershey LA, Colling C, Eskridge J, Deykin D, Winn HR: Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA, 1991; 266: 3289-3294 [PubMed] [Google Scholar]
- 24).North American Symptomatic Carotid Endarterectomy Trial Collaborators: Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med, 1991; 325: 445-453 [DOI] [PubMed] [Google Scholar]
- 25). Rothwell PM, Gutnikov SA, Warlow CP, European Carotid Surgery Trialist's Collaboration : Reanalysis of the final results of the European Carotid Surgery Trial. Stroke, 2003; 34: 514-523 [DOI] [PubMed] [Google Scholar]
- 26). Rothwell PM, Gibson RJ, Slattery J, Sellar RJ, Warlow CP: Equivalence of measurements of carotid stenosis. A comparison of three methods on 1001 angiograms. European Carotid Surgery Trialists' Collaborative Group. Stroke, 1994; 25: 2435-2439 [DOI] [PubMed] [Google Scholar]
- 27). Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, Warlow CP, Barnett HJ, Carotid Endarterectomy Trialists' Collaboration : Analysis of pooled data from the randomised controlled trials of end-arterectomy for symptomatic carotid stenosis. Lancet, 2003; 361: 107-116 [DOI] [PubMed] [Google Scholar]
- 28). Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ, Carotid Endarterectomy Trialists Collaboration : Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet, 2004; 363: 915-924 [DOI] [PubMed] [Google Scholar]
- 29). Hobson RW, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, Wright CB: Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med, 1993; 328: 221-227 [DOI] [PubMed] [Google Scholar]
- 30).Executive Committee for the Asymptomatic Carotid Atherosclerosis Study: Endarterectomy for asymptomatic carotid artery stenosis. JAMA, 1995; 273: 1421-1428 [PubMed] [Google Scholar]
- 31). Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D, MRC Asymptomatic Carotid Surgery Trial Collaborative Group : Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet, 2004; 363: 1491-1502 [DOI] [PubMed] [Google Scholar]
- 32). Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J, Pan H, Peto R, Potter J, Rahimi K, Rau A, Robertson S, Streifler J, Thomas D, Asymptomatic Carotid Surgery Trial Collaborative Group : 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet, 2010; 376: 1074-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Rothwell PM, Goldstein LB: Carotid endarterectomy for asymptomatic carotid stenosis: asymptomatic carotid surgery trial. Stroke, 2004; 35: 2425-2427 [DOI] [PubMed] [Google Scholar]
- 34). Moore WS, Vescera CL, Robertson JT, Baker WH, Howard VJ, Toole JF: Selection process for surgeons in the Asymptomatic Carotid Atherosclerosis Study. Stroke, 1991; 22: 1353-1357 [DOI] [PubMed] [Google Scholar]
- 35). Moore WS, Young B, Baker WH, Robertson JT, Toole JF, Vescera CL, Howard VJ: Surgical results: a justification of the surgeon selection process for the ACAS trial. The ACAS Investigators. J Vasc Surg, 1996; 23: 323-328 [DOI] [PubMed] [Google Scholar]
- 36). Hadar N, Raman G, Moorthy D, O'Donnell TF, Thaler DE, Feldmann E, Lau J, Kitsios GD, Dahabreh IJ: Asymptomatic carotid artery stenosis treated with medical therapy alone: temporal trends and implications for risk assessment and the design of future studies. Cerebrovasc Dis, 2014; 38: 163-173 [DOI] [PubMed] [Google Scholar]
- 37). Lanzino G, Rabinstein AA, Brown RD: Treatment of carotid artery stenosis: medical therapy, surgery, or stenting? Mayo Clin Proc, 2009; 84: 362-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Munster AB, Franchini AJ, Qureshi MI, Thapar A, Davies AH: Temporal trends in safety of carotid endarterectomy in asymptomatic patients: systematic review. Neurology, 2015; 85: 365-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Tulip HH, Rosero EB, Higuera AJ, Ilarraza A, Valentine RJ, Timaran CH: Cerebral embolization in asymptomatic versus symptomatic patients after carotid stenting. J Vasc Surg, 2012; 56: 1579-1584 [DOI] [PubMed] [Google Scholar]
- 40). Naylor AR, Bolia A, Abbott RJ, Pye IF, Smith J, Lennard N, Lloyd AJ, London NJ, Bell PR: Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg, 1998; 28: 326-334 [DOI] [PubMed] [Google Scholar]
- 41).CAVATAS investigators: Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet, 2001; 357: 1729-1737 [PubMed] [Google Scholar]
- 42). Roffi M, Sievert H, Gray WA, White CJ, Torsello G, Cao P, Reimers B, Mathias K, Setacci C, Schonholz C, Clair DG, Schillinger M, Grunwald I, Bosiers M, Abou-Chebl A, Moussa ID, Mudra H, Iyer SS, Scheinert D, Yadav JS, van Sambeek MR, Holmes DR, Cremonesi A: Carotid artery stenting versus surgery: adequate comparisons? Lancet Neurol, 2010; 9: 339-341 [DOI] [PubMed] [Google Scholar]
- 43). Ederle J, Bonati LH, Dobson J, Featherstone RL, Gaines PA, Beard JD, Venables GS, Markus HS, Clifton A, Sandercock P, Brown MM, Cavatas Investigators : Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol, 2009; 8: 898-907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Bonati LH, Dobson J, Featherstone RL, Ederle J, van der Worp HB, de Borst GJ, Mali WP, Beard JD, Cleveland T, Engelter ST, Lyrer PA, Ford GA, Dorman PJ, Brown MM, International Carotid Stenting Study investigators : Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet, 2015; 385: 529-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, Hennerici M, Stingele R, Fiehler J, Zeumer H, Jansen O: Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol, 2008; 7: 893-902 [DOI] [PubMed] [Google Scholar]
- 46). Space Collaborative Group. Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Stingele R, Zeumer H, Hacke W: 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet, 2006; 368: 1239-1247 [DOI] [PubMed] [Google Scholar]
- 47). International Carotid Stenting Study investigators. Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, Lo TH, Gaines P, Dorman PJ, Macdonald S, Lyrer PA, Hendriks JM, McCollum C, Nederkoorn PJ, Brown MM: Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet, 2010; 375: 985-997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lievre M, Leys D, Bonneville JF, Watelet J, Pruvo JP, Albucher JF, Viguier A, Piquet P, Garnier P, Viader F, Touze E, Giroud M, Hosseini H, Pillet JC, Favrole P, Neau JP, Ducrocq X, EVA-3S Investigators : Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med, 2006; 355: 1660-1671 [DOI] [PubMed] [Google Scholar]
- 49). Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, Bossavy JP, Denis B, Piquet P, Garnier P, Viader F, Touze E, Julia P, Giroud M, Krause D, Hosseini H, Becquemin JP, Hinzelin G, Houdart E, Henon H, Neau JP, Bracard S, Onnient Y, Padovani R, Chatellier G, EVA-3S investigators : Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol, 2008; 7: 885-892 [DOI] [PubMed] [Google Scholar]
- 50). Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K, Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators : Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med, 2004; 351: 1493-1501 [DOI] [PubMed] [Google Scholar]
- 51). Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Ansel G, Strickman NE, Wang H, Cohen SA, Massaro JM, Cutlip DE, SAPPHIRE Investigators : Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med, 2008; 358: 1572-1579 [DOI] [PubMed] [Google Scholar]
- 52). Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF, CREST Investigators : Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med, 2010; 363: 11-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, Moore WS, Hill MD, Mantese VA, Clark WM, Timaran CH, Heck D, Leimgruber PP, Sheffet AJ, Howard VJ, Chaturvedi S, Lal BK, Voeks JH, Hobson RW, CREST Investigators : Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N Engl J Med, 2016; 374: 1021-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Mas JL, Chatellier G, Beyssen B, EVA-3S Investigators : Carotid angioplasty and stenting with and without cerebral protection: clinical alert from the Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) trial. Stroke, 2004; 35: e18-20 [DOI] [PubMed] [Google Scholar]
- 55). Zahn R, Mark B, Niedermaier N, Zeymer U, Limbourg P, Ischinger T, Haerten K, Hauptmann KE, Leitner ER, Kasper W, Tebbe U, Senges J: Embolic protection devices for carotid artery stenting: better results than stenting without protection? Eur Heart J, 2004; 25: 1550-1558 [DOI] [PubMed] [Google Scholar]
- 56). Wholey MH, Al-Mubarek N, Wholey MH: Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv, 2003; 60: 259-266 [DOI] [PubMed] [Google Scholar]
- 57). Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB: Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke, 2003; 34: 813-819 [DOI] [PubMed] [Google Scholar]
- 58). Boulanger M, Cameliere L, Felgueiras R, Berger L, Rerkasem K, Rothwell PM, Touze E: Periprocedural myocardial infarction after carotid endarterectomy and stenting: systematic review and meta-analysis. Stroke, 2015; 46: 2843-2848 [DOI] [PubMed] [Google Scholar]
- 59). Carotid Stenting Trialists' Collaboration. Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, Fraedrich G, Mali WP, Zeumer H, Brown MM, Mas JL, Ringleb PA: Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet, 2010; 376: 1062-1073 [DOI] [PubMed] [Google Scholar]
- 60). Howard G, Roubin GS, Jansen O, Hendrikse J, Halliday A, Fraedrich G, Eckstein HH, Calvet D, Bulbulia R, Bonati LH, Becquemin JP, Algra A, Brown MM, Ringleb PA, Brott TG, Mas JL, Carotid Stenting Trialists' Collaboration : Association between age and risk of stroke or death from carotid endarterectomy and carotid stenting: a meta-analysis of pooled patient data from four randomised trials. Lancet, 2016; 387: 1305-1311 [DOI] [PubMed] [Google Scholar]
- 61). Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, Wechsler L, Jaff MR, Gray W, ACT I Investigators : Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med, 2016; 374: 1011-1020 [DOI] [PubMed] [Google Scholar]
- 62). ACST-2 Collaborative Group. Halliday A, Bulbulia R, Gray W, Naughten A, den Hartog A, Delmestri A, Wallis C, le Conte S, Macdonald S: Status update and interim results from the asymptomatic carotid surgery trial-2 (ACST-2). Eur J Vasc Endovasc Surg, 2013; 46: 510-518 [DOI] [PubMed] [Google Scholar]
- 63). Paraskevas KI, Mikhailidis DP, Veith FJ, Spence JD: Definition of best medical treatment in asymptomatic and symptomatic carotid artery stenosis. Angiology, 2016; 67: 411-419 [DOI] [PubMed] [Google Scholar]
- 64). Zhao XQ, Dong L, Hatsukami T, Phan BA, Chu B, Moore A, Lane T, Neradilek MB, Polissar N, Monick D, Lee C, Underhill H, Yuan C: MR imaging of carotid plaque composition during lipid-lowering therapy a prospective assessment of effect and time course. JACC Cardiovasc Imaging, 2011; 4: 977-986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65). van Lammeren GW, den Ruijter HM, Vrijenhoek JE, van der Laan SW, Velema E, de Vries JP, de Kleijn DP, Vink A, de Borst GJ, Moll FL, Bots ML, Pasterkamp G: Time-dependent changes in atherosclerotic plaque composition in patients undergoing carotid surgery. Circulation, 2014; 129: 2269-2276 [DOI] [PubMed] [Google Scholar]
- 66). Ramadan R, Dhawan SS, Binongo JN, Alkhoder A, Jones DP, Oshinski JN, Quyyumi AA: Effect of angiotensin II type I receptor blockade with valsartan on carotid artery atherosclerosis: a double blind randomized clinical trial comparing valsartan and placebo (EFFERVESCENT). Am Heart J, 2016; 174: 68-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67). Collins R, Armitage J, Parish S, Sleight P, Peto R, Heart Protection Study Collaborative Group : Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet, 2004; 363: 757-767 [DOI] [PubMed] [Google Scholar]
- 68). Heart Protection Study Collaborative Group : Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. Journal of Vascular Surgery, 2007; 45: 645-654 [DOI] [PubMed] [Google Scholar]
- 69). Amarenco P, Bogousslavsky J, Callahan A, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA, Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators : High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med, 2006; 355: 549-559 [DOI] [PubMed] [Google Scholar]
- 70). Gray WA, Chaturvedi S, Verta P: Thirty-day outcomes for carotid artery stenting in 6320 patients from 2 prospective, multicenter, high-surgical-risk registries. Circ Cardiovasc Interv, 2009; 2: 159-166 [DOI] [PubMed] [Google Scholar]
- 71). Eckstein HH, Reiff T, Ringleb P, Jansen O, Mansmann U, Hacke W, Space 2 Investigators : SPACE-2: A missed opportunity to compare carotid endarterectomy, carotid stenting, and best medical treatment in patients with asymptomatic carotid stenoses. Eur J Vasc Endovasc Surg, 2016; 51: 761-765 [DOI] [PubMed] [Google Scholar]
- 72). GALA Trial Collaborative Group. Lewis SC, Warlow CP, Bodenham AR, Colam B, Rothwell PM, Torgerson D, Dellagrammaticas D, Horrocks M, Liapis C, Banning AP, Gough M, Gough MJ: General anaesthesia versus local anaesthesia for carotid surgery (GALA): a multicentre, randomised controlled trial. Lancet, 2008; 372: 2132-214219041130 [Google Scholar]
- 73). Hye RJ, Voeks JH, Malas MB, Tom M, Longson S, Blackshear JL, Brott TG: Anesthetic type and risk of myocardial infarction after carotid endarterectomy in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). J Vasc Surg, 2016; 64: 3-8.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Rerkasem K, Rothwell PM: Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database Syst Rev, 2009: Cd000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75). Antonopoulos CN, Kakisis JD, Sergentanis TN, Liapis CD: Eversion versus conventional carotid endarterectomy: a meta-analysis of randomised and non-randomised studies. Eur J Vasc Endovasc Surg, 2011; 42: 751-765 [DOI] [PubMed] [Google Scholar]
- 76). Radak D, Tanaskovic S, Matic P, Babic S, Aleksic N, Ilijevski N: Eversion carotid endarterectomy--our experience after 20 years of carotid surgery and 9897 carotid endarterectomy procedures. Ann Vasc Surg, 2012; 26: 924-928 [DOI] [PubMed] [Google Scholar]
- 77). Nallamothu BK, Gurm HS, Ting HH, Goodney PP, Rogers MA, Curtis JP, Dimick JB, Bates ER, Krumholz HM, Birkmeyer JD: Operator experience and carotid stenting outcomes in Medicare beneficiaries. JAMA, 2011; 306: 1338-1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Calvet D, Mas JL: Recent advances in carotid angioplasty and stenting. Int J Stroke, 2016; 11: 19-27 [DOI] [PubMed] [Google Scholar]
- 79). Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, Hertzberg VS, McIff EB, Moore WS, Panagos PD, Riles TS, Rosenwasser RH, Taylor AJ: 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol, 2011; 57: e16-94 [DOI] [PubMed] [Google Scholar]
- 80). European Stroke Organisation. Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clement D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Rother J, Sievert H, van Sambeek M, Zeller T, ESC Committee for Practice Guidelines : ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J, 2011; 32: 2851-2906 [DOI] [PubMed] [Google Scholar]
- 81). Liapis CD, Bell PR, Mikhailidis D, Sivenius J, Nicolaides A, Fernandes e Fernandes J, Biasi G, Norgren L, ESVS Guidelines Collaborators : ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg, 2009; 37: 1-19 [DOI] [PubMed] [Google Scholar]
- 82). Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK, Society for Vascular Surgery : Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg, 2011; 54: e1-31 [DOI] [PubMed] [Google Scholar]
- 83). Tada H, Kawashiri MA, Nohara A, Inazu A, Kobayashi J, Yasuda K, Mabuchi H, Yamagishi M, Hayashi K. Lipid Management in a Japanese Community: Attainment Rate of Target Set by the Japan Atherosclerosis Society Guidelines for the Prevention of Atherosclerotic Cardiovascular Diseases 2012. J Atheroscler Thromb. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84). Ishiguro T, Yoneyama T, Ishikawa T, Yamaguchi K, Kawashima A, Kawamata T, Okada Y: Perioperative and long-term outcomes of carotid endarterectomy for japanese asymptomatic cervical carotid artery stenosis: a single institution study. Neurol Med Chir (Tokyo), 2015; 55: 830-837 [DOI] [PMC free article] [PubMed] [Google Scholar]


