Abstract
Spirometry is a physiological test for assessing the functional aspect of the lungs using an objective indicator to measure the maximum amount of air that a patient can inhale and exhale. Acceptable spirometry testing needs to be conducted three times by an acceptable and reproducible method for determining forced vital capacity (FVC). Until the results of three tests meet the criteria of reproducibility, the test should be repeated up to eight times. Interpretation of spirometry should be clear, concise, and informative. Additionally, spirometry should guarantee optimal quality prior to the interpreting spirometry results. Our guideline adopts a fixed normal predictive value instead of the lower limit of normal as the reference value because fixed value is more convenient and also accepts FVC instead of vital capacity (VC) because measurement of VC using a spirometer is impossible. The bronchodilator test is a method for measuring the changes in lung capacity after inhaling a short-acting β-agonist that dilates the airway. When an obstructive ventilatory defect is observed, this test helps to diagnose and evaluate asthma and chronic obstructive pulmonary disease by measuring reversibility with the use of an inhaled bronchodilator. A positive response to a bronchodilator is generally defined as an increase of ≥12% and ≥200 mL as an absolute value compared with a baseline in either forced expiratory volume at 1 second or FVC.
Keywords: Spirometry, Lung, Guideline, Bronchodilator Agents
Spirometry
Spirometry is a physiological test for assessing the functional aspect of the lungs using an objective indicator by measuring the amount of air that a patient can inhale and exhale to the maximum1. This report is written based on the guideline for pulmonary function test (PFT), recently released by The Korean Academy of Tuberculosis and Respiratory Diseases, which can be used on a clinical field2.
There are several types of PFTs. These tests cannot be used to diagnose a certain disease on their own. However, PFTs show unique features of a disease, and thus are helpful for diagnosing a disease at an early stage or for assessing the clinical stage or prognosis of the disease. These tests can help to monitor the response of treatment or therapeutic intervention3. PFTs should have acceptability and reproducibility4. The test technicians must ensure that subjects can undertake the test smoothly. Subjects must be instructed to meet the criteria of acceptability and reproducibility.
Spirometry is indicated for the following5. (1) Spirometry is used as a complementary tool for diagnosis of a disease. Therefore, spirometry helps make a diagnosis by differentiating between obstructive lung diseases, such as asthma or chronic obstructive pulmonary disease (COPD), and restrictive lung disorders, such as interstitial pneumonia. (2) Spirometry is used to identify respiratory impairment caused by occupational factors at an early stage. If the impairment is fixed, it provides objective data for the disability grade and its compensation. (3) Spirometry is used to evaluate the prognostic prospects or the response to treatment of a specific disease by serial tests. (4) Spirometry is used for preoperative evaluation to predict respiratory complications of surgery. (5) Spirometry is also used for management of national health. (6) Finally, spirometry is used for screening patients with pulmonary functional abnormalities among smokers.
The absolute contraindications of spirometry are patients with recent eye surgery, open heart surgery, laparotomy, stroke, cardiac arrest, myocardial infarction, pneumothorax, retinal detachment, and aortic aneurysm within 3 months. Other contraindications are patients with hyperventilation, diseases in which maximal ventilatory effort can be problematic (Moyamoya disease, repetitive spontaneous pneumothorax) or those with current respiratory infection, such as tuberculosis or their families were exposed to such infection, those with massive hemoptysis in the past 1 month, and those whose systolic blood pressure exceeds 200 mm Hg or diastolic blood pressure exceeds 140 mm Hg.
The relative contraindications of spirometry are patients with experience of urinary incontinence, those with chest or abdominal pain, those who have pain inside the mouth or on the face when biting the mouth piece, and those with dementia or decreased consciousness.
1. Test methods
Subjects should have accurate measurements of height and weight taken before spirometry. Additionally, the history of smoking, recent illness, and medications should be determined. Subjects are also asked whether they avoided any certain requests before the test, and they are asked to loosen tight clothing and to remove dentures4,6
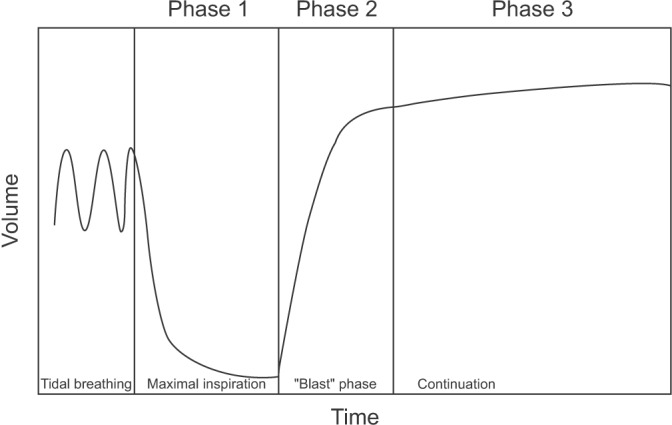
For the testing procedure, subjects are provided clear instructions and should be encouraged to breathe well following the instructions by each phase (Figure 1). At phase 1 of maximal inspiration, subjects are encouraged to take the deepest breath that they are able to. At phase 2 of the blast phase, subjects are asked to blast air into the spirometer as hard as possible, preferably for at least 6 seconds. At phase 3 of continuation, subjects are asked to maintain exhalation for at least 6 seconds (>3 seconds for children younger than 10 years old).
Figure 1. Forced expiration implementation method.
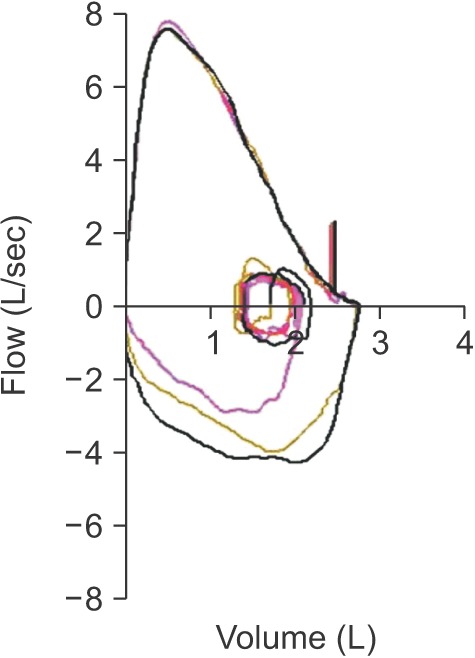
Subjects undergoing spirometry are asked to inhale the air quickly up to a total lung capacity (TLC) at once. Without any hesitation, subjects are then asked to exhale as hard as possible until they cannot exhale any longer. Thereafter, they inhale immediately as rapidly as possible. While conducting inhalation-exhalation with the maximum breathing capacity, a graph can be plotted with volume (X axis) and flow (Y axis), and this is called the flow-volume curve (Figure 2).
Figure 2. Flow-volume curve of a normal person.
2. Quality control
1) Acceptability
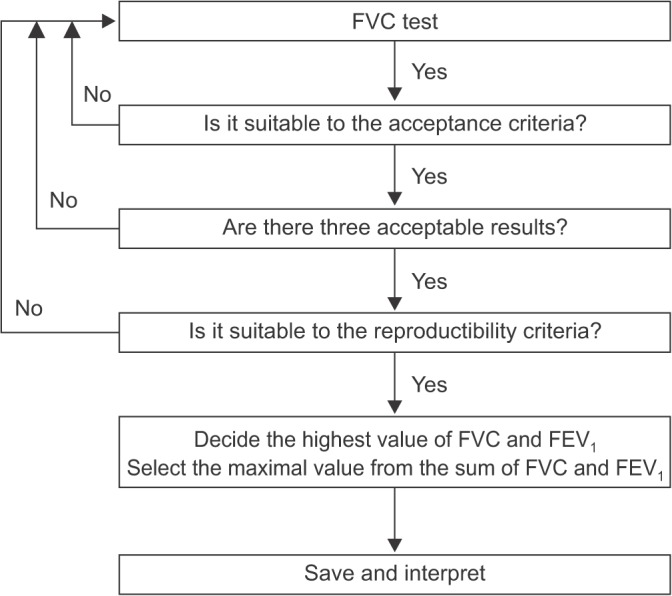
Acceptable spirometry testing needs to be conducted three times by an acceptable and reproducible method for determining forced vital capacity (FVC). FVC is obtained from a maximally forced expiratory effort. The tester must be able to verify the acceptability of the test. A testing method that is improperly conducted may be misinterpreted as a disease. Acceptability can be determined by direct confirmation on the flow-volume curve and the volume-time curve. An acceptable method is defined when the peak is sharp in the flow-volume curve and the exhalation time should be longer than 6 seconds in the volume-time curve. Two or three test curves satisfying acceptability are used for analysis.
2) Reproducibility
Reproducibility of the PFT is determined by comparing the values of FVC and forced expiratory volume at 1 second (FEV1) between tests, and should meet the following criteria. (1) The difference between the highest values of two FVCs should be within 5% or 150 mL. (2) When FVC is less than 1.0 L, the difference between the highest two values must be within 100 mL. (3) The difference between the two highest values of FEV1 should also be within 150 mL. (4) The highest FVC and FEV1 may be obtained from each different test5,7,8. Until the results of three tests meet the criteria of reproducibility, the test should be repeated up to eight times. When a subject cannot continue the test or does not want to do any more tests, and when good results are regarded as difficult to obtain, even if more tests are performed, the best three tests are selected (Figure 3)5.
Figure 3. Flow chart of acceptability and reproducibility. FVC: forced vital capacity; FEV1: forced expiratory volume at 1 second.
3) Selection of test values
According to the method described above, the scores of spirometry are evaluated on the basis of acceptability and reproducibility. The selection criteria are shown in Table 1.
Table 1. Selection of spirometry test values.
| Score | Adequate number of curves | Reproducibility gap* between two largest values | |
|---|---|---|---|
| A | 3 | and | <100 mL |
| B | 3 | and | <150 mL |
| C | 2 | and | <250 mL |
| D | 1 | ||
| F | 0 |
A and B are possible to interpret; C and D are possible to interpret with caution; F is impossible to interpret. When determined as “possible to interpret,” the values of FEV1 and FVC are adopted from the largest values. Each values of FVC and FEV1 may be obtained at different curves.
*Gap between the largest value minus the second largest value.
FVC: forced vital capacity; FEV1: forced expiratory volume at 1 second.
3. Interpretation
Interpretation of spirometry should be clear, concise, and informative, but a simple description as normal or abnormal only is not helpful. Additionally, this test should have optimal quality before interpretation of spirometry.
Even when the result of the test is not appropriate, useful information about the patient may be provided. The interpreter should be aware of the potential problem of the test performed and the possible inaccuracy of the interpretation. Most inappropriately performed spirometry tests are due to test subject-related factors. Therefore, an appropriate test performance can be ensured by carefully observing the subjects and the volume-time curve, as well as the flow-volume curve, during the test. Additionally, when interpreting spirometry results, they should be considered together with the clinical findings of symptoms. Those include respiratory symptoms, such as cough and dyspnea, past medical history, smoking history, examination results, and chest radiology reports.
Generally, spirometry provides the following important information. (1) FVC is defined as the total expiratory volume from one time of a maximally forced expiration maneuver. (2) FEV1 is defined as expiratory volume that has been exhaled at the end of the first second of a maximally forced expiration maneuver. (3) FEV1/FVC (FEV1 ratio) is defined as the ratio of FEV1 and FVC and is also expressed as a percentage. (4) Forced expiratory flow during the middle half of FVC (25%–75%) is defined as the average forced expiratory flow of 50% during the middle half of the test, except for the measurement of 25% at the beginning of the test and at the end of FVC. (5) Peak expiratory flow is defined as the maximal flow that is achieved during forced expiration. (6) Maximal voluntary volume, which is known as maximum voluntary ventilation, is a measure of the maximum volume of air that can be inhaled and exhaled within 1 minute by the maximal forced voluntary breathing of a patient. This is performed over a 12 or 15 second time period before being extrapolated to a value for 1 minute.
Interpretation of spirometry requires comparison between values that are measured from subjects and those that are measured in normal healthy subjects. Normal values of spirometry may differ depending on the physical requirements of the subjects, such as the race, sex, age, height and weight, measurement conditions, statistical methods, and socioeconomic or epidemiological requirements. Korea uses the Morris Asian Quanjer equation, described by Morris et al. in 19719, or normal predictive values of spirometry in the Korean population, which were based on 2001 National Health and Nutrition Examination Survey10.
Reference values vary by each proposed guideline for diagnostic criterion for ventilatory defects. A fixed value as the reference value can be a standard indicator for simple, independent clinical application. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define COPD as FEV1/FVC less than 0.7 from a post-bronchodilator test11. However, when considering FEV1/FVC less than 0.7 as the baseline for obstructive ventilatory defects, because FEV1/FVC decreases as age increases, false-positivity is likely to occur. False-positivity can result in the wrong diagnosis for obstructive pulmonary disease in healthy, older subjects who have never been exposed to risk12. When the same criteria are applied to young subjects, they may be diagnosed as being normal, even though they have obstructive pulmonary disease13. Therefore, a reference point of obstructive lung disease in young subjects, if they have clinical symptoms and a medical history, can be increased to FEV1/FVC <0.75–0.8013.
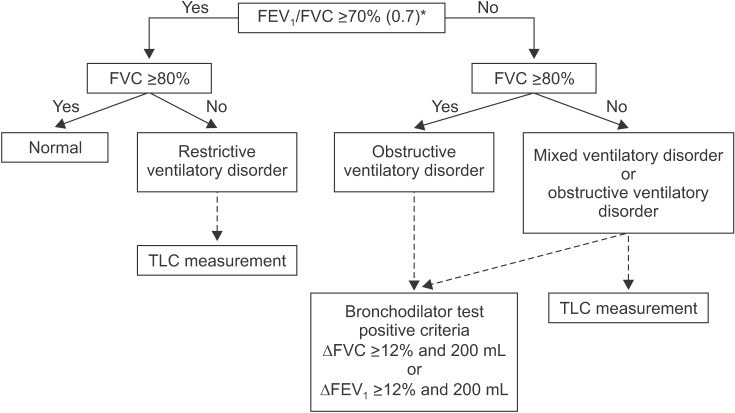
To overcome these problems, the American Thoracic Society and the European Respiratory Society defined the value corresponding to the lower fifth percentile of spirometry in the same age normal group that is 95 percentile method as the criteria of ventilation disorders as the lower limit of normal14. The American Thoracic Society and European Respiratory Society also recommend using vital capacity (VC) instead of FVC in the interpretation of spirometry14. VC is generally measured as greater than FVC. Additionally, VC is more accurate than FVC when the airway is flexible, such as in the case of emphysema, because it can diagnose airway obstruction with more certainty. However, our guideline adopts FVC instead of VC for interpretation in most other guidelines because the measurement of VC at spirometer is impossible. Ventilatory disorders can be divided into three main types. Obstructive ventilatory disorders occur when maximum flow rate is reduced compared with maximal volume, FVC, during a forced exhalation maneuver due to airway obstruction. When FEV1/FVC is less than 0.7 in spirometry, it is interpreted as having obstructive ventilatory defects (Figure 4). For the initial stage of obstructive pulmonary disease, initial forced expiratory flow remains normal, but late forced expiratory flow is decreased. Therefore, in this situation, FEV1/FVC may be maintained as normal. FEV1 is reduced when obstructive pulmonary disease further progresses causing severe airway obstruction. Representative diseases of obstructive pulmonary disease include asthma and COPD.
Figure 4. Flow chart of interpreting spirometry. *Possible to raise upward to 0.75–0.8 in young age group. FEV1: forced expiratory volume at 1 second; FVC: forced vital capacity; ΔFVC: change of FVC; ΔFEV1: change of FEV1; TLC: total lung capacity.
Restrictive ventilation disorders are characterized by a reduced TLC with normal FEV1/FVC (Figure 4). Spirometry can confirm that FVC is reduced, while FEV1 may be reduced secondarily to a reduction in FVC, or relatively maintains as normal. As a result, FEV1/FVC is normal or may increase slightly. FVC can also be reduced when a subject does not perform a full inspiratory–expiratory effort, whereas TLC is maintained as normal. Therefore, when restrictive ventilatory disorders are suspected when FVC is reduced as shown by spirometry, measurement of TLC is necessary for accurate diagnosis14. Pulmonary fibrosis, chest wall disease, or neuromuscular diseases can show restrictive ventilatory defects. Measurement of diffusing capacity is necessary to differentiate pulmonary parenchyma disease, chest wall disease, and neuromuscular disease.
Mixed ventilation disorders occur when obstructive and restrictive ventilatory defects occur together, in which FEV1/FVC decreases, as well as TLC. Obstructive or restrictive ventilatory defects can show a decrease in FVC from spirometry. Therefore, measurement of TLC is required to accurately determine the concurrence of restrictive and obstructive ventilatory defects (Figure 4). Mixed ventilatory defects are observed in fibrothorax accompanied by tuberculosis sequelae-induced airway obstruction or in smokers with COPD accompanied by pulmonary fibrosis.
PFT values in classification of lung function impairment show an association with daily life performance, severity of disease, and prognosis of patients with respiratory diseases. However, these values cannot predict exact symptoms or prognosis of individuals. When residual volume is increased, which leads to a reduction in FVC in obstructive pulmonary diseases, FEV1/FVC may not decrease at all. Therefore, FEV1/FVC helps to determine the presence of airway obstruction, but is not appropriate for identifying the severity of obstructive ventilation disorders.
To determine the severity of airway obstruction, a percentage of the predicted normal value of FEV1 is used. The severity of obstructive pulmonary diseases is shown in Table 211,14,15,16. The severity of restrictive ventilation disorders is related to the degree of pulmonary parenchyma disease and neuromuscular disease, but it cannot reflect the condition of the patient. Classification of the severity of restrictive ventilation disorders uses VC and TLC17. However, the American Thoracic Society and European Respiratory Society recommended using FEV1 for classification of the severity of all ventilation impairment, including restrictive ventilation disorders, in 2005 (Table 3).
Table 2. Classification of severity of obstructive ventilation disorders.
| Degree of severity (% of predicted FEV1) | GOLD11 | ATS/ERS14 | NICE15 | SEPAR/ALAT16 |
|---|---|---|---|---|
| Mild | ≥80 | ≥70 | 50–80 | ≥80 |
| Moderate | 50–79 | 60–69 | 30–49 | 50–79 |
| Moderately severe | - | 50–59 | - | - |
| Severe | 30–49 | 35–49 | <30 | 30–49 |
| Very severe | <30 | <35 | - | <30 |
FEV1: forced expiratory volume at 1 second; GOLD: Global Initiative for Chronic Obstructive Lung Disease; ATS: American Thoracic Society; ERS: European Respiratory Society; SEPAR: Spanish Society of Pulmonology and Thoracic Surgery; ALAT: Latin American Thoracic Society; NICE: National Institute for Health and Clinical Excellence.
Table 3. Classification of severity of restrictive ventilation disorders.
| Degree of severity | ATS17 (% of predicted VC) | ATS17 (% of predicted TLC) | ATS/ERS14 (% of predicted FEV1) |
|---|---|---|---|
| Mild | <LLN but ≥70 | <LLN but ≥70 | >70 |
| Moderate | 60–69 | 60–69 | 60–69 |
| Moderately severe | 50–59 | <60 | 50–59 |
| Severe | 34–49 | - | 35–49 |
| Very severe | <34 | - | <35 |
ATS: American Thoracic Society; VC: vital capacity; TLC: total lung capacity; ERS: European Respiratory Society; FEV1: forced expiratory volume at 1 second; LLN: lower limit of normal.
It can be regarded as respiratory failure in severe chronic lung diseases if the patient has been diagnosed as an underlying pulmonary disease for more than 1 year and if the lung disability of the patient is fixed in spite of sufficient treatment over the past 2 months. Table 414,17 shows the Criteria for Pulmonary Disability-Grading system provided by the Ministry of Health and Welfare in 201518.
Table 4. Criteria for decision of pulmonary disability grading in Korea18.
| Disability grade | Degree of disability |
|---|---|
| Class 1: Grade 1 | A person with severe dyspnea requiring oxygen therapy, even in a stable state, because of chronic dysfunction of the respiratory system, such as the lungs or bronchus (1) In a normal condition, FEV1 or diffusing capacity (DLCO) is ≤25% of normal predictive values or (2) In a normal condition, resting PaO2 is ≤55 mm Hg in a stable state |
| Class 1: Grade 2 | A person who maintains a tracheostomy tube and survives for 24 hours on a ventilator because of chronic respiratory disease |
| Class 2 | A person with dyspnea during normal physical activity at home because of chronic dysfunction of the respiratory system, such as the lungs or bronchus (1) In a normal condition, FEV1 or DLCO is >25% to ≤30% of normal predictive values or (2) In a normal condition, resting PaO2 is >55 to ≤ 60 mm Hg |
| Class 3 | A person with dyspnea when walking on plane level because of chronic dysfunction of the respiratory system, such as the lungs or bronchus (1) Normally, FEV1 or DLCO is >30% to ≤40% of normal predictive values or (2) Normally, resting PaO2 is ≤65 mm Hg in a stable state |
| Class 5: Grade 1 | A person who had lung transplantation |
| Class 5: Grade 2 | A person with bronchopleural fistula |
FEV1: forced expiratory volume at 1 second.
The descending curve of the normal air flow–volume curve forms a relatively straight line after reaching a peak. However, in obstructive pulmonary disease, the maximal forced expiratory flow rate is reduced and the descending curve shifts sharply downward with an increase in downward concavity after reaching the peak. Lung capacity is mainly reduced, but the airflow rate does not decrease much in restrictive ventilation disorders. Therefore, the flow-volume curve forms a tall and narrow shape. The maximal forced expiratory flow rate is relatively maintained, and the descending curve forms a straight line with a steep slope.
If a plateau is formed from inhalation or exhalation in a flow-volume curve, stenosis of the upper airway or central airway is suspected. If this condition is clinically suspected, its progress should be observed and the airway should be evaluated. Also, further evaluation including imaging study and bronchoscopy is recommended in this situation.
Bronchodilator Test
The bronchodilator test is a method for measuring the changes in lung capacity after inhaling a short-acting bronchodilator drug that dilates the airway. When an obstructive ventilatory defect is observed, this test helps to diagnose and evaluate asthma and COPD by measuring reversibility induced by the bronchodilator drug19.
1. Test methods
The bronchodilator test is conducted to determine the reversibility of airflow limitation after administration of a short-acting bronchodilator drug as a part of PFTs. Selection of the type of drug, dosage, and the route of administration to be used at this time are decided in accordance with what needs to be obtained from the bronchodilator reversibility test. If the purpose of the bronchodilator reversibility test is to determine if addition of a new drug to the regulated treatment can improve lung function of the patient, medication should be administered on a regular basis before the test can be continued. However, if the purpose of this test is to determine the reversibility of airflow limitation, the test should be conducted after suspending the medication for a certain period.
2. Test procedure
In the albuterol (short-acting β2-bronchodilator)19 bronchodilator test, the subject fully exhales slowly, and sprays an albuterol metered dose of 100 µg (1 puff) while biting a valved chamber. The subject then slowly and deeply inhales until reaching TLC over 3–5 seconds, holds the breath for 5–10 seconds, and exhales. This procedure is repeated four times (total 400 µg of albuterol), at intervals of 30 seconds. However, if there is any concern of affecting the pulse rate of the subject or causing occurrence of hand tremors, the dose may be decreased to 200 µg. After inhalation of the last medication, the spirometry test is conducted again between 10–20 minutes20.
3. Interpretation
A positive response to a bronchodilator is defined as follows. (1) Generally, a positive response is defined as an increase of ≥12% and ≥200 mL as an absolute value compared with baseline in either FEV1 or FVC. (2) When expressing a bronchodilator response with a percentage of the pre-inhalation indicator, it tends to be calculated as larger when the baseline value of VC is smaller, even if the amount of variation is the same. (3) When the bronchodilator response is positive, it suggests asthma in general. This conclusion is reached because the increase in post-inhalation flow rate and volume in patients with asthma is greater than that in patients with COPD. However, excluding COPD with the finding of a positive bronchodilator response only is difficult.
Recent Global Initiative for Asthma-GOLD guidelines state that in the case of a large increase of ≥12% and ≥400 mL in FEV1 after bronchodilator inhalation, the possibility of COPD is low, whereas the situation is more reasonable for asthma or asthma-COPD overlap syndrome21. Regardless of an increase in exhalation time after bronchodilator inhalation, when only FVC is increased (≥12% and ≥200 mL of the baseline), it may also be considered as a sign of bronchiectasis.
The information obtained from one time test can be useful with comparison with normal reference. However, performing a follow-up observation with repetitive spirometry, conducting treatment in specific individuals, and observation over time can provide clinically important information.
Acknowledgments
This work was supported by the Korean Academy of Tuberculosis and Respiratory Disease. The authors thank Kwang Ho In, M.D., Korea University and Jang Won Sohn, M.D., Hanyang University for their review.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.The BTS COPD Consortium. Spirometry in practice. A practical guide to using spirometry in primary care. 2nd ed. London: The BTS COPD Consortium; 2005. [Google Scholar]
- 2.The Korean Academy of Tuberculosis and Respiratory Diseases. 2016 Guideline of pulmonary function test [Internet] Seoul: The Korean Academy of Tuberculosis and Respiratory Diseases; 2016. [cited 2016 Nov 1]. Available from: http://www.lungkorea.org/bbs/index.html?code=guide&category=&gubun=&page=1&number=3487&mode=view&keyfield=&key= [Google Scholar]
- 3.Gold WM, Koth LL. Chapter 25. Pulmonary function testing. In: Broaddus VC, Mason RJ, Ernst JD, King TE, Lazarus SC, Murray JF, et al., editors. Murray and Nadel's textbook of respiratory medicine. 6th ed. Philadelphia: Saunders/Elsevier; 2015. pp. 407–435.e18. [Google Scholar]
- 4.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 5.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 6.Moore VC. Spirometry: step by step. Breathe. 2012;8:232–240. doi: 10.1183/20734735.5217-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. The Fifth Korea National Health and Nutrition Examination Survey (2010-2012) Manual [Internet] Cheongju: Centers for Disease Control and Prevention; 2012. [cited 2016 Nov 1]. Available from: http://cdc.go.kr/CDC/contents/CdcKrContentView.jsp?cid=60948&menuIds=HOME001-MNU1130-MNU1639-MNU1749-MNU1760. [Google Scholar]
- 8.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES): respiratory health spirometry procedures manual [Internet] Atlanta: Centers for Disease Control and Prevention; 2011. [cited 2016 Nov 1]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Spirometry_Procedures_Manual.pdf. [Google Scholar]
- 9.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis. 1971;103:57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- 10.Choi JK, Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis. 2005;58:230–242. [Google Scholar]
- 11.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 12.Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–1051. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 13.Cerveri I, Corsico AG, Accordini S, Niniano R, Ansaldo E, Anto JM, et al. Underestimation of airflow obstruction among young adults using FEV1/FVC <70% as a fixed cut-off: a longitudinal evaluation of clinical and functional outcomes. Thorax. 2008;63:1040–1045. doi: 10.1136/thx.2008.095554. [DOI] [PubMed] [Google Scholar]
- 14.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care [Internet] London: National Institute for Health and Care Excellence; 2004. [cited 2016 Nov 1]. Available from: http://www.nice.org.uk/guidance/cg12. [Google Scholar]
- 16.Peces-Barba G, Barbera JA, Agusti A, Casanova C, Casas A, Izquierdo JL, et al. Diagnosis and management of chronic obstructive pulmonary disease: joint guidelines of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Latin American Thoracic Society (ALAT) Arch Bronconeumol. 2008;44:271–281. [PubMed] [Google Scholar]
- 17.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health and Welfare. The criteria for decision of pulmonary disability-grading: notification No. 2015-188 [Internet] Sejong: Ministry of Health and Welfare; 2015. [cited 2016 Nov 1]. Available from: http://www.mohw.go.kr/front_new/jb/sjb0406ls.jsp?PAR_MENU_ID=03&MENU_ID=030406&page=30. [Google Scholar]
- 19.National Center for Health Statistics; Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES): respiratory health bronchodilator procedure manual [Internet] Atlanta: Centers for Disease Control and Prevention; 2008. [cited 2016 Nov 1]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/Bronchodilator.pdf. [Google Scholar]
- 20.van Schalkwyk EM, Schultz C, Joubert JR, White NW South African Thoracic Society Standards of Spirometry Committee. Guideline for office spirometry in adults, 2004. S Afr Med J. 2004;94(7 Pt 2):576–587. [PubMed] [Google Scholar]
- 21.Global Initiative for Asthma. Diagnosis of diseases of chronic airflow limitation: 2015 asthma, COPD and asthma-COPD overlap syndrome (ACOS) [Internet] Global Initiative for Asthma; 2015. [cited 2016 Oct 12]. Available from: http://ginasthma.org/asthma-copd-and-asthma-copd-overlap-syndrome-acos. [Google Scholar]