Abstract
To ensure correct spatial and temporal patterning, embryos must maintain pluripotent cell populations and control when cells undergo commitment. The newly identified nucleoprotein Akirin has been shown to modulate the innate immune response through epigenetic regulation and to play important roles in other physiological processes, but its role in neural development remains unknown. Here we show that Akirin2 is required for neural development in Xenopus and that knockdown of Akirin2 expands the expression of the neural progenitor marker Sox2 and inhibits expression of the differentiated neuronal marker N-tubulin. Akirin2 acts antagonistically to Geminin, thus regulating Sox2 expression, and maintains the neural precursor state by participating in the Brg1/Brm-associated factor (BAF) complex mediated by BAF53a. Additionally, Akirin2 also modulates N-tubulin expression by acting upstream of neuronal differentiation 1 (NeuroD) and in parallel with neurogenin-related 1 (Ngnr1) during terminal neuronal differentiation. Thus, our results reveal a novel model in which Akirin2 precisely coordinates and temporally controls Xenopus neural development.
Keywords: cell differentiation, chromatin remodeling, embryo, neurodevelopment, Xenopus
Introduction
Formation of the vertebrate neural system involves multiple processes, including an initial induction of neural tissue from the ectoderm, neural patterning along the dorsal-ventral and anterior-posterior axes, and neuron subtype specification (1–4). The initial neuroectoderm (also called neural plate) consists of multipotent neural progenitor cells that can generate a wide variety of cells needed to form the entire neural system (1, 5). These neural progenitors express Sox2, a SoxB1 subfamily member of the high mobility group (HMG) box transcription factor, which has been shown to be essential for the maintenance of the multipotency of proliferating neural precursors (6, 7).
Down-regulation of Sox2 is a key step during the transition of cells from proliferating neural progenitors into postmitotic differentiated neurons (6–9). A cascade of proneural genes is then activated, thus specifying the neuronal lineage and driving neuronal differentiation. Neurogenin-related 1 (Ngnr1), the first proneural factor of the cascade, induces the activation of later-acting basic helix-loop-helix (bHLH)7 factor neuronal differentiation 1 (NeuroD); this activation ultimately defines three prospective patches of primary neurons marked by the expression of N-tubulin in the neural plate in Xenopus (10–13).
The ATP-dependent SWI/SNF-like Brg1/Brm-associated factor (BAF) chromatin remodeling complexes are emerging as key regulators of the differentiation of neural precursor cells. The vertebrate BAF complex contains at least 15 different subunits, including two interchangeable ATPase subunits (Brg1 or Brm), a group of invariant core subunits, and a variety of lineage-specific subunits (14–16). In mice, the neural progenitor cell-specific BAF complex (npBAF), containing BAF53a, is involved in the self-renewal and proliferation of these cells, and neuron-specific BAF (nBAF) contains BAF53b and promotes neuronal differentiation (14). For neural progenitor maintenance, Brm/Brg1 activates Sox2 expression by directly binding to its N2 enhancer (17). It has also been reported that Geminin, a novel coiled-coil protein, is also recruited to the Sox2 N2 enhancer and promotes its expression through direct interaction with Brm/Brg1 (18, 19). The interaction between Geminin and Brm/Brg1 antagonizes the recruitment of the bHLH factors (Ngnr1 and NeuroD) to the npBAF complex (20, 21). During the transition from proliferating precursor to differentiated postmitotic neuron, Geminin is down-regulated, thus facilitating the assembly of the nBAF complex in which Ngnr1 and NeuroD physically interact with Brm/Brg1 (20–23).
Akirin is a recently discovered nuclear factor involved in many physiological and pathological processes (24–28). Akirin was first reported to interact with the Brahma-associated protein (BAP) complex subunit BAP60 and NF-κB, thus activating the expression of antimicrobial peptide in the Drosophila immune deficiency pathway (29). Studies in mammals have suggested that Akirin2 is involved in the innate immune response through bridging NF-κB and the chromatin-remodeling SWI/SNF complex by interacting with BAF60 and IκB-ζ and that it activates proinflammatory genes in macrophages (30). During Drosophila embryogenesis, Akirin interacts genetically and physically with Twist and consequently facilitates the expression of a number of Twist-regulated genes (26). Most vertebrates contain two Akirin genes (31). Although Akirin1-null mice appear normal, Akirin2 knock-out mice die by embryonic day 9.5 (24, 32, 33), thus suggesting an essential role of Akirin in embryonic development. A recent study has also shown that Akirin2 plays an essential role in controlling Sox2-positive progenitor expansion during cortical development in mice (33).
Here we show evidence that Akirin2 is involved in both neural precursor maintenance and terminal neural differentiation in Xenopus. In neural precursors, Akirin2 associates with BAF53a and antagonizes the activity of Geminin, thereby suppressing Sox2 expression. In addition, Akirin2 is required for proper activation of NeuroD and neuronal differentiation. Our results reveal that Akirin2 is a key regulator that balances neural progenitor self-renewal and neuronal differentiation in Xenopus.
Results
Akirins are highly expressed in the developing xenopus neural system
Two Akirin family members (Akirin1 and Akirin2) were isolated from Xenopus and shared 57% identity at the amino acid level (data not shown). Semiquantitative RT-PCR was used to investigate the temporal expression patterns of the Akirins during Xenopus laevis early development. Both Akirin1 and Akirin2 were maternally expressed, and the expression of both Akirins was maintained throughout the stages we examined (Fig. 1A and supplemental Fig. S1A). Whole-mount in situ hybridization was carried out to determine the spatial expression pattern of Xenopus Akirins. Both Akirin1 and Akirin2 transcripts were detected at the animal hemisphere (Fig. 1, B and C, and supplemental Fig. S1B). During the neurulation stage, their expression levels became enriched in the neural system, especially in the neural plate and subsequent neural tube region (Fig. 1, D–G, and supplemental Fig. S1, C–F). Additionally, cranial and trunk neural crest cells expressed Akirins (Fig. 1, E–G, and supplemental Fig. S1, D–F). At the tailbud and tadpole stages, Akirin transcripts were detected throughout the CNS, including the eye, brain, and spinal cord, and additionally in the branchial arches, pronephric tubule, and otic vesicle (Fig. 1, H–K, and supplemental Fig. S1, G–O). Akirin1 and Akirin2 had very similar expression patterns, but Akirin1 had weaker expression in the branchial arches than Akirin2 at the tadpole stage (supplemental Fig. S1K). These data showed that Xenopus Akirin1 and Akirin2 were highly expressed in the developing neural system, thus suggesting that they may have important roles in early neural development. Because Akrin2 appears to play a more important role during embryonic development (24, 32, 33), we focused on Akirin2 in the following study.
Figure 1.
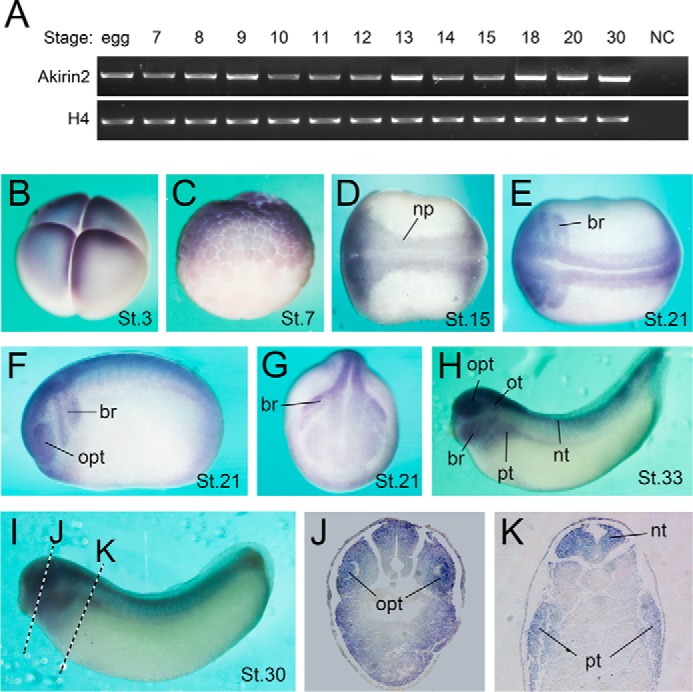
Expression pattern of Akirin2 during early Xenopus development. A, RT-PCR analysis of Akirin2 expression at different stages (stage (St.) 0 to St. 30). H4 is an internal reference. NC, negative control without reverse transcriptase in the RT reaction. B–I, whole-mount in situ hybridization of Akirin2. The Akirin2 transcript is detected in the animal pole at St. 3 (B) and St. 6.5 (C) (B and C, lateral view, animal pole at the top). At St. 15, Akirin2 is expressed at the neural plate (np, D, dorsal view). At late neurulation, Akirin2 is most abundant in branchial arches (br) and the optic vesicle (opt) (E, dorsal view; F, lateral view; G, frontal view). At the tailbud stage, Akirin2 is mainly expressed in the branchial arches, optic vesicle, otic vesicle (ot), pronephric tubule (pt), and neural tube (nt) (H and I, lateral view). J and K, transverse sections of a stage 30 embryo.
Akirin2 is required for xenopus neural development
To determine the potential function of endogenous Akirin2 during Xenopus development, knockdown experiments were carried out with specific morpholinos (MO) against Akirin2, which efficiently blocked the expression of a GFP reporter carrying its targeted sequence when co-injected into Xenopus embryos (supplemental Fig. S2).
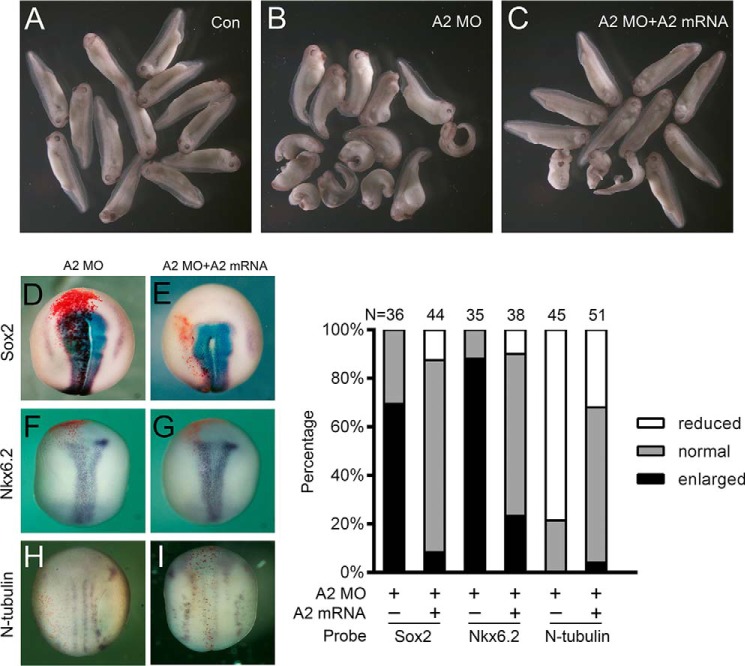
Interestingly, knockdown of Akirin2 led to slight dorsalization of the embryos, with enlarged dorsal structures and shortened trunks, an effect that was largely rescued by co-injection of Akirin2 mRNA (Fig. 2, A–C). At the neurula stage, the expression of Sox2 and Nkx6.2, the pan-neural and ventral neural progenitor markers, was expanded in Akirin2 MO-injected embryos, and this effect was also well rescued by co-injected Akirin2 mRNA (Fig. 2, D–G and J). The Akirin2 MO also inhibited the expression of the neuron marker N-tubulin, which was restored by Akirin2 mRNA (Fig. 2, H–J). No clear effect on Sox2 expression was observed when Akirin2 was overexpressed (data not shown). Akirin1 appears to work differently than Akirin2 during neural development because overexpression of Akirin1 expanded Sox2 expression and also did not rescue the effect of Akirin2 MO (supplemental Fig. S3, F–I, L, and M).
Figure 2.
Akirin2 knockdown disrupts neural development in Xenopus. A–C, morphology of tadpoles (stage 37/38) injected with standard MO (25 ng), Akirin2 MO (25 ng), Akirin2 MO (25 ng), and Akirin2 mRNA (0.6 ng). Embryos were injected in both blastomeres at the two-cell stage and raised to tadpole stage. Con, control. D–I, Akirin2 MO (25 ng) with or without Akirin2 mRNA (0.6 ng) was injected into one cell of four-cell-stage embryos, and whole-mount in situ hybridization with probes of Sox2, Nkx6.2 and N-tubulin was processed at St. 14–16. LacZ mRNA was co-injected to trace the injected sides (stained red on the left sides). J, quantification of the effects of the injection of Akirin2 MO or co-injection of Akirin2 MO and Akirin2 mRNA on the expression of Sox2, Nkx6.2, and N-tubulin as shown in D–I.
BAF53a interacts with akirin2 and is involved in xenopus neural development
Because Akirin interacts with the BAF complex subunits BAP60 and BAP55 in flies and BAF60 in mammals during the immune response (29, 30, 32), we examined whether the related Xenopus BAF subunits interact with Akirins and are involved in neural development.
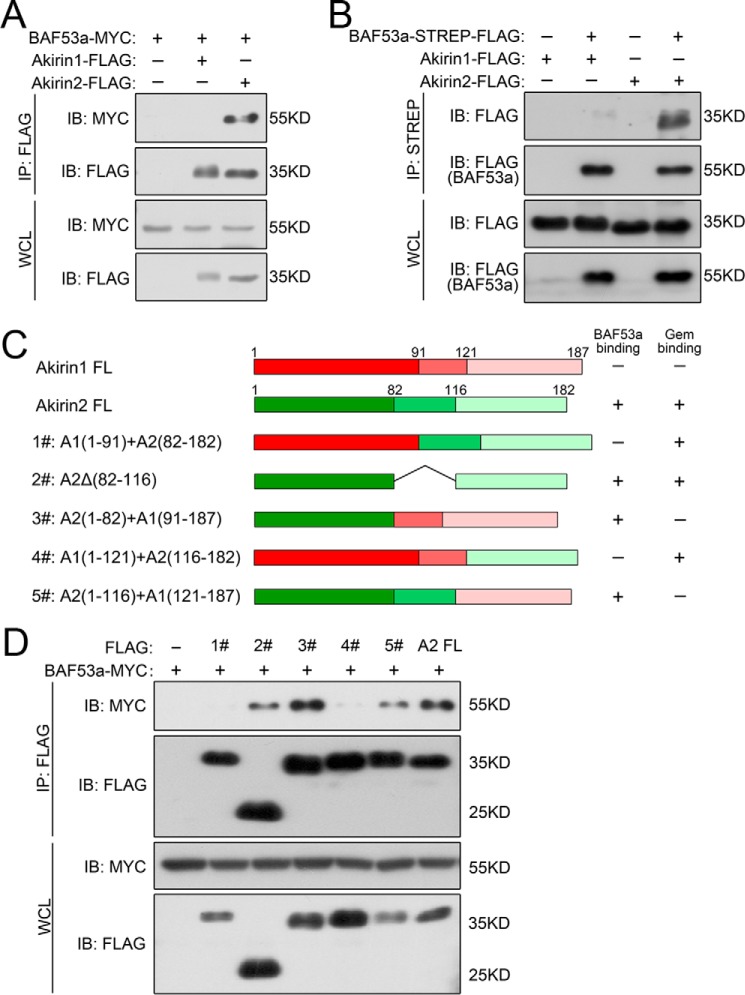
In our co-immunoprecipitation (co-IP) assays, neither XAkirin1 nor XAkirin2 pulled down XBAF60 (data not shown). However, Akirin2, but not Akirin1, did precipitate with BAF53a (the vertebrate homolog of Drosophila BAP55, Fig. 3A). In the reverse experiment, BAF53a also pulled down Akirin2 but not Akirin1 (Fig. 3B). We then investigated the domains involved in Akirin2-BAF53a interaction. Because Akirin1 and Akirin2 share >50% identity at the amino acid level, we constructed a series of Akirin1-Akirin2 chimeric proteins (Fig. 3C) and tested their BAF53a binding activities. The results showed that the N-terminal region (amino acids 1–82) of Akirin2 is responsible for its interaction with BAF53a because only the Akirin1-Akirin2 fusion proteins containing this fragment interacted with BAF53a (Fig. 3, C and D).
Figure 3.
Akirin2 interacts with BAF53a. A and B, co-IP assays of exogenous Akirin2 and BAF53a proteins in HEK293 cells. The cells were transfected with the indicated plasmids, and the cell extracts were immunoprecipitated and immunoblotted (IB) with the indicated antibodies. WCL, whole cell lysate. C, schematic of the Akirin1-Akirin2 fusion truncations with amino acid numbers indicated. FL, full-length. D, interactions of various Akirin1-Akirin2 fusion constructs with BAF53a in co-IP experiments.
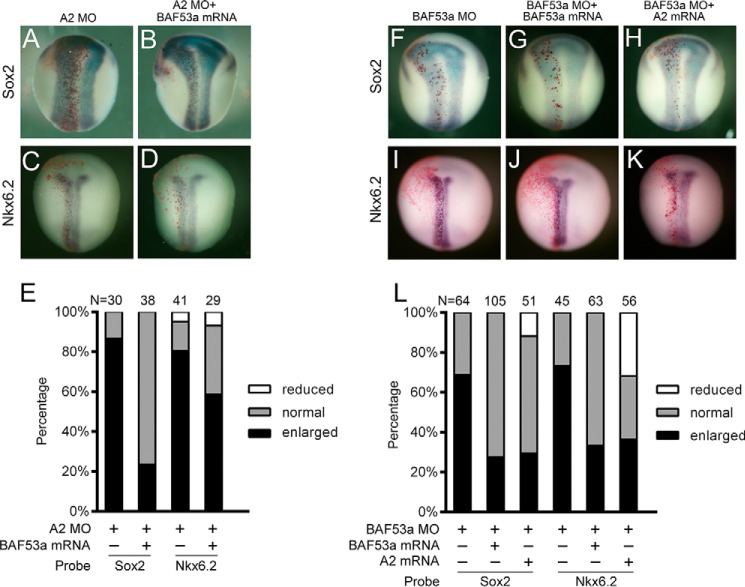
RT-PCR and whole-mount in situ hybridization analysis revealed that BAF53a is widely expressed in the developing nervous system in Xenopus embryos, similarly to Akirins (supplemental Fig. S4, A–J). Interestingly, co-injection of BAF53a mRNA rescued the expansion of Sox2 and Nkx6.2 expression in Akirin2 morphants (Fig. 4, A–E). In addition, MO-mediated knockdown of BAF53a produced a phenotype mimicking that of the Akirin2 morphants, including expanded Sox2 and Nkx6.2 expression, which was rescued by co-injection with BAF53a mRNA (Fig. 4, F, G, I, J, and L, and supplemental Fig. S4K). In addition, co-injection of Akirin2 mRNA restored the expansion of Sox2 and Nkx6.2 in the BAF53a morphants (Fig. 4, H, K, and L). Thus, Akirin2 interacts with BAF53a at a molecular and functional level.
Figure 4.
BAF53a and Akirin2 are involved in Xenopus neural development. A–D, co-injection with BAF53a mRNA (1.0 ng) rescues the expansion of Sox2 and Nkx6.2 in the Akirin2 morphants. LacZ mRNA was co-injected to trace the injected sides (stained red on the left sides). E, quantification of the effects of the injection of Akirin2 MO or co-injection of Akirin2 MO and BAF53a mRNA on the expression of Sox2 and Nkx6.2, as shown in A–D. F–K, co-injection with BAF53a mRNA (1.0 ng) or Akirin2 mRNA (1.0 ng) rescues the expansion of Sox2 and Nkx6.2 in the BAF53a morphants. LacZ mRNA was co-injected to trace the injected sides (stained red on the left sides). L, quantification of the effects of the injection of BAF53a MO or co-injection of BAF53a MO and BAF53a mRNA or Akirin2 mRNA on the expression of Sox2 and Nkx6.2 as shown in F–K.
Akirin2 and geminin antagonistically regulate Sox2 expression
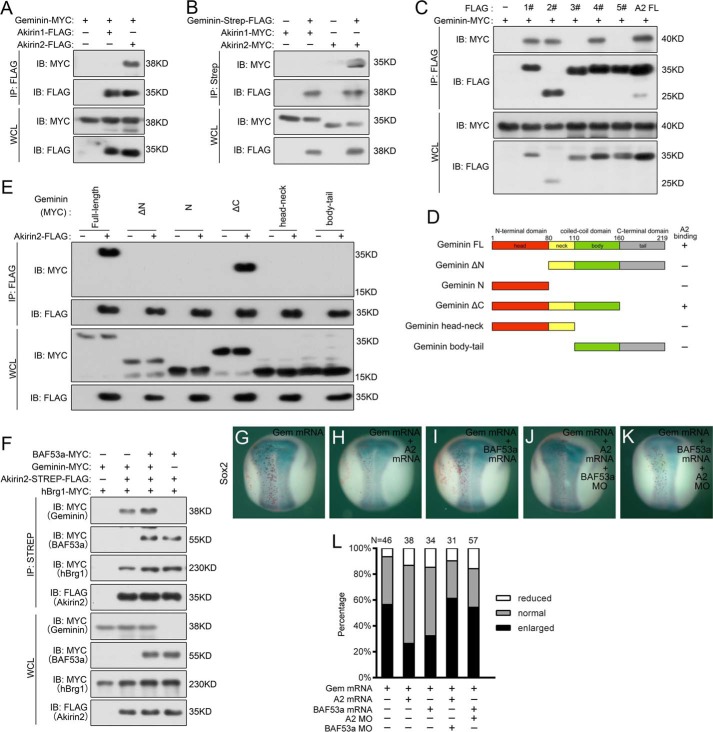
Geminin, another important interaction factor of the BAF complex, is also highly expressed in early embryonic neural precursor cells in Xenopus (20, 21, 23, 34). There is increasing evidence for the active involvement of Geminin in the induction of Sox2 expression through an SWI/SNF-dependent mechanism during vertebrate early neural development (17–19, 21, 34). Interestingly, co-IP experiments showed that Akirin2, but not Akirin1, pulled down Geminin and vice versa (Fig. 5, A and B). To define interacting regions within Akirin2 and Geminin, a series of the Akirin1-Akirin2 fusion constructs and Geminin deletion constructs were tested for their interaction (Figs. 3C and 5D). In co-IP experiments, the Akirin1-Akirin2 constructs containing the Akirin2 C-terminal region (amino acids 116–182) strongly associated with Geminin whereas other mutants did not, thus suggesting that the C-terminal region of Akirin2 is responsible for its interaction with Geminin (Figs. 3C and 5C). Analysis of the Geminin variants revealed that Geminin amino acids 1–160 were required for its interaction with Akirin2 (Fig. 5, D and E). Moreover, BAF53a enhanced the interaction of Akirin2 and Geminin (Fig. 5F), but BAF53a did not directly interact with Geminin (data not shown).
Figure 5.
Akirin2 and Geminin antagonistically regulate Sox2 expression. A and B, co-IP assays of exogenous Akirin2 and Geminin proteins in HEK293 cells. The cells were transfected with the indicated plasmids, and the cell extracts were immunoprecipitated and detected with the indicated antibodies. IB, immunoblot; WCL, whole cell lysate. C, interactions of various Akirin1-Akirin2 fusion constructs with Geminin in co-IP experiments. D, schematic of the Geminin truncations with amino acid numbers indicated. FL, full-length. E, interactions of various Geminin truncation constructs with Akirin2 in co-IP experiments. F, BAF53a enhances the interaction of Akirin2 and Geminin. G–K, co-injection with either Akirin2 or BAF53a mRNA rescues the expansion of Sox2 in Geminin misexpression embryos, and this effect was reversed by co-injection of BAF53a MO or Akirin2 MO. LacZ mRNA was co-injected to trace the injected sides (stained red on the left sides). L, quantification of the effect of the injection of mRNA or co-injection of MO as shown in G–J.
Geminin overexpression results in excess neural progenitor cells as marked by Sox2 expansion, as reported previously (Fig. 5, G and L) (23, 34). Interestingly, co-injection of Akirin2 or BAF53a mRNA alone inhibited the ability of Geminin to stimulate Sox2 expression, and this effect was reversed by co-injection of BAF53a MO or Akirin2 MO (Fig. 5, H–L). These data suggested that Akirin2 recruits BAF53a, thus resulting in antagonism of Geminin during Xenopus neural development.
Akirin2 functions in parallel with Ngnr1 during early neurogenesis in xenopus
Knockdown of Akirin2 also inhibited neurogenesis, as indicated by loss of N-tubulin expression (Fig. 2, H–J). Early neurogenesis in Xenopus is controlled by the Ngnr1-NeuroD-N-tubulin gene activation relay cascade (10–13). We found that, in addition to N-tubulin, the expression of NeuroD, but not Ngnr1, was also affected in the Akrin2 morphants, an effect that was largely rescued by co-injection with Akirin2 mRNA (Fig. 6, A, B, and I, and supplemental Fig. S5A). When overexpressed, Ngnr1 efficiently induced ectopic expression of NeuroD and N-tubulin (Fig. 6, C, E, and I). However, its activity was strongly inhibited in the Akirin2 morphants (Fig. 6, D, F, and I). In contrast, the ability of NeuroD to induce N-tubulin was not affected when Akirin2 was knocked down (Fig. 6, G–I). Additionally, injection of Ngnr1 mRNA had no effect on the expression of Akirin2 (supplemental Fig. S5B). Thus, Akirin2 functions in parallel with Ngnr1 in inducing NeuroD, regulating early neurogenesis in Xenopus.
Figure 6.
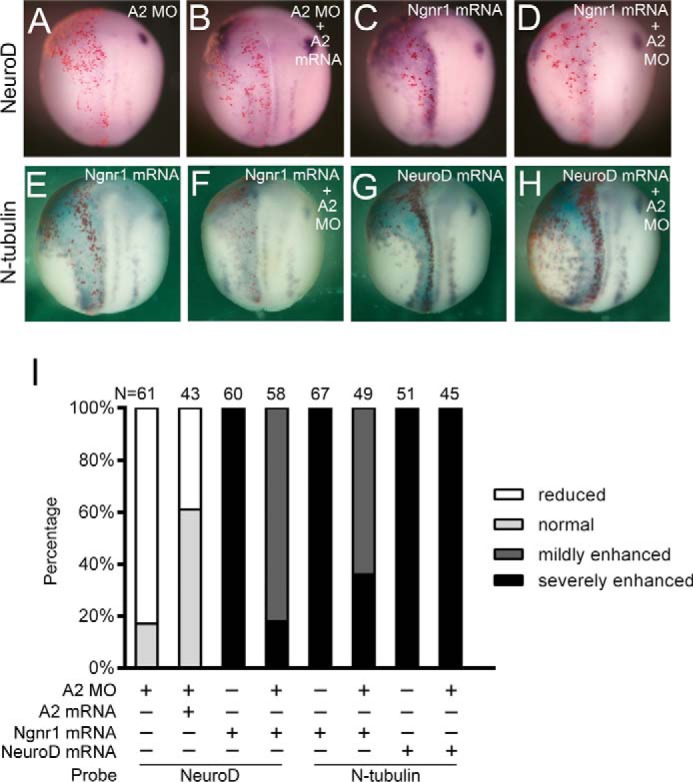
Akirin2 functions in parallel with Ngnr1 during early neurogenesis in Xenopus. A and B, co-injection with Akirin2 mRNA (0.6 ng) rescues the reduction of NeuroD in the Akirin2 morphants. LacZ mRNA was co-injected to trace the injected sides (stained red on the left sides). C–F, co-injection with Akirin2 MO (0.6 ng) inhibits the activation of NeuroD (C and D) and N-tubulin (E and F) in Ngnr1 misexpression embryos. LacZ mRNA was co-injected to trace the injected sides (stained red on the left sides). G and H, co-injection with Akirin2 MO (0.6 ng) cannot inhibit the activation of N-tubulin in NeuroD misexpression embryos. LacZ mRNA was co-injected to trace the injected sides (stained red on the left sides). I, quantification of the effects of the injection of A2 MO, Ngnr1 mRNA, or NeuroD mRNA or co-injection of A2 MO mRNA and Akirin2/Ngnr1/NeuroD mRNA on the expression of NeuroD or N-tubulin as shown in A–H.
Discussion
Together, our data suggest two independent roles of Akirin2 in Xenopus neural development: modulation of Sox2 expression through recruitment to the BAF remodeling complex in the neural precursor cells, thereby antagonizing Geminin activity, and promotion of NeuroD and N-tubulin expression in parallel with Ngnr1 during early neurogenesis (Fig. 7).
Figure 7.
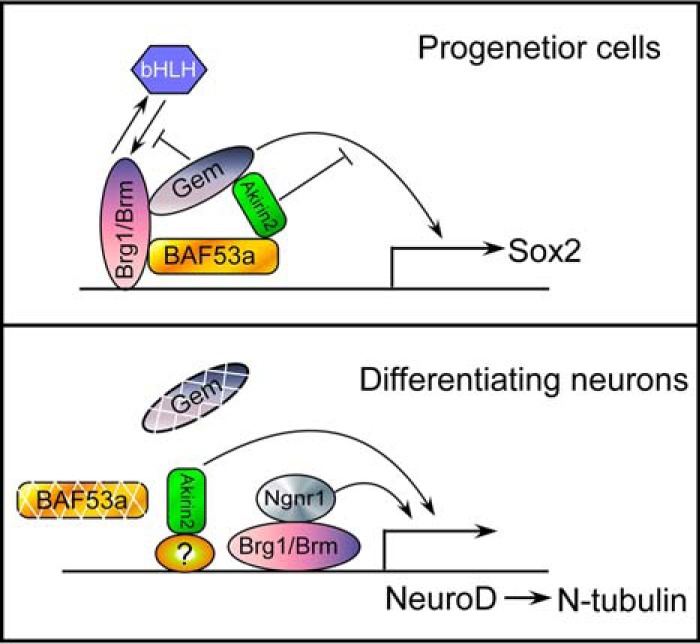
Proposed model for the coordination and temporal control of Xenopus neural development by Akirin2. In neural progenitor cells, Akirin2 acts antagonistically to Geminin in regulating Sox2 expression, and it maintains the neural precursor state after being recruited into BAF by BAF53a. Akirin2 also modulates N-tubulin expression by acting upstream of NeuroD, functioning in parallel with Ngnr1 during terminal neuronal differentiation when BAF53a and Geminin disappear.
Akirin1 and Akirin2 are paralogs that arose through gene duplication in a common ancestor to the vertebrate lineage (31). Mammals and amphibians retain both paralogs, whereas avians have lost Akirin1 (31). The current view is that Akirin1 has diverged because of its significantly faster rate of evolution in multiple sites relative to Akirin or Akirin2 (31). A series of related morphological defects ranging from apparent truncation of anterior structures to reduction of the eye or no eye were observed in Akirin1 morphants (supplemental Figs. S2 and S3, A–E). The Sox2 expression reduced rather than expanded, as in Akirin2 morphants, and co-injection with Akirin1 mRNAs can partly rescue the reduced Sox2 expression in Akirin1 morphants (supplemental Fig. S3, F, G, and L). However, as Akitin2 MO-injection did, Akirin1 MO-injection also inhibited the expression of N-tubulin, which could be rescued by co-injection with its mRNAs (supplemental Fig. S3, J–L). The functions of Akirins also appear to have diverged during vertebrate evolution, as suggested by our study and previous studies in mice (24, 32, 35).
It has been suggested that Akirin functions as a general cofactor for gene expression through interactions with SWI/SNF chromatin remodeling complexes. For Xenopus Akirins, we did not observe direct interaction with BAF60, as has been observed for its homologs in flies and mice (data not shown) (26, 29, 30), but we cannot rule out their potential context-dependent interactions because the protein-protein interactions were tested in mammalian cells in this study. However, Xenopus Akirin2, but not Akirin1, did interact with BAF53a (the homolog of Drosophila BAP55) both molecularly and functionally (Figs. 3 and 4). The BAF53a interacting N-terminal portion (amino acids 1–82) of XAkirin2 shares high identity among Akirin2 proteins of other species but is less conserved among Akirin1 and Akirin2 proteins of different species (31, 37–39). During neurogenesis, npBAF is replaced with nBAF in mice, in which BAF53a is replaced with BAF53b (40, 41). It is also possible that Akirin2 may regulate NeuroD expression through interaction with nBAF during neurogenesis. However, we were unable to identify the BAF53b homolog in the Xenopus genome, and whether such a mechanism has been conserved during evolution remains unknown.
Geminin is a bifunctional interactor of the npBAF complex and helps to maintain neural progenitors in an undifferentiated state by directly promoting Sox2 expression and antagonizing the preneural bHLH factors being recruited to drive neurogenesis (Fig. 7) (20, 21, 23, 42). Geminin blocks the association of Brg1 and proneural bHLH proteins, thus inhibiting neurogenesis, probably through occupation of a functional bHLH-binding site (20, 21). It remains unknown whether BAF53a and Geminin are down-regulated before or during neuronal differentiation in Xenopus as it is in mice (19, 40, 41, 43–45).
Moreover, a recent study in mice has reported decreases in Tuj1+ neurons and normal expression of Ngn2 (the homolog of Ngnr1 in Xenopus) during cortical development in Akirin2 conditional knock-out mice (33). Although significant apoptosis and decreased cell proliferation have been observed in Akirin2-null mice, their Sox2-positive progenitors have been found to expand and spill out of their territory (33). Therefore, these data suggest a conserved role of Akirin2 in vertebrates during neural development. An interesting line of future investigation will be to further examine the role played by Akirin in regulating gene expression and signaling cascades in neural development.
Experimental procedures
Ethics statement
The care of X. laevis (Nasco), in vitro fertilization procedures, and manipulation of embryos were performed according to standard protocols. All animal protocols were approved by the Ethics Committee of Kunming Institute of Zoology, Chinese Academy of Sciences (permit no. SYDW-2006-006).
Plasmid construction
Full-length X. laevis Akirin1, Akirin2, and BAF53a and the Geminin coding region were obtained by PCR according to sequences in NCBI (Akirin1, NM_001095776.1; Akirin2, NM_001092015.1; BAF53a, NM_001086982.1; Geminin, NM_001090747.1) and then cloned into pCS2+-N-FLAG/pCS2+-N-Myc/pCS2+-N-Strep-FLAG vectors for co-IP assays. The hBrg1 plasmid was a kind gift from Dr. Kristen L. Kroll (Washington University School of Medicine).
RT-PCR
Embryonic total RNAs were extracted using TRIzol total RNA extract reagent (Tiangen) and were reverse-transcribed using a Fermentas RevertAidTM first strand cDNA synthesis kit to prepare templates for semiquantitative PCR. The primers used were as follows: Akirin1, 5′-TCTCCTCAGAGATGCGCCATTA-3′ and 5′-CGCATGATCTGGTCATGTGTG-3′; Akirin2, 5′-ATGGCGTGTGGAGCCACACTTAAA-3′ and 5′-TCATGAAACGTAGCTAGCTGGCTG-3′; and BAF53a, 5′-AGGCGTTTATGGCGGAGATGA-3′ and 5′AGCCACTATCCAAGATGAGCC-3′. H4 was used as a loading control.
Embryo microinjection and whole-mount in situ hybridization
In vitro fertilization, embryo culture, whole-mount in situ hybridization, preparation of mRNA, and microinjection were carried out as described previously (46). The sequences of the antisense MO for Akirin2 and BAF53a, which were obtained from Gene Tools, were as follows: Akirin2 MO, 5′-CACCTAGAAACACAACAATGCCCAC-3′; BAF53a MO, 5′-CATAAACGCCTCCGCTCATATCCAG-3′. MO and mRNA were injected into the dorsal region of two- to four-cell stage embryos. 25 ng of MO was injected per stitch, and 0.8 ng of Akirin1 mRNA or 50 pg of Geminin/Ngnr1/NeuroD mRNA was injected in overexpression experiments. 0.8 ng of Akirin1 mRNA, 0.6 ng of Akirin2 mRNA, or 1.0 ng of BAF53a mRNA was co-injected in rescue experiments. For in situ hybridization, the probes of Sox2, Nkx6.2, N-tubulin, Akirin1, Akirin2, BAF53a, NeuroD, and Ngnr1 were used as described previously (46, 47). Expression plasmids for Akirin1, Akirin2, BAF53a, Geminin, Ngnr1, and NeuroD were all cloned into the pCS2+ vector. Capped mRNAs for microinjection were synthesized with an SP6 mMessage mMachine kit (Ambion).
Cell culture, transfection, Co-IP assay, and immunoblotting
HEK293 cells were maintained in DMEM containing 10% FBS. Plasmids were transfected using Lipofectamine 2000 (Invitrogen) according to the instructions of the manufacturer. Co-IP assays and Western blotting analysis were conducted as described previously (48). The antibodies used were as follows: anti-FLAG (M2, Sigma) and anti-Myc (Sigma). Horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (Pierce) was used as the secondary antibody.
Author contributions
B. M., X. L., Y. X., and P. M. conceived and designed the experiments. X. L., Y. X., P. M., J. T., L. M., and C. L. performed the experiments. X. L., Y. X., and P. M. analyzed the data. X. L., Y. X., P. M., J. T., L. M., and C. L. contributed reagents/materials/analysis tools. B. M., X. L., Y. X., and P. M. wrote the paper.
Supplementary Material
Acknowledgment
We thank Dr. Kristen L. Kroll (Washington University School of Medicine) for the hBrg1 plasmid.
This work was supported by National Natural Science Foundation of China Grant 31171404 (to B. Y. M.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figs. S1–S5.
- bHLH
- basic helix-loop-helix
- BAF
- Brg1/Brm-associated factor
- npBAF
- neural progenitor cell-specific Brg1/Brm-associated factor
- nBAF
- neuron-specific Brg1/Brm-associated factor
- BAP
- Brahma-associated protein
- MO
- morpholino(s)
- co-IP
- co-immunoprecipitation
- St.
- stage.
References
- 1. Cremisi F., Philpott A., and Ohnuma S. (2003) Cell cycle and cell fate interactions in neural development. Curr. Opin. Neurobiol. 13, 26–33 [DOI] [PubMed] [Google Scholar]
- 2. Bally-Cuif L., and Hammerschmidt M. (2003) Induction and patterning of neuronal development, and its connection to cell cycle control. Curr. Opin. Neurobiol. 13, 16–25 [DOI] [PubMed] [Google Scholar]
- 3. Doniach T. (1993) Planar and vertical induction of anteroposterior pattern during the development of the amphibian central nervous system. J. Neurobiol. 24, 1256–1275 [DOI] [PubMed] [Google Scholar]
- 4. Mathis L., and Nicolas J. F. (2002) Cellular patterning of the vertebrate embryo. Trends Genet. 18, 627–635 [DOI] [PubMed] [Google Scholar]
- 5. Edlund T., and Jessell T. M. (1999) Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell 96, 211–224 [DOI] [PubMed] [Google Scholar]
- 6. Graham V., Khudyakov J., Ellis P., and Pevny L. (2003) SOX2 functions to maintain neural progenitor identity. Neuron 39, 749–765 [DOI] [PubMed] [Google Scholar]
- 7. Avilion A. A., Nicolis S. K., Pevny L. H., Perez L., Vivian N., and Lovell-Badge R. (2003) Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17, 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kishi M., Mizuseki K., Sasai N., Yamazaki H., Shiota K., Nakanishi S., and Sasai Y. (2000) Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm. Development 127, 791–800 [DOI] [PubMed] [Google Scholar]
- 9. Neilson K. M., Klein S. L., Mhaske P., Mood K., Daar I. O., and Moody S. A. (2012) Specific domains of FoxD4/5 activate and repress neural transcription factor genes to control the progression of immature neural ectoderm to differentiating neural plate. Dev. Biol. 365, 363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma Q., Kintner C., and Anderson D. J. (1996) Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87, 43–52 [DOI] [PubMed] [Google Scholar]
- 11. Lee J. E., Hollenberg S. M., Snider L., Turner D. L., Lipnick N., and Weintraub H. (1995) Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science 268, 836–844 [DOI] [PubMed] [Google Scholar]
- 12. Bertrand N., Castro D. S., and Guillemot F. (2002) Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 3, 517–530 [DOI] [PubMed] [Google Scholar]
- 13. Farah M. H., Olson J. M., Sucic H. B., Hume R. I., Tapscott S. J., and Turner D. L. (2000) Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development 127, 693–702 [DOI] [PubMed] [Google Scholar]
- 14. Narayanan R., and Tuoc T. C. (2014) Roles of chromatin remodeling BAF complex in neural differentiation and reprogramming. Cell Tissue Res. 356, 575–584 [DOI] [PubMed] [Google Scholar]
- 15. Yoo A. S., and Crabtree G. R. (2009) ATP-dependent chromatin remodeling in neural development. Curr. Opin. Neurobiol. 19, 120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho L., and Crabtree G. R. (2010) Chromatin remodelling during development. Nature 463, 474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kondo T., and Raff M. (2004) Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev. 18, 2963–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papanayotou C., Mey A., Birot A. M., Saka Y., Boast S., Smith J. C., Samarut J., and Stern C. D. (2008) A mechanism regulating the onset of Sox2 expression in the embryonic neural plate. PLoS Biol. 6, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang V. S., Carter S. A., Hyland S. J., Tachibana-Konwalski K., Laskey R. A., and Gonzalez M. A. (2011) Geminin escapes degradation in G1 of mouse pluripotent cells and mediates the expression of Oct4, Sox2, and Nanog. Curr. Biol. 21, 692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seo S., Herr A., Lim J. W., Richardson G. A., Richardson H., and Kroll K. L. (2005) Geminin regulates neuronal differentiation by antagonizing Brg1 activity. Genes Dev. 19, 1723–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seo S., and Kroll K. L. (2006) Geminin's double life chromatin connections that regulate transcription at the transition from proliferation to differentiation. Cell Cycle 5, 374–379 [DOI] [PubMed] [Google Scholar]
- 22. Aigner S., and Gage F. H. (2005) A small gem with great powers: geminin keeps neural progenitors thriving. Dev. Cell 9, 171–172 [DOI] [PubMed] [Google Scholar]
- 23. Kroll K. L. (2007) Geminin in embryonic development: coordinating transcription and the cell cycle during differentiation. Front. Biosci. 12, 1395–1409 [DOI] [PubMed] [Google Scholar]
- 24. Goto A., Matsushita K., Gesellchen V., El Chamy L., Kuttenkeuler D., Takeuchi O., Hoffmann J. A., Akira S., Boutros M., and Reichhart J. M. (2008) Akirins are highly conserved nuclear proteins required for NF-κB-dependent gene expression in Drosophila and mice. Nat. Immunol. 9, 97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salerno M. S., Dyer K., Bracegirdle J., Platt L., Thomas M., Siriett V., Kambadur R., and Sharma M. (2009) Akirin1 (Mighty), a novel promyogenic factor regulates muscle regeneration and cell chemotaxis. Exp. Cell Res. 315, 2012–2021 [DOI] [PubMed] [Google Scholar]
- 26. Nowak S. J., Aihara H., Gonzalez K., Nibu Y., and Baylies M. K. (2012) Akirin links twist-regulated transcription with the Brahma chromatin remodeling complex during embryogenesis. PLoS Genet. 8, e1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu N., Wang X. W., Sun J. J., Wang L., Zhang H. W., Zhao X. F., and Wang J. X. (2016) Akirin interacts with Bap60 and 14-3-3 proteins to regulate the expression of antimicrobial peptides in the kuruma shrimp (Marsupenaeus japonicus). Dev. Comp. Immunol. 55, 80–89 [DOI] [PubMed] [Google Scholar]
- 28. Nowak S. J., and Baylies M. K. (2012) Akirin: a context-dependent link between transcription and chromatin remodeling. Bioarchitecture 2, 209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonnay F., Nguyen X. H., Cohen-Berros E., Troxler L., Batsche E., Camonis J., Takeuchi O., Reichhart J. M., and Matt N. (2014) Akirin specifies NF-κB selectivity of Drosophila innate immune response via chromatin remodeling. EMBO J. 33, 2349–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tartey S., Matsushita K., Vandenbon A., Ori D., Imamura T., Mino T., Standley D. M., Hoffmann J. A., Reichhart J. M., Akira S., and Takeuchi O. (2014) Akirin2 is critical for inducing inflammatory genes by bridging IκB-ζ and the SWI/SNF complex. EMBO J. 33, 2332–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Macqueen D. J., and Johnston I. A. (2009) Evolution of the multifaceted eukaryotic akirin gene family. BMC Evol. Biol. 9, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tartey S., and Takeuchi O. (2015) Chromatin remodeling and transcriptional control in innate immunity: emergence of Akirin2 as a novel player. Biomolecules 5, 1618–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bosch P. J., Fuller L. C., Sleeth C. M., and Weiner J. A. (2016) Akirin2 is essential for the formation of the cerebral cortex. Neural Dev. 11, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kroll K. L., Salic A. N., Evans L. M., and Kirschner M. W. (1998) Geminin, a neuralizing molecule that demarcates the future neural plate at the onset of gastrulation. Development 125, 3247–3258 [DOI] [PubMed] [Google Scholar]
- 35. Liu T., Gao Y., and Xu T. (2015) Evolution of akirin family in gene and genome levels and coexpressed patterns among family members and rel gene in croaker. Dev. Comp. Immunol. 52, 17–25 [DOI] [PubMed] [Google Scholar]
- 36. Deleted in proof
- 37. Chen X., Huang Z., Jia G., Wu X., and Wu C. (2012) Molecular cloning, tissue distribution, and functional analysis of porcine Akirin2. Anim. Biotechnol. 23, 124–131 [DOI] [PubMed] [Google Scholar]
- 38. Hou F., Wang X., Qian Z., Liu Q., Liu Y., He S., Mi X., Bai C., Sun C., and Liu X. (2013) Identification and functional studies of Akirin, a potential positive nuclear factor of NF-κB signaling pathways in the Pacific white shrimp, Litopenaeus vannamei. Dev. Comp. Immunol. 41, 703–714 [DOI] [PubMed] [Google Scholar]
- 39. Yan J., Dong X., Kong Y., Zhang Y., Jing R., and Feng L. (2013) Identification and primary immune characteristics of an amphioxus akirin homolog. Fish Shellfish Immunol. 35, 564–571 [DOI] [PubMed] [Google Scholar]
- 40. Lessard J., Wu J. I., Ranish J. A., Wan M., Winslow M. M., Staahl B. T., Wu H., Aebersold R., Graef I. A., and Crabtree G. R. (2007) An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron 55, 201–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu J. I., Lessard J., Olave I. A., Qiu Z., Ghosh A., Graef I. A., and Crabtree G. R. (2007) Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron 56, 94–108 [DOI] [PubMed] [Google Scholar]
- 42. Yellajoshyula D., Lim J. W., Thompson D. M. Jr, Witt J. S., Patterson E. S., and Kroll K. L. (2012) Geminin regulates the transcriptional and epigenetic status of neuronal fate-promoting genes during mammalian neurogenesis. Mol. Cell. Biol. 32, 4549–4560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yellajoshyula D., Patterson E. S., Elitt M. S., and Kroll K. L. (2011) Geminin promotes neural fate acquisition of embryonic stem cells by maintaining chromatin in an accessible and hyperacetylated state. Proc. Natl. Acad. Sci. U.S.A. 108, 3294–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gonzalez M. A., Tachibana K. E., Adams D. J., van der Weyden L., Hemberger M., Coleman N., Bradley A., and Laskey R. A. (2006) Geminin is essential to prevent endoreduplication and to form pluripotent cells during mammalian development. Genes Dev. 20, 1880–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lim J. W., Hummert P., Mills J. C., and Kroll K. L. (2011) Geminin cooperates with Polycomb to restrain multi-lineage commitment in the early embryo. Development 138, 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ma P., Xia Y., Ma L., Zhao S., and Mao B. (2013) Xenopus Nkx6.1 and Nkx6.2 are required for mid-hindbrain boundary development. Dev. Genes Evol. 223, 253–259 [DOI] [PubMed] [Google Scholar]
- 47. Seo S., Richardson G. A., and Kroll K. L. (2005) The SWI/SNF chromatin remodeling protein Brg1 is required for vertebrate neurogenesis and mediates transactivation of Ngn and NeuroD. Development 132, 105–115 [DOI] [PubMed] [Google Scholar]
- 48. Ma P., Yang X., Kong Q., Li C., Yang S., Li Y., and Mao B. (2014) The ubiquitin ligase RNF220 enhances canonical Wnt signaling through USP7-mediated deubiquitination of β-catenin. Mol. Cell. Biol. 34, 4355–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.