Abstract
Sialidase cleaves sialic acids on the extracellular cell surface as well as inside the cell and is necessary for normal long-term potentiation (LTP) at mossy fiber-CA3 pyramidal cell synapses and for hippocampus-dependent spatial memory. Here, we investigated in detail the role of sialidase in memory processing. Sialidase activity measured with 4-methylumbelliferyl-α-d-N-acetylneuraminic acid (4MU-Neu5Ac) or 5-bromo-4-chloroindol-3-yl-α-d-N-acetylneuraminic acid (X-Neu5Ac) and Fast Red Violet LB was increased by high-K+-induced membrane depolarization. Sialidase activity was also increased by chemical LTP induction with forskolin and activation of BDNF signaling, non-NMDA receptors, or NMDA receptors. The increase in sialidase activity with neural excitation appears to be caused not by secreted sialidase or by an increase in sialidase expression but by a change in the subcellular localization of sialidase. Astrocytes as well as neurons are also involved in the neural activity-dependent increase in sialidase activity. Sialidase activity visualized with a benzothiazolylphenol-based sialic acid derivative (BTP3-Neu5Ac), a highly sensitive histochemical imaging probe for sialidase activity, at the CA3 stratum lucidum of rat acute hippocampal slices was immediately increased in response to LTP-inducible high-frequency stimulation on a time scale of seconds. To obtain direct evidence for sialic acid removal on the extracellular cell surface during neural excitation, the extracellular free sialic acid level in the hippocampus was monitored using in vivo microdialysis. The free sialic acid level was increased by high-K+-induced membrane depolarization. Desialylation also occurred during hippocampus-dependent memory formation in a contextual fear-conditioning paradigm. Our results show that neural activity-dependent desialylation by sialidase may be involved in hippocampal memory processing.
Keywords: hippocampus, imaging, rat, sialic acid, sialidase
Introduction
Sialic acid, an acidic monosaccharide, is expressed most frequently at the ends of glycans and creates a negative charge on the cell surface. Certain neural functions including memory processing depend on sialic acid bound to glycans in glycoproteins and gangliosides (1–3). Sialic acid contained in gangliosides such as the tetrasialoganglioside GQ1b2 is crucial for synaptic plasticity and hippocampal memory (4, 5). The sialic acid polymer (polysialic acid (PSA)), having a large negative charge, that is attached to neural cell adhesion molecules (NCAM) regulates neural circuit formation during memory formation and brain development (6, 7). Thus, sialic acid residues in sialylglycoconjugates are essential for hippocampal memory processing.
The sialylation level of glycans changes markedly during brain development, during acquisition processes of neurological disorders such as epilepsy, and during an inflammatory response of microglia (8–11). Sialic acid residues in sialylglycoconjugates are removed by sialidase, which is one of the regulators for the sialylation level of glycans (12). We previously found that mossy fiber (MF) terminals showed relatively intense sialidase activity in the hippocampus (13). Sialidase is necessary for normal long-term potentiation (LTP) at MF-CA3 pyramidal cell synapses and for hippocampus-dependent spatial memory (14). However, little is known about the detailed role of sialidase in memory processing.
Mammalian sialidase isozymes are designated as Neu1, Neu2, Neu3, and Neu4. All sialidase isozymes, the main subcellular locations of which are different, show enzyme activity on the plasma membrane or in the extracellular space (11, 12, 15). Because exogenous sialidase extracellularly applied to the hippocampus influences many neural functions including memory and synaptic plasticity (16–20), endogenous sialidase would also play a crucial role in hippocampal memory processing on the membrane surface. Desialylation on the cell surface by sialidase can change cell surface properties quickly and dramatically.
In the present study, we investigated the change of sialidase activity and sialic acid removal from glycans during memory processing. We previously developed a highly sensitive fluorescent probe, a benzothiazolylphenol-based sialic acid derivative (BTP3-Neu5Ac), for histochemical sialidase activity imaging (21). BTP3-Neu5Ac can monitor the rapid change of sialidase activity in mammalian tissues. In this study, we found that sialidase activity detected with BTP3-Neu5Ac at the rat CA3 stratum lucidum was immediately increased in response to LTP-inducible high-frequency stimulation. In vivo monitoring of sialic acid removal in the hippocampal extracellular space showed that desialylation was significantly enhanced during hippocampus-dependent memory formation in a contextual fear-conditioning paradigm. The results suggest that neural activity-dependent desialylation by sialidase occurs during hippocampal memory processing.
Results
Increase in sialidase activity in response to neural excitation
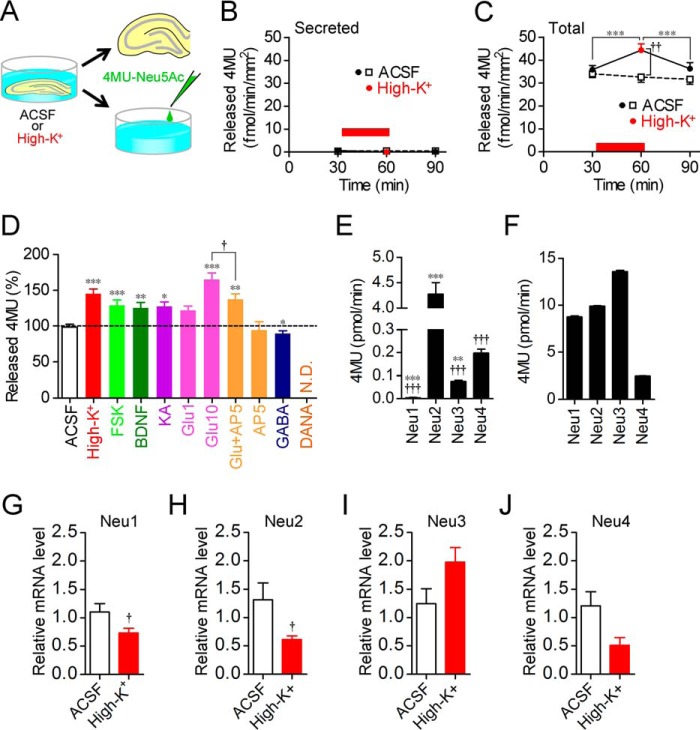
We first investigated the influence of neural excitation on sialidase activity. In rat acute hippocampal slices, sialidase activity measured using 4-methylumbelliferyl-α-d-N-acetylneuraminic acid (4MU-Neu5Ac), a fluorescent sialidase substrate for quantitative analysis of sialidase activity, was increased by high-K+-induced membrane depolarization at pH 7.3 (one-way repeated measures ANOVA; F3,21 = 33.04, p < 0.001) (Fig. 1, A and B). Increased sialidase activity returned to the basal level immediately after stimulation. If not stimulated, sialidase activity in artificial cerebrospinal fluid (ACSF; pH 7.3) was stable during the experimental period. Galactosidase activity measured using 4MU-β-d-galactopyranoside (4MU-Gal) was not increased by high-K+ stimulation (Fig. 1C).
Figure 1.
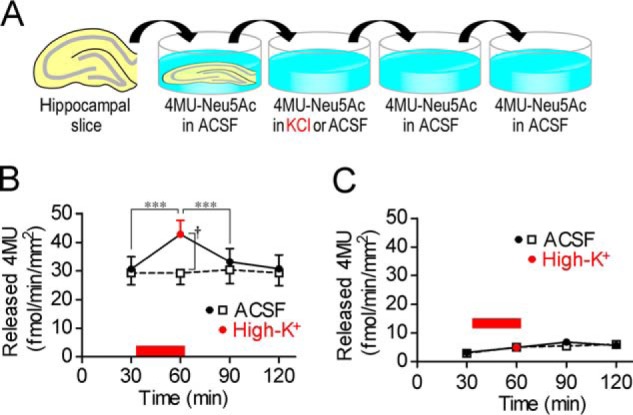
Increase in sialidase activity by neural excitation. A, schematic of sialidase activity measurement with 4MU-Neu5Ac. Rat hippocampal acute slices were incubated in ACSF or high-K+ solution (pH 7.3) containing 10 μm 4MU-Neu5Ac for 30 min. B, sialidase activity was increased by neural excitation with high-K+. High-K+ solution was applied at the time point indicated by a red bar. ACSF, n = 10; high-K+, n = 8. ***, p < 0.001 (one-way repeated measures ANOVA); †, p < 0.05 (unpaired t test). C, β-galactosidase activity was measured using 10 μm 4MU-Gal. ACSF, n = 8; high-K+, n = 8. Error bars represent S.E.
We previously developed a fluorescent histochemical imaging method for sialidase by using X-Neu5Ac and FRV LB (13). When rat acute hippocampal slices were stained in ACSF containing X-Neu5Ac and FRV LB, fluorescence was observed throughout the hippocampus (Fig. 2A). Fluorescence intensities in various hippocampal regions were increased by staining in high-K+ solution containing X-Neu5Ac and FRV LB (Fig. 2, A and B). When the staining was performed in the presence of 1 mm 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (DANA), a sialidase inhibitor, fluorescence was remarkably reduced, indicating that staining with X-Neu5Ac and FRV LB is specific for sialidase activity.
Figure 2.
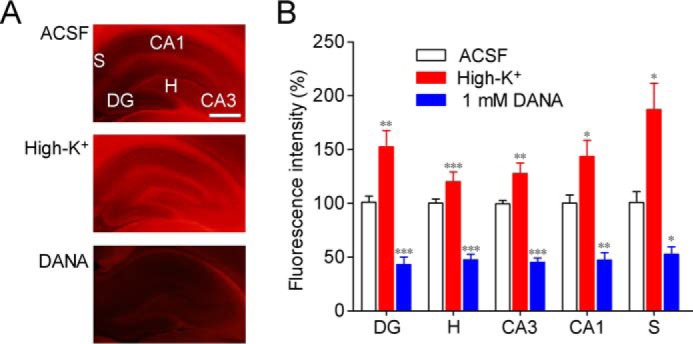
Sialidase activity imaging with X-Neu5Ac and FRV LB. A, sialidase activity in rat hippocampal acute slices was imaged using X-Neu5Ac and FRV LB in ACSF, high-K+ solution, or ACSF containing 1 mm DANA. B, relative fluorescence intensities in each region are shown. DG, dentate gyrus; H, hilus, CA, cornus ammonis, S, subiculum. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 versus control (one-way ANOVA or Kruskal-Wallis test with Dunn's multiple comparison test). Scale bar, 1 mm. Error bars represent S.E.
Factors responsible for increase in sialidase activity
To determine whether increased sialidase activity during neural excitation was caused by secreted sialidase, secreted sialidase activity was monitored by measuring the sialidase activity in incubated solutions after each incubation (Fig. 3A). Secreted sialidase activity was barely detected and was not increased by high-K+ stimulation (Fig. 3B). In contrast, total sialidase activity including cell surface sialidase activity, which was measured in hippocampal slices prepared from the same rats as those used for the secreted sialidase activity measurement, was high enough to be detected before stimulation and was increased by high-K+ stimulation (one-way repeated measures ANOVA; F2,22 = 17.0) (Fig. 3C).
Figure 3.
Factors responsible for increase in sialidase activity. A, schematic of secreted sialidase activity measurement with 4MU-Neu5Ac. B, secreted sialidase activity was not changed by neural excitation with high-K+. n = 12 each. C, total sialidase activity in slices, which were prepared from the same rats as those used for the secreted sialidase activity measurement, was monitored in the same manner as in Fig. 1. n = 12 each. ***, p < 0.001 (one-way repeated measures ANOVA); ††, p < 0.01 (unpaired t test). High-K+ solution was applied at the time point indicated by a red bar. D, sialidase activities were measured for 30 min in the presence of 100 mm KCl (high-K+; n = 8), 50 μm forskolin (FSK; n = 15), 200 ng/ml BDNF (n = 16), 5 μm kainite (KA; n = 8), 1 (n = 8) or 10 (n = 19) mm glutamate (Glu), 10 mm glutamate with 50 μm AP5 (n = 13), 50 μm AP5 (n = 8), 10 μm GABA (n = 8), or 1 mm DANA (n = 10) after measurement of basal sialidase activity levels for 30 min. The dashed line shows basal sialidase activity levels. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 versus baseline level (two-way repeated measures ANOVA); †, p < 0.05 (one-way ANOVA). N.D., not detected. E and F, cleavage of 4MU-Neu5Ac with each of the recombinant rat sialidase isozymes. C-terminal Myc-tagged rat sialidase isozymes Neu1, Neu2, Neu3, and Neu4 were expressed in C6 rat glioma cells. Each lysate equalized with the amount of Myc was incubated in ACSF (E; pH 7.3; n = 4) or sodium acetate buffer (F; pH 4.6; n = 4) containing 200 μm 4MU-Neu5Ac. The amount of hydrolyzed 4MU is shown as a bar graph. †††, p < 0.001 versus Neu2; **, p < 0.01; and ***, p < 0.001 versus Neu4 (one-way ANOVA) in E. All combinations in F had significance (p < 0.001, one-way ANOVA). G–J, quantitative measurement of Neu1 (G), Neu2 (H), Neu3 (I), and Neu4 (J) mRNA levels in hippocampal slices after incubation with ACSF or 100 mm KCl. †, p < 0.05 (unpaired t test). Error bars represent S.E.
We pharmacologically identified the signaling pathways that increase sialidase activity. Sialidase activity measured with 4-methylumbelliferyl-α-d-N-acetylneuraminic acid (4MU-Neu5Ac) was increased by forskolin, a chemical LTP inducer (22); by BDNF, a regulator of synaptic plasticity (23); and by activation of glutamate receptors using kainate and glutamate (Fig. 3D). The increase in sialidase activity induced by glutamate was partially abolished by 2-amino-5-phosphonopentanoic acid (AP5), an NMDAR antagonist, although AP5 alone did not have any effect on sialidase activity. In contrast, sialidase activity was decreased by GABA. We confirmed that sialidase activity measured with 4MU-Neu5Ac was completely inhibited by DANA.
Next, we identified the sialidase isozymes for which sialidase activity was detected by 4MU-Neu5Ac. C-terminal Myc-tagged rat sialidase isozymes were constructed in C6 rat glioma cells. At pH 7.3 in ACSF, 4MU-Neu5Ac was hydrolyzed preferentially by Neu2 and Neu4 and weakly by Neu1 and Neu3 (Fig. 3E). At pH 4.6, 4MU-Neu5Ac was hydrolyzed more efficiently by all sialidase isozymes compared with the neutral pH condition (Fig. 3F).
To investigate the change in sialidase expression caused by neural excitation, mRNA levels of sialidase isozymes were measured by using real time quantitative reverse transcription-polymerase chain reaction (RT-PCR). Expression levels of mRNA in acute hippocampal slices were significantly decreased in Neu1 and Neu2 by high-K+ stimulation and were not significantly changed in Neu3 and Neu4 (Fig. 3, G–J).
Influence of neural excitation on subcellular localization of sialidase
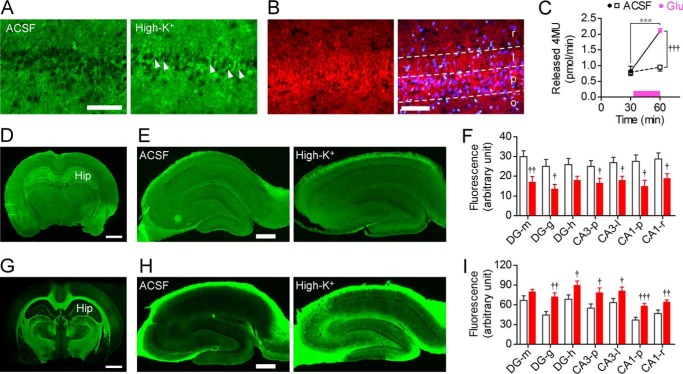
We investigated the influence of neural excitation on subcellular localization of sialidase. Hippocampal slices (400 μm in thickness) were incubated with ACSF or 100 mm KCl for 30 min and then immediately frozen with liquid nitrogen. After cutting into 20-μm-thick sections, the hippocampal slices were stained with 1 mm BTP3-Neu5Ac (pH 7.3; 27 °C). Intense fluorescence appeared in the cell membrane of pyramidal cells with neural excitation by high-K+ (Fig. 4A). Immunohistochemical staining of the hippocampus showed that Neu4 was expressed in the stratum lucidum where MFs terminate and in the stratum pyramidale where the cell bodies of pyramidal neurons are located (Fig. 4B).
Figure 4.
Influence of neural excitation on subcellular localization of sialidase activity and sialylated motif detected by MAA lectin. A, hippocampal slices (20 μm in thickness) were prepared after incubation with ACSF (left panel) or 100 mm KCl (right panel) for 30 min and then stained with 100 μm BTP3-Neu5Ac (pH 7.3). Arrowheads indicate intense fluorescence in the cell membrane of pyramidal cells. Scale bar, 100 μm. B, immunohistochemical staining of Neu4 (red). Counterstaining was performed using DAPI (blue). r, stratum radiatum; l, stratum lucidum; p, stratum pyramidale; o, stratum oriens. Scale bar, 100 μm. C, effect of 100 μm glutamate on sialidase activity measured by 4MU-Neu5Ac in cultured primary rat hippocampal astrocytes. Glutamate (Glu) was applied at the time point indicated by a bar. ACSF, n = 7; glutamate, n = 8. ***, p < 0.001 (paired t test); †††, p < 0.05 (unpaired t test). D–I, cleavage of sialic acid residues by neural excitation. Brain slices (D and G; scale bar, 2 mm) and hippocampal slices after incubation with ACSF (left) or 100 mm KCl (right) for 30 min (E and H; scale bar, 0.5 mm) were stained using FITC-labeled lectins MAA (D and E) and PNA (G and H), respectively. DG, dentate gyrus; Hip, hippocampus; m, stratum moleculare; g, stratum granulosum; h, hilus. Quantification of the binding of MAA and PNA lectins to the hippocampal slices (F, MAA, n = 11 in ACSF, n = 8 in high-K+; I, PNA, n = 18 in ACSF and high-K+). †, p < 0.05; ††, p < 0.01; and †††, p < 0.001 versus ACSF (unpaired t test). Error bars represent S.E.
We also investigated the influence of glutamate on sialidase activity in rat primary cultured astrocytes. Sialidase activity measured with 4MU-Neu5Ac was significantly increased by incubation with 100 μm glutamate for 30 min (Fig. 4C).
Influence of neural excitation on hippocampal sialylation level
Maackia amurensis agglutinin (MAA) and peanut agglutinin (PNA) mainly recognize α2–3-linked sialic acid (Neu5Acα2–3Gal-β(1–3)-GalNAc) and galactose residue (Gal-β(1–3)-GalNAc), respectively. The results of lectin staining showed that MAA bound abundantly to the hippocampus compared with the binding in other brain regions (Fig. 4D). The binding of MAA to the hippocampus was attenuated after neural excitation with high-K+ (Fig. 4, E and F). In contrast, PNA showed weak binding to the hippocampus (Fig. 4G). The binding of PNA to the hippocampus was strengthened after neural excitation (Fig. 4, H and I).
Spatiotemporal dynamics of sialidase activity by LTP-inducible high-frequency stimulation
Because forskolin induced an increase in sialidase activity, we investigated the effect of electrophysiological LTP induction stimuli on sialidase activity. BTP3-Neu5Ac can detect a change of sialidase activity on a living tissue directly (21). To elucidate the spatiotemporal dynamics of sialidase activity during LTP induction, the rapid change in sialidase activity in response to LTP-inducible high-frequency stimulation was measured by using BTP3-Neu5Ac. When the hippocampal CA3 region was stained with BTP3-Neu5Ac (pH 7.3), the stratum lucidum showed relatively intense fluorescence in the CA3 region (Fig. 5A). BTP3-Neu5Ac did not cause fluorescence in the presence of DANA, indicating that BTP3-Neu5Ac specifically detected sialidase activity (Fig. 5B). The fluorescence intensities at the CA3 stratum lucidum were rapidly increased by an LTP-inducible high-frequency stimulus of 5, 25, or 100 Hz for 5 s (Fig. 5, C and D) with a latency of less than 2 s. The increase in sialidase activity by 25-Hz stimulation was attenuated by inhibition of action potentials with tetrodotoxin (TTX), a voltage-gated sodium channel blocker.
Figure 5.
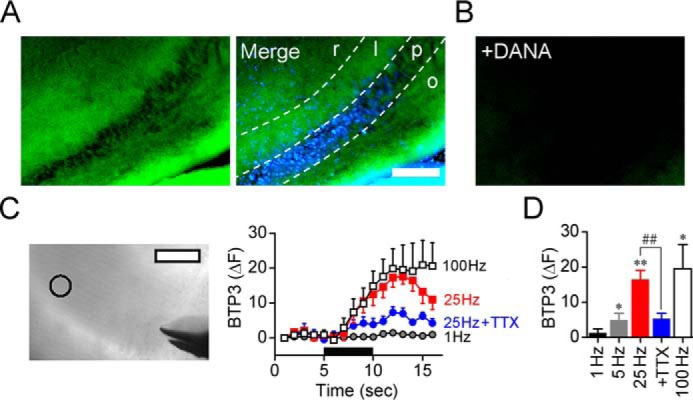
Spatiotemporal dynamics of sialidase activity in LTP induction. A, sialidase activity in the hippocampal CA3 region was visualized with 100 μm BTP3-Neu5Ac (pH 7.3) by using rat acute hippocampal slices (400 μm in thickness). BTP3-Neu5Ac, green; DAPI, blue. Scale bar, 200 μm. r, stratum radiatum; l, stratum lucidum; p, stratum pyramidale; o, stratum oriens. B, BTP3-Neu5Ac staining with 10 mm DANA. C, fluorescence intensity was monitored at the CA3 stratum lucidum (open circle in transmitted light image). Scale bars, 100 μm. The black bar represents a time period in which stimulation (1–100 Hz) was applied to the MF. D, the bar graph shows averaged fluorescence intensities measured after 5 s of stimulation. 1 Hz, n = 5; 5 Hz, n = 5; 25 Hz, n = 7; 25 Hz + 1 μm TTX, n = 7; 100 Hz, n = 5. *, p < 0.05; and **, p < 0.01 versus baseline level (paired t test); ##, p < 0.01 (unpaired t test). Error bars represent S.E.
Desialylation during neural excitation
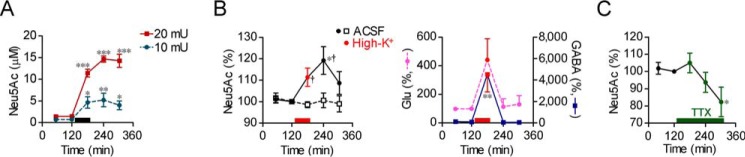
For in vivo monitoring of desialylation on the cell surface, the amount of free sialic acid collected from the hippocampal extracellular space was measured using in vivo microdialysis. To confirm that free sialic acid collected through the dialysis membrane is increased by enhancement of sialidase activity, the hippocampus was perfused with ACSF and then injected with exogenous sialidase from Arthrobacter ureafaciens (AUSA) through a cannula placed beside the dialysis membrane. Neu5Ac, the main molecular species of sialic acid, in the perfusate was increased depending on the concentration of AUSA (one-way repeated measures ANOVA; 10 milliunits, F4,20 = 9.07, p < 0.001; 20 milliunits, F4,12 = 57.7, p < 0.001) (Fig. 6A).
Figure 6.
In vivo sialidase activity measurement. A, free Neu5Ac collected from the hippocampal extracellular space was monitored using in vivo microdialysis. AUSA was injected into the hippocampus at the time points indicated by a black bar. 10 milliunits, n = 6; 20 milliunits, n = 4. B, effect of high-K+ stimulation (red bars and points) on hippocampal extracellular free Neu5Ac, glutamate (Glu), and GABA levels (n = 10 each). ACSF, n = 4. †, p < 0.05 versus ACSF (unpaired t test with Welch's correction). C, effect of 1 μm TTX on extracellular free Neu5Ac level. n = 5. A–C, *, p < 0.05; **, p < 0.01, and ***, p < 0.001 versus basal level (one-way repeated measures ANOVA). Error bars represent S.E.
To obtain direct evidence for sialic acid removal by neural excitation, we evaluated the effect of neural excitation on desialylation. The Neu5Ac level in the perfusate was increased by perfusion with high-K+ solution for 30 min (one-way repeated measures ANOVA; F4,36 = 3.65, p < 0.001) but was stable by perfusion with ACSF (Fig. 6B). Glutamate and GABA levels in the perfusate, which are indices of neural excitation, were also increased by high-K+ (one-way repeated measures ANOVA; glutamate, F4,36 = 3.96, p < 0.01; GABA, F4,36 = 7.34, p < 0.001) (Fig. 6B). In contrast, the Neu5Ac level was decreased by perfusion with TTX (one-way repeated measures ANOVA; F4,16 = 3.14, p < 0.05) (Fig. 6C).
Desialylation during hippocampus-dependent memory formation
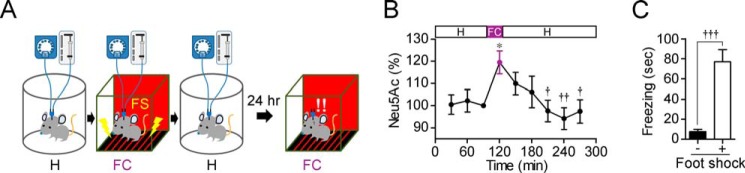
We next evaluated desialylation during hippocampus-dependent memory formation in a contextual fear-conditioning paradigm. In contextual fear conditioning, rats learn the association between a specific environment (context) and an aversive stimulus such as a foot shock. Under the condition of hippocampal perfusion with ACSF, rats were transferred from a home cage to a novel context (foot shock chamber) and presented with a foot shock (1 s, 0.2 mA) every 20 s for 15 min and then returned to the home cage (Fig. 7A). The Neu5Ac level in the hippocampal extracellular space was significantly increased by contextual fear conditioning and decreased to the basal level after the conditioning (one-way repeated measures ANOVA; F8,72 = 3.11, p < 0.01) (Fig. 7B). The rats were transferred to the context again without foot shock 24 h later, and their freezing behavior was observed. Acquisition of contextual fear memory was confirmed by an increase in the freezing time in the foot shock group (Fig. 7C).
Figure 7.
Neu5Ac cleavage during contextual fear conditioning. A, in vivo microdialysis was performed during contextual fear conditioning. H, home cage; FC, foot shock chamber; FS, foot shock. B, Neu5Ac level in the hippocampal extracellular space was monitored during contextual fear conditioning. n = 13. *, p < 0.05 versus baseline level; †, p < 0.05; and ††, p < 0.01 versus 120 min (one-way repeated measures ANOVA). C, 24 h after the conditioning, freezing behaviors in the context were compared in the foot shock-delivered group (+; n = 13) and non-delivered group (−; n = 8). †††, p < 0.001 (unpaired t test with Welch's correction). Error bars represent S.E.
Discussion
In the present study, we first investigated the change of sialidase activity by neural activity. Sialidase activity measured with 4MU-Neu5Ac or X-Neu5Ac and FRV LB was increased by high-K+-induced membrane depolarization. Sialidase activity was also increased by chemical LTP induction with forskolin and activation of BDNF signaling, non-NMDARs, or NMDARs. Because these neural activations are involved in calcium signaling, one of the triggers for increment in sialidase activity would be intracellular Ca2+ elevation. In contrast, sialidase activity was decreased by GABA receptor activation, suggesting that sialidase activity is up-regulated and down-regulated depending on the neural activity state.
Sialidase activity in incubated solutions was barely detected and was not increased by high-K+ stimulation. Expression levels of sialidase mRNA were significantly decreased in Neu1 and Neu2 by high-K+ stimulation and not significantly changed in Neu4. Although the Neu3 mRNA level showed a tendency to increase (no significance), BTP3-Neu5Ac hardly detected Neu3 activity at pH 7.3 (14). Thus, we concluded that the increase in sialidase activity with neural excitation appears to be caused not by secreted sialidase or by an increase in sialidase expression.
Sialidase changes its subcellular localization with extracellular stimulation. The localization of Neu3 rapidly changes in response to stimulation with epidermal growth factor (24). Neu1 expressed in T lymphocytes is also relocated to the cell surface by activation with concanavalin A (25). Because intense sialidase activity appeared in the cell membrane of hippocampal CA3 pyramidal cells after neural excitation, the increase in sialidase activity on the surface of neurons may be caused by a change in the subcellular localization of sialidase in response to neural activities. Fast monitoring of sialidase activity with BTP3-Neu5Ac indicated that sialidase activity is immediately increased in response to LTP-inducible high-frequency stimulation with a delay of a few seconds. Sialidase activity may be regulated on the time scale of seconds.
The glial-neuronal signaling is regulated by activation of the glutamate receptor in astrocytes. We investigated the effect of glutamate on sialidase activity in astrocytes. Because glutamate increases sialidase activity in rat primary cultured astrocytes, astrocytes are also involved in the neural activity-dependent increase in sialidase activity.
In the present study, we investigated the influence of neural excitation on the hippocampal sialylation level. The results of lectin staining showed that the binding of MAA, which recognizes sialylated moieties, to the hippocampus was attenuated after neural excitation. In contrast, the binding of PNA, which recognizes unsialylated moieties, to the hippocampus was strengthened after neural excitation. These findings suggest that the increase in sialidase activity with neural excitation causes desialylation. Because desialylation caused by neural excitation was observed in not only the pyramidal cell layer but also the whole hippocampal region, multiple cell types as well as neurons may be affected by neural activity-dependent desialylation.
We also investigated the sialic acid removal from glycans during memory processing. The amount of free sialic acid collected from the hippocampal extracellular space by using in vivo microdialysis was increased depending on the concentration of exogenously applied sialidase, indicating that the desialylation level on the cell surface can be estimated by the amount of extracellular free sialic acid. By using this in vivo desialylation monitoring method, we found that the desialylation level was increased by high-K+ stimulation. We conclude that increment in sialidase activity by neural excitation is sufficient for sialic acid removal from glycans on the extracellular cell surface. In contrast, the desialylation level was decreased by reducing the spontaneous firing rate of neurons with TTX, indicating that sialidase activity is up-regulated even by spontaneous spikes. Removal of sialic acid residues also occurred during hippocampus-dependent memory formation in the contextual fear-conditioning paradigm. Because sialidase is necessary for normal LTP at MF-CA3 pyramidal cell synapses and hippocampal memory (14), neural activity-dependent desialylation by sialidase would be essential for hippocampal synaptic plasticity and memory.
At neutral pH, 4MU-Neu5Ac and BTP3-Neu5Ac were hydrolyzed preferentially by Neu2 and Neu4 in ACSF (14). Neu4 is expressed predominantly in the brain, particularly in the hippocampus (26, 27). Our immunohistochemical staining showed that Neu4 was expressed in the stratum lucidum and stratum pyramidale in the hippocampus. Neu4 has broad pH dependence with an optimal pH of 3.5–4.6 and shows sialidase activity even at neutral pH (28, 29). In addition to lysosomes, mitochondria, and endoplasmic reticulum, Neu4 is localized at the cell surface (12, 15, 29, 30). In contrast, Neu2 is poorly expressed in the brain (31, 32). Thus, Neu4 may mainly contribute to the neural activity-dependent increase in sialidase activity detected by 4MU-Neu5Ac and BTP3-Neu5Ac. Because Neu4 knockdown impairs hippocampus-dependent spatial memory in Morris water maze tasks (14), neural activity-dependent regulation of sialyl signaling by Neu4 may be involved in hippocampal memory processing.
Neu4 catalyzes degradation of PSA as well as removal of sialic acid from glycoproteins, gangliosides, and oligosaccharides (26, 27, 30). PSA plays an important role in hippocampal synaptic plasticity and memory (2, 3, 6). In hippocampal MFs, the expression level of PSA on NCAM is related to synaptic maturation. Although immature MF boutons express PSA on NCAM, most of the mature MF boutons do not (33). These findings support the idea that synaptic maturation at MF boutons is induced by neural activity-dependent PSA removal during memory formation. In addition, memory and synaptic plasticity are impaired by removal of PSA by endoneuraminidase beforehand (16–20), indicating that appropriate timing of PSA removal may be important for memory formation.
Eckhardt et al. (34) showed that the entire CA3 area, representing the MF termination field on pyramidal neurons, expresses large amounts of PSA in wild-type mice at 6 months of age. However, mice lacking ST8SiaIV/PST-1, one of the polysialyltransferases responsible for addition of PSA to NCAM, showed an almost undetectable level of PSA in the CA3 area at 6 months of age but showed a normal level of LTP at MF-CA3 pyramidal cell synapses (34). Thus, the role of PSA in synaptic plasticity at MF terminals is still controversial. PSA regulates the activity of voltage-gated sodium channels and glutamate receptors (35, 36) and has the ability for capturing BDNF and dopamine (37, 38). Neural activity-dependent degradation of PSA seems to play a multidimensional role in hippocampal neurotransmission.
Overall, although structural regulation of glycoconjugates has been studied on a time scale of hours to days, our observations show that sialidase rapidly increases its enzyme activity in response to neural activity on a time scale of seconds. Furthermore, sialidase removes sialic acid on the extracellular cell surface during hippocampal memory formation in a contextual fear-conditioning paradigm. Therefore, neural activity-dependent desialylation by sialidase may be involved in hippocampal memory processing. Activity-dependent desialylation on the cell surface may markedly change cell surface properties and give fast feedback on sialyl signaling-dependent neural activities such as synaptic plasticity (2, 3, 6), hippocampal memory (2, 3, 6), neurotransmission (39), neural cell adhesion (2, 6, 7), and AMPA receptor trafficking (7, 40). Because abnormal sialyl signaling causes epilepsy (1, 41, 42), Alzheimer's disease (6, 7, 43, 44), and neuropsychiatric disorders (6, 7, 45) such as schizophrenia and bipolar disorder, sialidase may provide a novel therapeutic target for these neural disorders.
Experimental procedures
Experimental animals
Male Wistar rats (8–9 weeks old) and a pregnant Wistar rat were purchased from Japan SLC (Shizuoka, Japan). The rats were housed under standard laboratory conditions (23 ± 1 °C, 55 ± 5% humidity) and had access to tap water and diet ad libitum. The lights were automatically turned on at 8:00 and off at 20:00. The rats were handled for at least 10 min everyday for at least 7 days before use in the behavioral experiments. All experiments were performed in accordance with the guidelines established by the University of Shizuoka for the care and use of laboratory animals. The protocols were preapproved by the Animal Ethics Committee of the University of Shizuoka.
Preparation of acute hippocampal slices
The procedure for acute hippocampal slice preparation was described previously (13). Briefly, the rats were deeply anesthetized with halothane and decapitated. The hippocampus was quickly removed and immersed in ice-cold ACSF (pH 7.3) containing 119 mm NaCl, 2.5 mm KCl, 2.5 mm CaCl2, 1.3 mm MgCl2, 1.0 mm NaH2PO4, 26.2 mm NaHCO3, and 11 mm d-glucose to suppress excessive neuronal excitation and damage. Acute hippocampal slices (400 μm in thickness) were prepared using a vibratome tissue slicer (LinearSlicer PRO-7, Dosaka, Kyoto, Japan) in ice-cold ACSF and placed in a humidified interface-type holding chamber for at least 1 h. If necessary, hippocampal slices were incubated in ACSF or high-K+ solution (21.5 mm NaCl, 100 mm KCl, 2.5 mm CaCl2, 1.3 mm MgCl2, 1.0 mm NaH2PO4, 26.2 mm NaHCO3, and 11 mm d-glucose) for 30 min at 27 °C. In the acute slice experiments, a gas mixture of 95% O2 and 5% CO2 was continuously bubbled through all solutions.
Measurement of enzyme activity in hippocampal slices
Hippocampal slices were incubated in 250 μl of ACSF containing 10 μm 4MU-Neu5Ac (Nacalai Tesque, Kyoto, Japan) for 30 min at 27 °C and then transferred to the following incubation buffers containing 10 μm 4MU-Neu5Ac: ACSF, high-K+ solution, or ACSF containing 50 μm forskolin, 5 μm kainate, 1 or 10 mm glutamate, 10 mm glutamate with 50 μm AP5, 50 μm AP5, 10 μm GABA, or 1 mm DANA. After incubation for 30 min, the slices were incubated with ACSF containing 10 μm 4MU-Neu5Ac for 30 min twice. Immediately after each incubation, 200 μl of the supernatant was transferred to a 96-well black microplate (Corning) filled with 50 μl of sodium carbonate buffer (500 mm; pH 10.7). Fluorescence intensities of 4MU were measured using a microplate reader (ex/em, 355/460 nm; Infinite M200, Tecan, Männedorf, Switzerland). In the case of galactosidase activity measurement, 10 μm 4MU-Gal was used instead of 4MU-Neu5Ac. At the end of the experiments, the area (mm2) of each slice was measured using a stereoscopic microscope (SZX-7, Olympus, Tokyo, Japan) and Photoshop CS4 (Adobe Systems, San Jose, CA).
To measure the secreted sialidase activity, hippocampal slices were incubated in 250 μl of ACSF or high-K+ solution for 30 min. The supernatant (200 μl) was transferred to another incubation chamber, and 4MU-Neu5Ac was added to give a concentration of 10 μm. After incubation for 30 min, the enzyme reaction was stopped by adding sodium carbonate buffer, and then the fluorescence intensities were measured.
Imaging of sialidase activity
The procedure for sialidase activity imaging was described previously (13, 14). Briefly, rat acute hippocampal slices were incubated with 400 μl of ACSF (pH 7.3) containing 100 μm BTP3-Neu5Ac at 27 °C for 60 min. For high-resolution imaging of sialidase activity, acute hippocampal slices were frozen with liquid nitrogen, cut into 20-μm-thick sections using a cryotome (Leica Microsystems), and then stained with ACSF containing 1 mm BTP3-Neu5Ac at 27 °C for 60 min. After washing with ice-cold ACSF, fluorescence was observed using a fluorescence microscope (IX71, Olympus) by using a filter set (ex/em, BP330–385/BA510IF). The background level of fluorescence was determined using a non-stained brain slice. In all observations with the fluorescence microscope, the gain of the DP70 digital microscope camera (Olympus) was set to not detect background fluorescence. After obtaining pictures, the slices were incubated in ACSF containing 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI; Dojindo Laboratories, Kumamoto, Japan) for nuclear staining and observed through a filter set (ex/em, BP330–385/DM400). In cases requiring staining with X-Neu5Ac and FRV LB, the hippocampal slices were incubated with 400 μl of ACSF or high-K+ solution containing 1 mm X-Neu5Ac and 0.1 mg/ml FRV LB at 27 °C for 60 min. After washing with ACSF, fluorescence was observed using a filter set (ex/em, BP530–550/BA575IF). The background level of fluorescence was determined using a slice incubated in ACSF containing only 0.1 mg/ml FRV LB. To confirm the specificity of sialidase activity imaging, the staining was also performed in the presence of 1–10 mm DANA.
Hydrolysis of 4MU-Neu5Ac with each rat sialidase isozyme
The procedure for rat sialidase isozyme expression was described previously (14). Briefly, C-terminal Myc-tagged rat sialidase isozymes Neu1, Neu2, Neu3, and Neu4 were stably expressed in C6 rat glioma cells. Each lysate equalized with the amount of Myc was incubated in ACSF or 100 mm sodium acetate buffer (pH 4.6) containing 200 μm 4MU-Neu5Ac at 27 °C for 120 min. The released 4MU was measured using a microplate reader (ex/em, 355/460 nm).
Real time quantitative RT-PCR
The procedure for real time quantitative RT-PCR was described previously (14). Briefly, total RNA was isolated by using the guanidinium phenol reagent (TRIzol reagent, Life Technologies) according to the manufacturer's instructions. Neu4 cDNA copies were evaluated using a thermal cycler system (Thermal Cycler Dice® Real Time System Lite, TaKaRa Bio), a One Step SYBR PrimeScript PLUS RT-PCR kit (Perfect Real Time, TaKaRa Bio), and primer pairs (5′-CCCATCCCGAGTACCGAGT-3′ and 5′-CCCGGCCACAACTGGAC-3′ for Neu1, 5′-GAGCCACCAACCATGTCAAG-3′ and 5′-AAGGGACATGGATTCATGGAG-3′ for Neu2, 5-CGGAGCTGGTGAGCTGAG-3′ and 5′-CCTGCTGGAACAGAGTGCTG-3′ for Neu3, 5′-TCTGGAGAGTGCCAACTGGC-3′ and 5′-AAGGAAGTGCCTTCATCAGCAC-3′ for Neu4, and 5′-TGAACGGATTTGGCCGTATCGG-3′ and 5′-TCAATGAAGGGGTCGTTGATGG-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH)). To normalize for sample variation, GAPDH mRNA was used as an internal standard.
Immunohistochemical staining
The procedure for immunohistochemistry was described previously (13). Briefly, the rats were anesthetized using halothane and transcardially perfused with PBS. After embedding, brains were cut into 10-μm-thick sections at −20 °C using a cryotome. After fixation and antigen retrieval treatment, blocking was performed in Tris-buffered saline with 0.1% Tween 20 (pH 7.4) containing 2% BSA. The sections were stained with goat anti-human Neu4 polyclonal antibody (1:50; Santa Cruz Biotechnology, Dallas, TX) having cross-reactivity against rat Neu4 and with HiLyte Fluor 555-conjugated rabbit anti-goat IgG (1:50; AnaSpec). The sections were also stained using 1 μg/ml DAPI.
Images were acquired using a fluorescence microscope and a filter set (ex/em, BP530–550/BA575IF for HiLyte Fluor 555 and BP330–385/BA420 for DAPI). The background level of fluorescence was determined by using sections stained with only the secondary antibody. All imaging was performed at least twice using different rats, and the reproducibility was confirmed. The specificity of the anti-Neu4 antibody was determined by using a rat brain that was inoculated with glioma cells overexpressing rat Neu4 (data not shown).
Sialidase activity measurement in rat primary cultured astrocytes
Cell suspensions were isolated from the rat fetal hippocampus (embryonic day 17) by using a nerve cell culture system (Sumitomo Bakelite, Tokyo, Japan) according to the manufacturer's instructions and incubated in a nerve cell culture medium (Sumitomo Bakelite) for 5 days on poly-l-lysine-coated culture dishes. After removal of neurons and microglia, astrocytes were subcultured twice using 0.25% trypsin. After preincubation with serum-free neuron assay medium (Sumitomo Bakelite), sialidase activity was measured by incubation with serum-free neuron assay medium containing 4MU-Neu5Ac or 4MU-Neu5Ac and 100 μm glutamate for 30 min at 37 °C. The released 4MU was measured in the same manner as before.
Lectin staining
An acute hippocampal slice incubated with ACSF and an acute hippocampal slice incubated with high-K+ solution were embedded in the same block to avoid staining variation. Frozen blocks were cut into 20-μm-thick sections using a cryotome. After fixation, blocking was performed in Carbo-Free Blocking Solution (Vector Laboratories, Burlingame, CA). The sections were stained with FITC-labeled MAA (0.1 mg/ml; EY Laboratories, San Mateo, CA) or FITC-labeled PNA (0.02 mg/ml; J-Oil Mills). Fluorescence intensity was measured using Photoshop CS4. Images were acquired using a fluorescence microscope (BZ-X710, Keyence, Osaka, Japan) and a filter set (ex/em, BP450–490/BA500–550). The background level of fluorescence was determined by using non-stained sections.
Time lapse imaging of sialidase activity
Rat acute hippocampal slices were transferred to glass bottom dishes that were each filled with 50 μl of ACSF (pH 7.3; 27 °C) containing 100 μm BTP3-Neu5Ac or 10 mm 4MU-Neu5Ac. Regions of interest (100 μm in diameter) were set at the CA3 stratum lucidum, and fluorescence intensity was monitored using a confocal laser scanning microscope system (LSM 510, Carl Zeiss; 1 frame/s) through a 10× objective lens and a filter set (ex, 364 nm; em, 530–600 nm; dichroic mirror, 375 nm) for BTP3-Neu5Ac. Electrical stimuli (1–100 Hz, 5 s, 300 μA) were delivered to MFs through a tungsten bipolar electrode.
In vivo microdialysis
The procedure for in vivo microdialysis was described previously (46). Briefly, a microdialysis probe (3-mm membrane CMA12 Elite microdialysis probe; cutoff, 20,000 daltons; CMA Microdialysis, Stockholm, Sweden) was attached to a cannula, and the dialysis membrane was surgically implanted in the rat hippocampal CA3 (AP = −5.6 mm; ML = 4.6 mm; DV = 6.9 mm) (47) using a stereotaxic instrument (Narishige, Tokyo, Japan). The hippocampus was perfused with ACSF at 2.3 μl/min over 240 min to stabilize the dialysis. Then 2 μl of 5 or 10 units/ml AUSA in ACSF was injected into the hippocampus at 0.3 μl/min through the cannula. Perfusate samples were collected every 60 min.
To investigate the effect of high-K+ solution or TTX on desialylation, a guide tube was surgically implanted in the rat hippocampus (AP = −5.6 mm; ML = 4.6 mm; DV = 3.9 mm) under anesthesia and fixed with dental cement (Shofu, Kyoto, Japan). Forty-eight hours after implantation, a microdialysis probe was inserted into the hippocampal CA3 through the guide tube under anesthesia. The hippocampus was perfused with ACSF at 2.3 μl/min over 4 h to stabilize the dialysis. Then the hippocampus was perfused with high-K+ solution or ACSF containing 1 μm TTX. Perfusate samples were collected every 60 min.
Contextual fear conditioning
A guide tube was surgically implanted in the rat hippocampus (AP = −5.6 mm; ML = 4.6 mm; DV = 3.9 mm) and fixed using dental cement. Three days after implantation, a microdialysis probe was inserted into the hippocampal CA3 through the guide tube in the awakened state. After hippocampal perfusion (2.3 μl/min) with ACSF for 3.5 h in the home cage, the rats were placed in a fear-conditioning chamber (28 (width) × 29.5 (depth) × 29 cm (height); Muromachi Kikai) with shock grid floors (bars of 5.0 mm in diameter spaced 1.5 cm apart) followed by foot shock training (1 s, 0.2 mA (the feeble electric current of limit that can be sensed)) every 20 s for 15 min and then returned to the home cage. Hippocampal perfusion was continued for 2.5 h after each rat had been returned to the home cage. Perfusate samples were collected every 30 min. On the next day, the rats were placed in the conditioning chamber again. Behavior was recorded by an overhead camera, and freezing time was assessed for 3 min.
HPLC measurement
HPLC measurement of sialic acid (Neu5Ac), glutamate, and GABA was performed using precolumn fluorescence derivatization as described previously (46, 48). Neu5Ac in the perfusate (20 μl) was derivatized with 1,2-diamino-4,5-methylenedioxybenzene and analyzed using an HPLC system (LC-2000 Plus series, Jasco, Tokyo, Japan) with an octadecylsilyl column (TSKgel ODS-100V column, Tosoh, Tokyo, Japan) and a mobile phase of methanol/water (25:75, v/v). Fluorescence was monitored at excitation and emission wavelengths of 373 and 448 nm, respectively. Glutamate and GABA in the perfusate (10 μl) were derivatized using o-phthaldialdehyde and analyzed using an octadecylsilyl column (CrestPak C18S, Jasco) and gradient mobile phase of 50 mm sodium acetate and methanol. Fluorescence was monitored at excitation and emission wavelengths of 340 and 450 nm, respectively.
Statistical analysis
Statistical significance was assessed using one-way ANOVA, one-way repeated measures ANOVA, or two-way repeated measures ANOVA with Bonferroni's multiple comparison test, Kruskal-Wallis test with Dunn's multiple comparison test, two-tailed unpaired t test with or without Welch's correction, and two-tailed paired t test. Statistical analysis was performed using Prism 5 (GraphPad, La Jolla, CA). Error bars are expressed as S.E.
Author contributions
A. M. and T. S. contributed to the study design and wrote the paper. Under the supervision of A. M., the authors contributed as follows: Y. M., M. S., R. T., and T. T., biochemical analyses; S. I. and S. Sa., in vivo microdialysis; Y. H. and S. Sh., behavior experiments; A. I., H. S., and H. F., histochemical staining. T. O. and K. I. provided BTP3-Neu5Ac. All of the authors discussed and commented on the manuscript.
Acknowledgment
We are grateful to Dr. Tomoya Hikita for construction of recombinant rat sialidase isozymes.
This work was supported by Grant-in-aid for Young Scientists (B) KAKENHI 24790080 and for Scientific Research (C) KAKENHI 16K08277, the Hokuto Foundation for Bioscience, Futaba Electronics Memorial Foundation, and the Takahashi Industrial and Economic Research Foundation. The authors declare that they have no conflicts of interest with the contents of this article.
- GQ1b
- N-acetylneuraminyl-N-acetylneuraminylgalactosyl-N-acetylgalactosaminyl[N-acetylneuraminyl-N-acetylneuraminyl]-galactosylglucosylceramide
- PSA
- polysialic acid
- ACSF
- artificial cerebrospinal fluid
- AP5
- 2-amino-5-phosphonopentanoic acid
- AUSA
- sialidase from A. ureafaciens
- BTP3-Neu5Ac
- benzothiazolylphenol-based sialic acid derivative
- DANA
- 2,3-dehydro-2-deoxy-N-acetylneuraminic acid
- DAPI
- 4′,6-diamidino-2-phenylindole
- LTP
- long-term potentiation
- MF
- mossy fiber
- MAA
- M. amurensis agglutinin
- NCAM
- neural cell adhesion molecules
- Neu5Ac
- N-acetylneuraminic acid
- NMDAR
- N-methyl-d-aspartate receptor
- PNA
- peanut agglutinin
- TTX
- tetrodotoxin
- 4MU
- 4-methylumbelliferone
- 4MU-Gal
- 4MU-β-d-galactopyranoside
- 4MU-Neu5Ac
- 4-methylumbelliferyl-α-d-N-acetylneuraminic acid
- X-Neu5Ac
- 5-bromo-4-chloroindol-3-yl-α-d-N-acetylneuraminic acid
- FRV LB
- Fast Red Violet LB
- ANOVA
- analysis of variance
- ex
- excitation
- em
- emission
- AP
- anterior-posterior
- ML
- medial-lateral
- DV
- dorsal-ventral.
References
- 1. Schnaar R. L., Gerardy-Schahn R., and Hildebrandt H. (2014) Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 94, 461–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sato C., and Kitajima K. (2013) Disialic, oligosialic and polysialic acids: distribution, functions and related disease. J. Biochem. 154, 115–136 [DOI] [PubMed] [Google Scholar]
- 3. Minami A., and Suzuki T. (2012) Distribution of sialidase activity and the role of sialidase in the brain. Trends Glycosci. Glycotechnol. 24, 112–121 [Google Scholar]
- 4. Fujii S., Igarashi K., Sasaki H., Furuse H., Ito K., Kaneko K., Kato H., Inokuchi J., Waki H., and Ando S. (2002) Effects of the mono- and tetrasialogangliosides GM1 and GQ1b on ATP-induced long-term potentiation in hippocampal CA1 neurons. Glycobiology 12, 339–344 [DOI] [PubMed] [Google Scholar]
- 5. Jung W. R., Kim H. G., and Kim K. L. (2008) Ganglioside GQ1b improves spatial learning and memory of rats as measured by the Y-maze and the Morris water maze tests. Neurosci. Lett. 439, 220–225 [DOI] [PubMed] [Google Scholar]
- 6. Gascon E., Vutskits L., and Kiss J. Z. (2007) Polysialic acid-neural cell adhesion molecule in brain plasticity: from synapses to integration of new neurons. Brain Res. Rev. 56, 101–118 [DOI] [PubMed] [Google Scholar]
- 7. Senkov O., Tikhobrazova O., and Dityatev A. (2012) PSA-NCAM: synaptic functions mediated by its interactions with proteoglycans and glutamate receptors. Int. J. Biochem. Cell Biol. 44, 591–595 [DOI] [PubMed] [Google Scholar]
- 8. Yu R. K., Macala L. J., Taki T., Weinfield H. M., and Yu F. S. (1988) Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J. Neurochem. 50, 1825–1829 [DOI] [PubMed] [Google Scholar]
- 9. Di Cristo G., Chattopadhyaya B., Kuhlman S. J., Fu Y., Bélanger M. C., Wu C. Z., Rutishauser U., Maffei L., and Huang Z. J. (2007) Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat. Neurosci. 10, 1569–1577 [DOI] [PubMed] [Google Scholar]
- 10. Kato K., Iwamori M., and Hirabayashi Y. (2008) Increase of GQ1b in the hippocampus of mice following kindled-seizures. Neurosci. Lett. 441, 286–290 [DOI] [PubMed] [Google Scholar]
- 11. Sumida M., Hane M., Yabe U., Shimoda Y., Pearce O. M., Kiso M., Miyagi T., Sawada M., Varki A., Kitajima K., and Sato C. (2015) Rapid trimming of cell surface polysialic acid (polySia) by exovesicular sialidase triggers release of preexisting surface neurotrophin. J. Biol. Chem. 290, 13202–13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miyagi T., and Yamaguchi K. (2012) Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology 22, 880–896 [DOI] [PubMed] [Google Scholar]
- 13. Minami A., Shimizu H., Meguro Y., Shibata N., Kanazawa H., Ikeda K., and Suzuki T. (2011) Imaging of sialidase activity in rat brain sections by a highly sensitive fluorescent histochemical method. Neuroimage 58, 34–40 [DOI] [PubMed] [Google Scholar]
- 14. Minami A., Saito M., Mamada S., Ieno D., Hikita T., Takahashi T., Otsubo T., Ikeda K., and Suzuki T. (2016) Role of sialidase in long-term potentiation at mossy fiber-CA3 synapses and hippocampus-dependent spatial memory. PLoS One 11, e0165257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiozaki K., Yamaguchi K., Takahashi K., Moriya S., and Miyagi T. (2011) Regulation of sialyl Lewis antigen expression in colon cancer cells by sialidase NEU4. J. Biol. Chem. 286, 21052–21061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Becker C. G., Artola A., Gerardy-Schahn R., Becker T., Welzl H., and Schachner M. (1996) The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J. Neurosci. Res. 45, 143–152 [DOI] [PubMed] [Google Scholar]
- 17. Muller D., Wang C., Skibo G., Toni N., Cremer H., Calaora V., Rougon G., and Kiss J. Z. (1996) PSA-NCAM is required for activity-induced synaptic plasticity. Neuron 17, 413–422 [DOI] [PubMed] [Google Scholar]
- 18. Kochlamazashvili G., Senkov O., Grebenyuk S., Robinson C., Xiao M. F., Stummeyer K., Gerardy-Schahn R., Engel A. K., Feig L., Semyanov A., Suppiramaniam V., Schachner M., and Dityatev A. (2010) Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors. J. Neurosci. 30, 4171–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dityatev A., Dityateva G., Sytnyk V., Delling M., Toni N., Nikonenko I., Muller D., and Schachner M. (2004) Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J. Neurosci. 24, 9372–9382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopez-Fernandez M. A., Montaron M. F., Varea E., Rougon G., Venero C., Abrous D. N., and Sandi C. (2007) Upregulation of polysialylated neural cell adhesion molecule in the dorsal hippocampus after contextual fear conditioning is involved in long-term memory formation. J. Neurosci. 27, 4552–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Minami A., Otsubo T., Ieno D., Ikeda K., Kanazawa H., Shimizu K., Ohata K., Yokochi T., Horii Y., Fukumoto H., Taguchi R., Takahashi T., Oku N., and Suzuki T. (2014) Visualization of sialidase activity in mammalian tissues and cancer detection with a novel fluorescent sialidase substrate. PLoS One 9, e81941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weisskopf M. G., Castillo P. E., Zalutsky R. A., and Nicoll R. A. (1994) Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science 265, 1878–1882 [DOI] [PubMed] [Google Scholar]
- 23. Edelmann E., Lessmann V., and Brigadski T. (2014) Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology 76, 610–627 [DOI] [PubMed] [Google Scholar]
- 24. Yamaguchi K., Hata K., Wada T., Moriya S., and Miyagi T. (2006) Epidermal growth factor-induced mobilization of a ganglioside-specific sialidase (NEU3) to membrane ruffles. Biochem. Biophys. Res. Commun. 346, 484–490 [DOI] [PubMed] [Google Scholar]
- 25. Lukong K. E., Seyrantepe V., Landry K., Trudel S., Ahmad A., Gahl W. A., Lefrancois S., Morales C. R., and Pshezhetsky A. V. (2001) Intracellular distribution of lysosomal sialidase is controlled by the internalization signal in its cytoplasmic tail. J. Biol. Chem. 276, 46172–46181 [DOI] [PubMed] [Google Scholar]
- 26. Shiozaki K., Koseki K., Yamaguchi K., Shiozaki M., Narimatsu H., and Miyagi T. (2009) Developmental change of sialidase neu4 expression in murine brain and its involvement in the regulation of neuronal cell differentiation. J. Biol. Chem. 284, 21157–21164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Comelli E. M., Amado M., Lustig S. R., and Paulson J. C. (2003) Identification and expression of Neu4, a novel murine sialidase. Gene 321, 155–161 [DOI] [PubMed] [Google Scholar]
- 28. Seyrantepe V., Landry K., Trudel S., Hassan J. A., Morales C. R., and Pshezhetsky A. V. (2004) Neu4, a novel human lysosomal lumen sialidase, confers normal phenotype to sialidosis and galactosialidosis cells. J. Biol. Chem. 279, 37021–37029 [DOI] [PubMed] [Google Scholar]
- 29. Shiozaki K., Ryuzono S., Matsushita N., Ikeda A., Takeshita K., Chigwechokha P. K., Komatsu M., and Miyagi T. (2014) Molecular cloning and biochemical characterization of medaka (Oryzias latipes) lysosomal neu4 sialidase. Fish Physiol. Biochem. 40, 1461–1472 [DOI] [PubMed] [Google Scholar]
- 30. Takahashi K., Mitoma J., Hosono M., Shiozaki K., Sato C., Yamaguchi K., Kitajima K., Higashi H., Nitta K., Shima H., and Miyagi T. (2012) Sialidase NEU4 hydrolyzes polysialic acids of neural cell adhesion molecules and negatively regulates neurite formation by hippocampal neurons. J. Biol. Chem. 287, 14816–14826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Monti E., Preti A., Rossi E., Ballabio A., and Borsani G. (1999) Cloning and characterization of NEU2, a human gene homologous to rodent soluble sialidases. Genomics 57, 137–143 [DOI] [PubMed] [Google Scholar]
- 32. Hasegawa T., Feijoo Carnero C., Wada T., Itoyama Y., and Miyagi T. (2001) Differential expression of three sialidase genes in rat development. Biochem. Biophys. Res. Commun. 280, 726–732 [DOI] [PubMed] [Google Scholar]
- 33. Seki T., and Arai Y. (1999) Different polysialic acid-neural cell adhesion molecule expression patterns in distinct types of mossy fiber boutons in the adult hippocampus. J. Comp. Neurol. 410, 115–125 [DOI] [PubMed] [Google Scholar]
- 34. Eckhardt M., Bukalo O., Chazal G., Wang L., Goridis C., Schachner M., Gerardy-Schahn R., Cremer H., and Dityatev A. (2000) Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J. Neurosci. 20, 5234–5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hammond M. S., Sims C., Parameshwaran K., Suppiramaniam V., Schachner M., and Dityatev A. (2006) Neural cell adhesion molecule-associated polysialic acid inhibits NR2B-containing N-methyl-d-aspartate receptors and prevents glutamate-induced cell death. J. Biol. Chem. 281, 34859–34869 [DOI] [PubMed] [Google Scholar]
- 36. Hoffman K. B., Kessler M., and Lynch G. (1997) Sialic acid residues indirectly modulate the binding properties of AMPA-type glutamate receptors. Brain Res. 753, 309–314 [DOI] [PubMed] [Google Scholar]
- 37. Isomura R., Kitajima K., and Sato C. (2011) Structural and functional impairments of polysialic acid by a mutated polysialyltransferase found in schizophrenia. J. Biol. Chem. 286, 21535–21545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanato Y., Kitajima K., and Sato C. (2008) Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology 18, 1044–1053 [DOI] [PubMed] [Google Scholar]
- 39. Ando S., Tanaka Y., Waki H., Kon K., Iwamoto M., and Fukui F. (1998) Gangliosides and sialylcholesterol as modulators of synaptic functions. Ann. N.Y. Acad. Sci. 845, 232–239 [DOI] [PubMed] [Google Scholar]
- 40. Prendergast J., Umanah G. K., Yoo S. W., Lagerlöf O., Motari M. G., Cole R. N., Huganir R. L., Dawson T. M., Dawson V. L., and Schnaar R. L. (2014) Ganglioside regulation of AMPA receptor trafficking. J. Neurosci. 34, 13246–13258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Isaev D., Isaeva E., Shatskih T., Zhao Q., Smits N. C., Shworak N. W., Khazipov R., and Holmes G. L. (2007) Role of extracellular sialic acid in regulation of neuronal and network excitability in the rat hippocampus. J. Neurosci. 27, 11587–11594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boyzo A., Ayala J., Gutiérrez R., and Hernández-R J. (2003) Neuraminidase activity in different regions of the seizing epileptic and non-epileptic brain. Brain Res. 964, 211–217 [DOI] [PubMed] [Google Scholar]
- 43. Hong S., Ostaszewski B. L., Yang T., O'Malley T. T., Jin M., Yanagisawa K., Li S., Bartels T., and Selkoe D. J. (2014) Soluble Aβ oligomers are rapidly sequestered from brain ISF in vivo and bind GM1 ganglioside on cellular membranes. Neuron 82, 308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Annunziata I., Patterson A., Helton D., Hu H., Moshiach S., Gomero E., Nixon R., and d'Azzo A. (2013) Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis. Nat. Commun. 4, 2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barbeau D., Liang J. J., Robitalille Y., Quirion R., and Srivastava L. K. (1995) Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc. Natl. Acad. Sci. U.S.A. 92, 2785–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takeda A., Sakurada N., Kanno S., Minami A., and Oku N. (2006) Response of extracellular zinc in the ventral hippocampus against novelty stress. J. Neurochem. 99, 670–676 [DOI] [PubMed] [Google Scholar]
- 47. Paxinos G., and Watson C. (2007) The Rat Brain in Stereotaxic Coordinates, 6th Ed., p. 80, Academic Press/Elsevier, Boston [Google Scholar]
- 48. Minami A., Ishibashi S., Ikeda K., Ishitsubo E., Hori T., Tokiwa H., Taguchi R., Ieno D., Otsubo T., Matsuda Y., Sai S., Inada M., and Suzuki T. (2013) Catalytic preference of Salmonella typhimurium LT2 sialidase for N-acetylneuraminic acid residues over N-glycolylneuraminic acid residues. FEBS Open Bio. 3, 231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]