Abstract
G protein-coupled receptors (GPCRs) regulate many animal behaviors. GPCR signaling is mediated by agonist-promoted interactions of GPCRs with heterotrimeric G proteins, GPCR kinases (GRKs), and arrestins. To further elucidate the role of GRKs in regulating GPCR-mediated behaviors, we utilized the genetic model system Caenorhabditis elegans. Our studies demonstrate that grk-2 loss-of-function strains are egg laying-defective and contain low levels of serotonin (5-HT) and high levels of the 5-HT metabolite 5-hydroxyindole acetic acid (5-HIAA). The egg laying defect could be rescued by the expression of wild type but not by catalytically inactive grk-2 or by the selective expression of grk-2 in hermaphrodite-specific neurons. The addition of 5-HT or inhibition of 5-HT metabolism also rescued the egg laying defect. Furthermore, we demonstrate that AMX-2 is the primary monoamine oxidase that metabolizes 5-HT in C. elegans, and we also found that grk-2 loss-of-function strains have abnormally high levels of AMX-2 compared with wild-type nematodes. Interestingly, GRK-2 was also found to interact with and promote the phosphorylation of AMX-2. Additional studies reveal that 5-HIAA functions to inhibit egg laying in a manner dependent on the 5-HT receptor SER-1 and the G protein GOA-1. These results demonstrate that GRK-2 modulates 5-HT metabolism by regulating AMX-2 function and that 5-HIAA may function in the SER-1 signaling pathway.
Keywords: G protein, G protein-coupled receptor (GPCR), protein kinase, serotonin, signal transduction, 5-hydroxyindole acetic acid, egg laying, monoamine oxidase
Introduction
G protein-coupled receptor (GPCR)2 signaling regulates multiple behaviors in eukaryotic organisms. Agonist binding to GPCRs promotes conformational changes that drive interaction with three protein families, heterotrimeric G proteins, GPCR kinases (GRKs), and arrestins. Heterotrimeric G proteins function to regulate downstream effectors and signaling, whereas GRKs and arrestins function to attenuate G protein signaling and facilitate GPCR trafficking and arrestin-mediated signaling (1, 2). GRKs are serine/threonine protein kinases that play a central role in receptor regulation by their ability to selectively bind and phosphorylate agonist-occupied GPCRs. The seven mammalian GRKs can be divided into three subfamilies, GRK1 and -7; GRK2 and -3; and GRK4, -5, and -6. Although these kinases have been extensively studied in vitro and implicated in a number of diseases (3), the link between GRK structure and function in normal physiology and disease is less well defined.
Caenorhabditis elegans is a powerful model organism for identifying the physiological role of GPCR signaling. GPCRs coordinately regulate behaviors such as egg laying, locomotion, and feeding via activation of the Gq ortholog EGL-30, which stimulates these behaviors, and the Go ortholog GOA-1, which attenuates these behaviors (4, 5). Although the C. elegans genome contains a large number of GPCRs (∼1600) and heterotrimeric G proteins (20 Gα subunits, two β subunits, and two γ subunits), it has only two GRKs (GRK-1 and GRK-2) and a single arrestin (ARR-1) (6). GRK-2 is an ortholog for mammalian GRK2 and GRK3 and has been shown to play a role in chemosensation (7), whereas GRK-1, an ortholog for mammalian GRK4–6, has a role in dopamine-mediated behavior (8). Although the mechanism mediating GRK-2 function in chemosensation is poorly defined, recent studies have shown that the kinase activity of GRK-2 and its interactions with GPCRs, Gβγ subunits, and phospholipids are critical to this function (9). Moreover, reduced chemosensory responsiveness in grk-2 loss-of-function strains may involve gene expression changes that alter bitter taste responses (10). In addition, although the loss of GRK-2 impairs NaCl sensing, GRK-2 also appears to desensitize NaCl avoidance signaling in nociceptive neurons (11). Thus, GRK-2 plays an important role in regulating chemoresponsiveness in sensory neurons.
One of the better characterized behaviors in C. elegans is egg laying. The egg laying cellular machinery includes hermaphrodite-specific neurons (HSNs) that are regulated by ventral type C motor neurons, which regulate the release of serotonin (5-hydroxytryptamine (5-HT)) and neuropeptides that modulate the egg laying response. The eight vulval and eight uterine muscle cells are also essential for egg laying (5). The 5-HT that is released from the HSNs plays a central role in this process by activating 5-HT receptors on vulval muscle cells to increase the frequency of calcium transients, likely via activation of the Ca2+ channel EGL-19 that promotes muscle contraction (12, 13).
Because GPCRs are critically involved in egg laying and because GRKs play an important role in regulating GPCR function, we hypothesized that GRKs would regulate egg laying behavior in C. elegans. We initially focused on GRK-2 and found that grk-2 loss-of-function (lf) strains were egg laying-defective due to reduced levels of 5-HT. Interestingly, grk-2(lf) worms have significantly increased levels of the 5-HT metabolite 5-hydroxyindole acetic acid (5-HIAA), which appears to contribute to the egg laying defect. The egg laying defect can be rescued by the addition of 5-HT, by inhibiting 5-HT metabolism, or by expression of GRK-2 in HSNs. We also found that AMX-2 is the primary monoamine oxidase for 5-HT in C. elegans and that the grk-2 mutant strains have significantly increased expression of AMX-2. These results demonstrate that GRK-2 controls the metabolism of 5-HT by regulating monoamine oxidase function.
Results
Loss-of-function grk-2 mutants are egg laying-defective
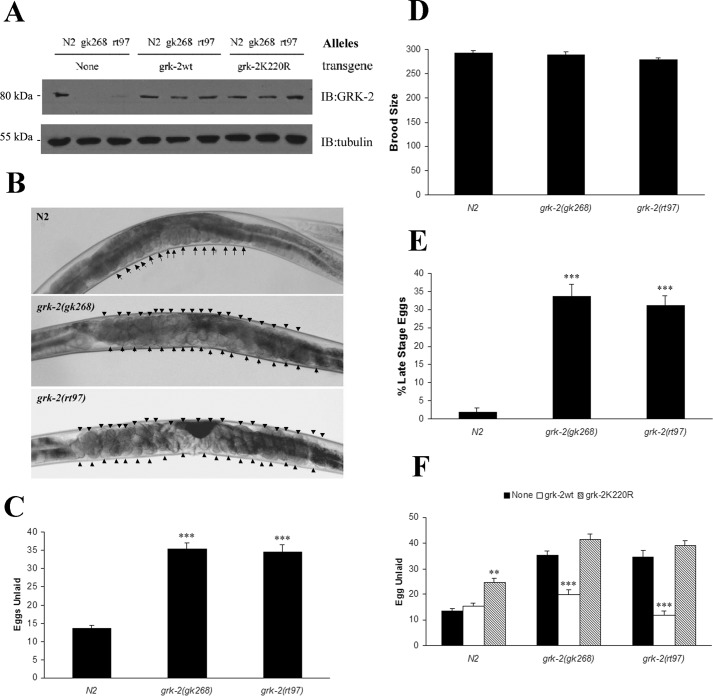
Prior studies have shown that C. elegans GRK-2 plays a critical role in chemosensory responsiveness (7, 9). To further investigate the role of GRK-2 in C. elegans, we evaluated the role of two grk-2 alleles in egg laying behavior. The first allele is from a grk-2-null strain bearing deletion of the first three exons of grk-2 (grk-2(gk268)), whereas the other allele contains a T354I point mutation in grk-2 (grk-2(rt97)) that results in significantly reduced expression and activity (7) (Fig. 1A). Because mammalian GRK2 effectively attenuates Gq signaling (14), we investigated whether C. elegans GRK-2 regulates egg laying considering that this behavior is stimulated by Gq signaling (15). Although we anticipated that loss of GRK-2 would result in enhanced Gq signaling and constitutive egg laying, both grk-2(lf) alleles were egg laying-defective as each mutant retained ∼35 eggs compared with 13–14 eggs in the wild-type N2 worms (Figs. 1B and C). Although there was no difference in the brood size between N2 and grk-2 mutants (Fig. 1D), a significant number of the laid eggs were late stage in the grk-2 loss-of-function strains (Fig. 1E). Because we did not observe a significant number of unfertilized eggs or dead embryos in the grk-2 mutant strains, this suggests that the sperm capacity and development of embryos are normal in these strains.
Figure 1.
grk-2 mutants are egg laying-defective. A, Western blot using a GRK2/3 antibody to evaluate GRK-2 protein levels in wild-type (N2), grk-2 mutants (gk268 and rt97), and transgenic worms (None, grk-2wt, and grk-2K220R). B, confocal microscopy of wild type and grk-2 mutants. Eggs in the various strains are marked with an arrow or arrowhead. C, analysis of egg number in N2 and grk-2 mutants. Wild-type worms contain 13–14 eggs, whereas both grk-2 mutants contain ∼35 eggs. The egg laying assay was performed at 40 h after vulva induction in worms grown at 24.5 °C (***, p < 0.001 compared with N2). D, brood size analysis reveals no significant changes in the brood sizes for N2 and grk-2 mutants. E, analysis of the developmental stage of freshly laid eggs reveals that ∼35% of the eggs are comma or later stage in grk-2 mutants compared with ∼2% in N2 (***, p < 0.001 compared with N2). F, the egg laying defect in grk-2 mutants can be rescued by integrated array of wild-type GRK-2 (***, p < 0.001 compared with None) but not by the catalytically inactive GRK-2(K220R). GRK-2(K220R) functions as a dominant negative and inhibits egg laying in wild-type (N2) worms (**, p < 0.01 compared with None). All error bars indicate S.E. IB, immunoblotting.
To assess whether we could rescue the egg laying defect in the grk-2 mutants, we generated transgenic strains expressing either wild-type GRK-2 or catalytically inactive GRK-2(K220R) in the N2 and grk-2 mutant strains (Fig. 1A). We found that the egg laying defect in the grk-2(lf) strains could be effectively rescued by expression of wild-type GRK-2 at levels comparable with those of endogenous GRK-2, whereas there was no rescue in strains expressing GRK-2(K220R) (Fig. 1F). In fact, GRK-2(K220R) appeared to function as a dominant negative to inhibit egg laying when expressed in N2 (Fig. 1F). Although we cannot rule out the possibility that GRK-2(K220R) has a low level of activity, previous studies have shown that it cannot rescue the chemosensory defect in the grk-2(gk268) allele (9). Thus, GRK-2 functions to stimulate egg laying, and the catalytic activity of the kinase is essential for this process.
GRK-2 expression in HSNs functions to regulate egg laying behavior
We next determined the cellular location where GRK-2 may be functioning to regulate egg laying. Previous studies have shown that GRK-2 is expressed in various sensory and ventral nerve chord neurons, interneurons, and vulval muscle (7). We further characterized GRK-2 expression in the vulval area utilizing a grk-2::GFP transgenic strain and found that it was expressed in HSNs, VC4/VC5 neurons, and vulval muscle cells (Fig. 2, A and B). Because each of these cells has a significant role in regulating egg laying, we next evaluated which specific cells contributed to the egg laying defect in the grk-2 mutant strains by driving expression of GRK-2 using cell-specific promoters (5, 16). Expression of GRK-2 in HSNs fully rescued the egg laying defect in grk-2(rt97) and partially rescued the defect in grk-2(gk268) worms, whereas GRK-2 expression in VC neurons was without effect on egg laying (Fig. 2C). Similarly, GRK-2 expression in vulval muscle did not rescue the egg laying defect in grk-2 mutants, and it appeared to cause a modest egg laying defect in the N2 worms (Fig. 2C). Considering that our initial studies demonstrated that the catalytic activity of GRK-2 was essential in rescuing the egg laying defect (Fig. 1F), these results suggest that GRK-2 may phosphorylate proteins in HSNs that regulate egg laying. Moreover, because HSNs regulate the production and release of 5-HT and neuropeptides that modulate egg laying, it is possible that GRK-2 is functioning in these pathways. Thus, HSNs appear to be the primary cell type involved in mediating the effect of GRK-2 on egg laying.
Figure 2.
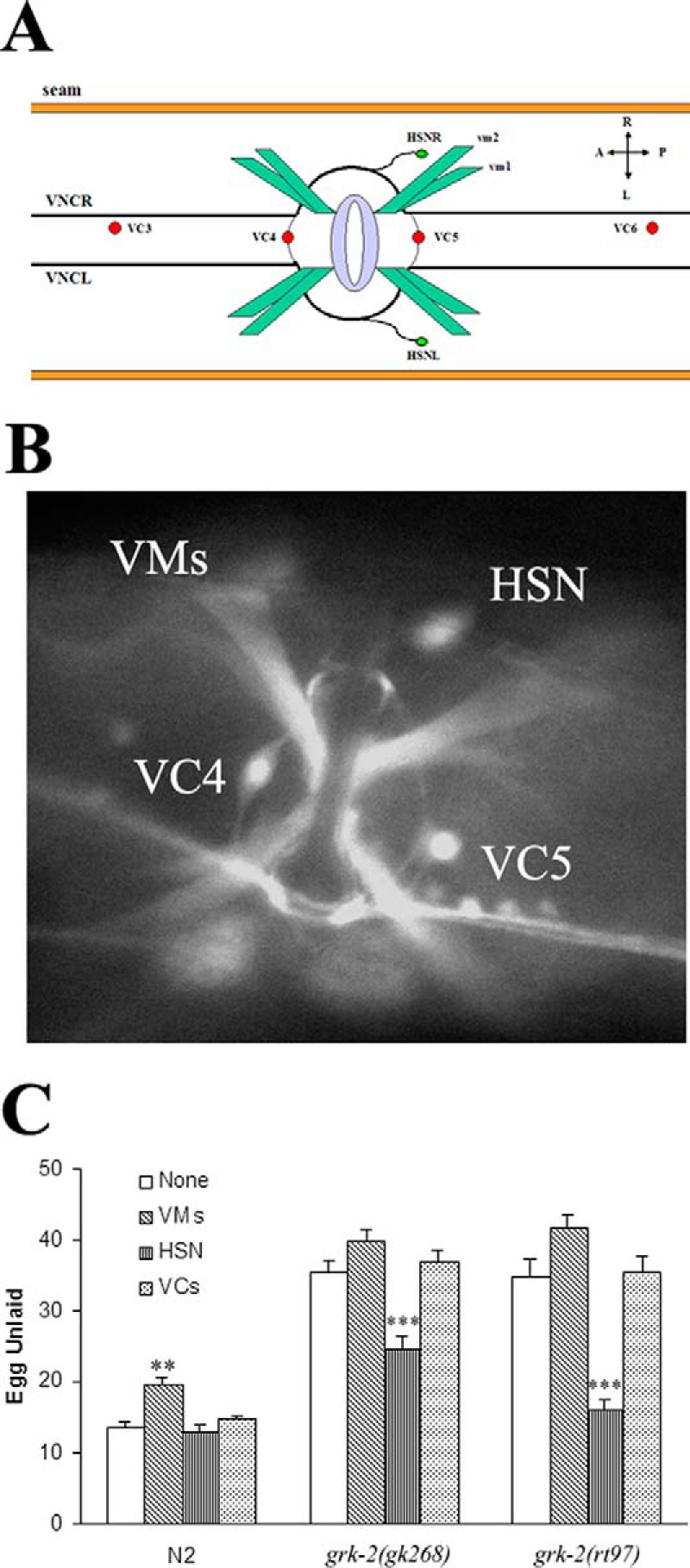
Cell-specific expression of grk-2 demonstrates that expression in HSNs is sufficient to rescue the egg laying defect in grk-2 mutants. A, model of the cells around the vulva region in C. elegans. B, expression of grk-2::GFP can be detected in vulval muscles (VMs), VC4, VC5, and HSN. C, grk-2 expression using cell-specific promoters demonstrates that grk-2 expression in HSN significantly rescues egg laying in both grk-2 mutants, suggesting that the loss of GRK-2 in the HSN is responsible for the egg laying defect (***, p < 0.001 compared with None). Because ∼65% of the worms expressing grk-2 in vulval muscle were multivulva, we only collected egg laying data from the ∼35% of worms containing a single normal vulva. The data also show that grk-2 overexpression in vulval muscle inhibits egg laying in wild-type worms (**, p < 0.01 compared with None). All error bars indicate S.E. VNC, ventral nerve cord; A, anterior; P, posterior; L, left; R, right
grk-2 loss of function can suppress egg laying-constitutive mutants
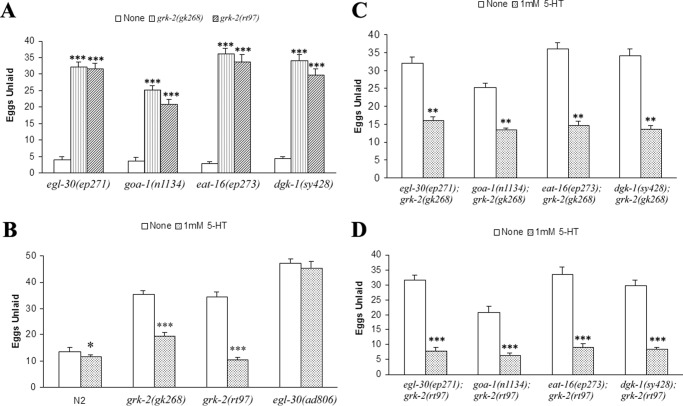
Although GRKs are largely known for their ability to phosphorylate activated GPCRs, these kinases also regulate the phosphorylation and function of numerous other proteins (3). Thus, GRK-2 may function to regulate egg laying at a GPCR or potentially at other points in the pathway. We evaluated this question by crossing the grk-2(gk268) and grk-2(rt97) alleles with strains that are constitutive egg layers. These strains include a gain-of-function strain that has activated Gαq (egl-30(ep271)) as well as loss-of-function strains that are defective in Gαo (goa-1(n1134)), the G protein that inhibits egg laying; EAT-16 (eat-16(ep273)), the regulator of G protein signaling (RGS) protein of Gαq; or diacylglycerol kinase (dgk-1(sy428)), a potential effector enzyme that phosphorylates diacylglycerol. Interestingly, the constitutive egg laying phenotype in these strains could be effectively suppressed by grk-2(lf) (Fig. 3A), suggesting that GRK-2 may function downstream of these proteins in the egg laying pathway.
Figure 3.
Egg laying-constitutive mutants can be suppressed by grk-2, and the egg laying defect can be rescued by exogenous serotonin. A, egl-30(ep271), goa-1(n1134), eat-16(ep273), and dgk-1(sy428) are egg laying-constitutive, and all are suppressed by the grk-2 mutants (***, p < 0.001 compared with None). B, addition of 1 mm 5-HT to NGM plates and overnight incubation of worms enhances wild-type egg laying (*, p < 0.05 compared with None) and rescues the egg laying defect in both grk-2 mutants (***, p < 0.001 compared with None). In contrast, the egg laying defect in the loss-of-function egl-30(ad806) is not rescued by 5-HT. C, the double mutants with grk-2(gk268) can be partially rescued by exogenous 5-HT (**, p < 0.01 compared with None). D, the double mutants with grk-2(rt97) are completely rescued by 5-HT and are egg laying-constitutive (***, p < 0.001 compared with None). All error bars indicate S.E.
5-HT is an important neurotransmitter that regulates egg laying. Mutant strains defective in egg laying are often divided into classes based on their response to 5-HT and the 5-HT transporter inhibitor imipramine (17). The addition of 1 mm 5-HT completely rescued the egg laying defect seen in grk-2(rt97) and it partially rescued the defect in grk-2(gk268) (Fig. 3B). As a control, the Gαq loss-of-function strain egl-30(ad806) was egg laying-defective and did not respond to exogenous 5-HT (Fig. 3B) as has been shown previously (15). In addition, double mutants of grk-2(gk268) with the constitutive egg laying mutants egl-30(ep271), goa-1(n1134), eat-16(ep273), and dgk-1(sy428) were partially rescued with exogenous 5-HT (Fig. 3C), whereas double mutants with grk-2(rt97) were completely rescued (Fig. 3D). Together, these results suggest that there may be a 5-HT deficiency in the grk-2 mutants because exogenous 5-HT was able to rescue the egg laying defects in the grk-2 loss-of-function alleles.
grk-2 mutants have a defect in serotonin metabolism
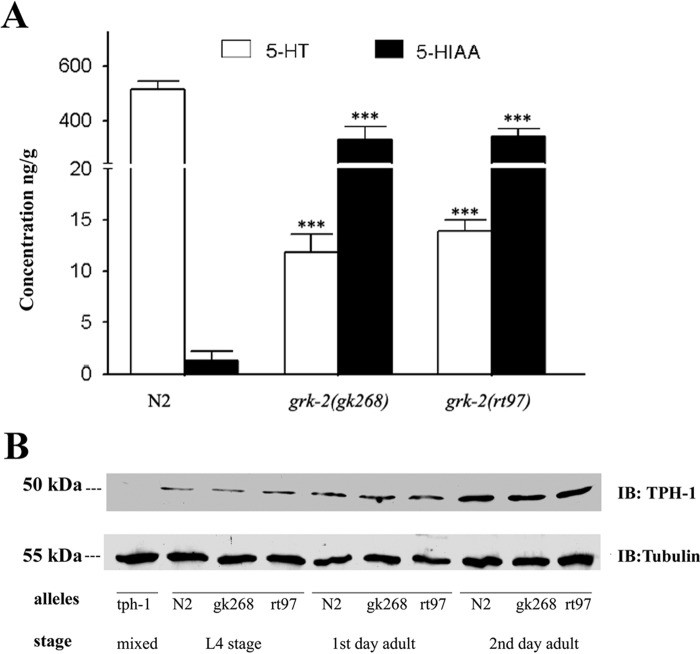
We next evaluated whether there was any change in 5-HT levels in the grk-2 mutants. To quantify the 5-HT levels in N2 and grk-2 mutant strains, we made lysates from purified 2nd day adult worms and determined 5-HT levels by HPLC with electrochemical (EC) detection. These studies revealed an ∼40-fold reduction in 5-HT levels in the grk-2(lf) strains (Fig. 4A). Reduced 5-HT levels in the grk-2 mutants could be from reduced synthesis or enhanced degradation of 5-HT. Degradation of 5-HT involves initial oxidation by monoamine oxidase (MAO) to 5-hydroxyindole acetaldehyde followed by further oxidation by aldehyde dehydrogenase to 5-HIAA (18). To quantify 5-HIAA levels, we also used HPLC-EC analysis to measure 5-HIAA in lysates from N2 and grk-2 mutant strains. These studies revealed a >250-fold increase in 5-HIAA levels in the grk-2 mutants compared with N2 (Fig. 4A). Thus, there appears to be a significant increase in 5-HT metabolism in the grk-2 mutant strains.
Figure 4.
Serotonin is metabolized to 5-hydroxyindole acetic acid in grk-2 mutants. A, HPLC-EC analysis of 5-HT and 5-HIAA in crude worm lysates shows that 5-HT levels are decreased (F(2,21) = 342.0, p < 0.001), and 5-HIAA levels are increased (F(2,21) = 48.4, p < 0.001) in the two grk-2 mutants (Bonferroni p values <0.001) compared with wild-type (N2) worms. n = 7–8 samples/genotype for HPLC-EC. B, Western blot using a mammalian Tph1 antibody shows that TPH-1 protein levels are similar between grk-2 mutants and wild-type worms at different developmental stages. No TPH-1 staining is observed in the tph-1 mutant strain. All error bars indicate S.E. IB, immunoblotting.
To determine whether there were any changes in 5-HT synthesis, we measured tph-1 expression in N2 and grk-2 mutants by generating transgenic strains expressing GFP using the tph-1 promoter (tph-1::GFP). No differences in tph-1::GFP expression were observed in the N2 and grk-2 mutant strains as assessed by fluorescence (data not shown). We also evaluated TPH-1 using a mammalian Tph1 antibody and found that TPH-1 protein levels are similar in wild type and grk-2 mutants at several developmental stages (Fig. 4B). It is worth noting, however, that TPH-1 levels appear to significantly increase from L4 to 2nd day young adult in both wild-type and mutant strains. Because prior studies have shown a direct correlation between tph-1 expression and 5-HT synthesis (16), these results suggest that 5-HT synthesis is similar in N2 and grk-2 mutants.
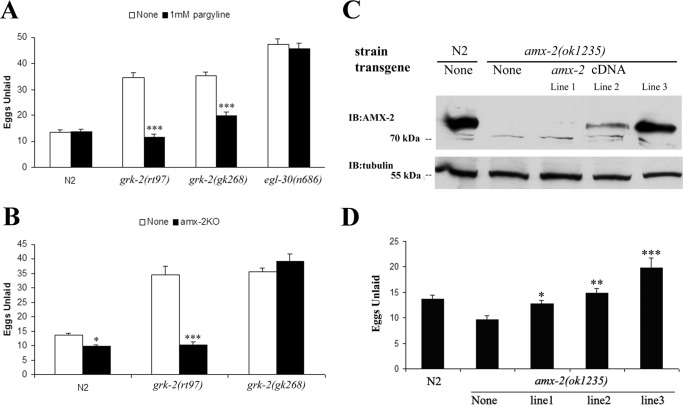
Considering that 5-HT can be oxidized by monoamine oxidases, we next evaluated the role of MAOs in 5-HT metabolism in the grk-2 mutants by treating animals with pargyline, a general MAO inhibitor used to treat depression in humans (19, 20). Although pargyline had no significant effect on egg laying in the N2 or egl-30(n686) strains, the inhibitor rescued the egg laying defect in the grk-2 mutants (Fig. 5A). We also used a genetic approach to try to disrupt MAO activity. There appear to be three potential MAOs in C. elegans (AMX-1, AMX-2, and AMX-3) based on homology with human MAO-A and MAO-B (21). AMX-2 is most related to human MAOs with 40.1% amino acid similarity with MAO-A and 39.2% with MAO-B, whereas AMX-1 has 37.7% similarity with MAO-A and 39.5% with MAO-B, and AMX-3 has 35.1% similarity with MAO-A and 36.0% with MAO-B. Moreover, recent studies support a role for AMX-2 in 5-HT metabolism as an amx-2(lf) strain has elevated levels of 5-HT compared with wild-type worms (21).
Figure 5.
The grk-2 mutants can be rescued by inhibiting monoamine oxidase activity or by knocking out the monoamine oxidase amx-2. A, the monoamine oxidase inhibitor pargyline rescues the egg laying defect in both grk-2 mutants but not in egl-30(n686) (***, p < 0.001 compared with None). B, the knock-out of the monoamine oxidase ortholog amx-2 completely rescues the egg laying defect in grk-2(rt97) but not in grk-2(gk268) (*, p < 0.05; ***, p < 0.001 compared with grk-2(rt97)). C, Western blot shows AMX-2 is absent in the knock-out strain. Multiple transgenic lines show different expression levels of amx-2. D, the rescue of amx-2(ok1235) is dependent on the protein level (**, p < 0.01). All error bars indicate S.E. IB, immunoblotting.
To characterize the potential role of AMX-2 in egg laying, we analyzed responses in an amx-2 mutant strain, amx-2(ok1235). This strain showed a weak constitutive egg laying phenotype and when crossed with the grk-2(rt97) strain amx-2(ok1235) effectively suppressed the grk-2(rt97) egg laying defect (Fig. 5B). In contrast, it did not suppress the egg laying defect of grk-2(gk268). To assess whether the rescue of egg laying by amx-2(ok1235) correlated with a rescue of 5-HT levels, we analyzed 5-HT levels in the various strains by HPLC-EC analysis. amx-2(ok1235) had an ∼28% higher level of 5-HT compared with N2, and it effectively rescued 5-HT levels when crossed with grk-2(rt97) (Table 1). In contrast, 5-HT levels were only partially rescued when crossed with grk-2(gk268). A similar trend was found with 5-HIAA levels, which were ∼8-fold lower in amx-2(ok1235) compared with N2 and were fully rescued in amx-2;grk-2(rt97) and partially rescued in amx-2;grk-2(gk268). These results support the role of 5-HT metabolism in mediating the egg laying defect in grk-2 loss-of-function alleles, and they also suggest that AMX-2 is the primary monoamine oxidase for 5-HT in C. elegans given the significant increase in 5-HT and decrease in 5-HIAA in the amx-2(ok1235) strain. However, because the stronger grk-2(gk268) allele was not rescued by loss of amx-2, there appear to be additional factors that contribute to the egg laying defect in this strain.
TABLE 1.
5-HT and 5-HIAA levels in N2, grk-2 mutant, and amx-2 mutant strains
| Genotype | 5-HTa |
5-HIAAb |
||||
|---|---|---|---|---|---|---|
| Mean | S.E. | n | Mean | S.E. | n | |
| ng/g | ng/g | ng/g | ng/g | |||
| N2 | 258 | ±14 | 7 | 0.64 | ±0.45 | 7 |
| grk-2(gk268) | 5.9c | ±0.9 | 7 | 168d | ±22 | 7 |
| grk-2(rt97) | 6.9c | ±0.6 | 8 | 174d | ±11 | 8 |
| amx-2(ok1235) | 330c,e | ±14 | 8 | 0.08f | ±0.01 | 8 |
| amx-2;grk-2(gk268) | 114c,e,g | ±5.4 | 7 | 28.3f | ±1.1 | 7 |
| amx-2;grk-2(rt97) | 274e,g | ±20 | 8 | 0.15f | ±0.01 | 8 |
a One-way ANOVA: F(5,44) = 155.67, p < 0.001.
b One-way ANOVA: F(5,44) = 77.58, p < 0.001.
c For 5-HT, Bonferroni p < 0.001, N2 versus grk-2(gk268), grk-2(rt97), amx-2(ok1235), and amx-2;grk-2(gk268).
d For 5-HIAA, Bonferroni p < 0.001, N2 versus grk-2(gk268) and grk-2(rt97).
e For 5-HT, Bonferroni p < 0.001, grk-2(gk268) and grk-2(rt97) versus amx-2(ok1235), amx-2;grk-2(gk268), and amx-2;grk-2(rt97).
f For 5-HIAA, Bonferroni p < 0.001, grk-2(gk268) and grk-2(rt97) versus amx-2(ok1235), amx-2;grk-2(gk268), and amx-2;grk-2(rt97).
g For 5-HT, Bonferroni p < 0.021, amx-2(ok1235) versus amx-2;grk-2(gk268) and amx-2;grk-2(rt97).
To determine the expression level of AMX-2, we generated antibodies against AMX-2 and initially evaluated their ability to detect the endogenous protein in N2 lysates. Western blotting analysis of AMX-2, which is 724 amino acids, reveals that the antibody detects an ∼75-kDa protein that is absent in amx-2(ok1235) (Fig. 5C). To assess whether we can rescue the amx-2(ok1235) egg laying phenotype, we used a transgenic amx-2 construct, which includes its promoter (∼3 kb) and cDNA sequence (yk1326h5), to generate three transgenic amx-2 strains with low, medium, and high levels of AMX-2 expression (Fig. 5C). AMX-2 expression effectively rescued the egg laying-constitutive phenotype of amx-2(ok1235) with high levels of expression inducing a partial egg laying defect (Fig. 5D).
grk-2 regulates the function of AMX-2
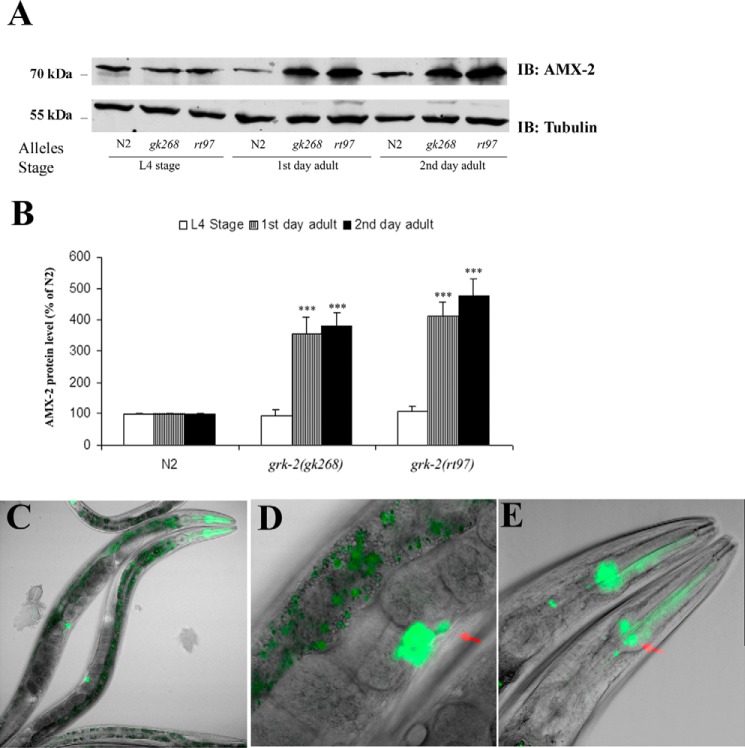
Because potential changes in AMX-2 activity might contribute to altered 5-HT metabolism, we next assessed whether loss of GRK-2 regulates expression of AMX-2. A comparison of AMX-2 expression in the wild-type and grk-2 mutant strains reveals that AMX-2 levels are comparable at the L4 stage, whereas the levels are significantly increased in both grk-2 mutant strains in the 1st and 2nd day young adults (Fig. 6A). The protein level of AMX-2 in wild type does not change in the different stages, whereas in grk-2 mutants it increases 3.5–5-fold at the early adult stages (Fig. 6B). To assess where amx-2 is expressed, we generated a transgenic strain using an amx-2 promoter (∼3 kb)-driven GFP construct (amx-2::GFP). This approach revealed localization in pharyngeal muscle, head neurons including neurosecretory motor neurons, vulva, HSN, intestine, and anus (Fig. 6, C–E). amx-2 expression has significant overlap with grk-2 especially in the HSN and vulva. Thus, GRK-2 appears to regulate the function of AMX-2, most likely in HSN, and thereby it regulates 5-HT metabolism and egg laying behavior.
Figure 6.
Expression of the monoamine oxidase AMX-2 is regulated by GRK-2. A, AMX-2 levels significantly increase in adult grk-2 mutant worms compared with N2. B, AMX-2 protein level increases ∼4–5-fold in grk-2 mutants in the adult stage (***, p < 0.001; n = 6). C, the amx-2 promoter-driven GFP shows amx-2 gene expression in the head region, vulva, intestine, and anus. D, the vulva region has strong GFP expression including expression in HSN (red arrow). E, GFP is visible in pharyngeal muscle and head region neurons including neurosecretory motor neurons (red arrow). All error bars indicate S.E. IB, immunoblotting.
To dissect the potential mechanism of increased expression of AMX-2, we evaluated amx-2 mRNA in N2 and grk-2 mutant strains using quantitative RT-PCR. These studies revealed there was an ∼50% increase in amx-2 mRNA levels in the grk-2 mutants; however, this enhancement was not statistically significant (Fig. 7A). These results suggest that changes in mRNA are unlikely to be the cause of the increased AMX-2 overexpression in the grk-2 mutant strains, although it is possible that this contributes to the altered expression. We next tried to evaluate whether GRK-2 regulates AMX-2 function through a post-translational process. Because our initial rescue studies revealed that the catalytic activity of GRK-2 was important for egg laying (Fig. 1F), it is possible that GRK-2 phosphorylation of AMX-2 may be involved in regulating the activity and/or stability of this protein. To test this possibility, we initially evaluated whether GRK-2 interacts with AMX-2. Interestingly, immunoprecipitation of AMX-2 resulted in significant co-immunoprecipitation of GRK-2, and this was modestly enhanced in the presence of aluminum fluoride, which activates heterotrimeric G proteins (Fig. 7B). For controls, we found that no co-immunoprecipitation was observed in either the amx-2 or grk-2 mutant strains. To test whether GRK-2 phosphorylates AMX-2, we immunoprecipitated AMX-2 from the N2 and grk-2 mutant strains and performed Western blotting using phospho-Thr antibodies. These results reveal that AMX-2 is phosphorylated on Thr and that the phosphorylation is reduced in the two grk-2 mutant strains even though AMX-2 levels are higher in these strains compared with N2 (Fig. 7C). As a control, we found no phospho-Thr band in the amx-2(ok1235) mutant strain. Taken together, these studies demonstrate that GRK-2 interacts with AMX-2 and plays a role in mediating AMX-2 phosphorylation.
Figure 7.
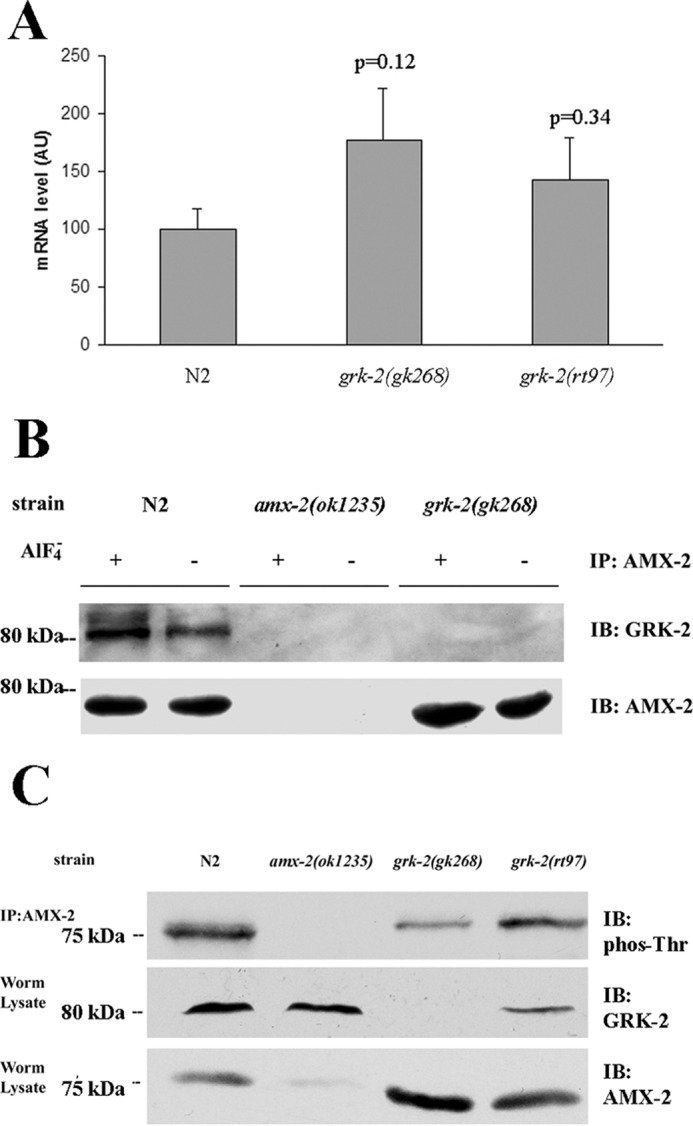
Interaction of GRK-2 with AMX-2. A, quantitative RT-PCR analysis indicates that the mRNA level of amx-2 is not significantly changed in the grk-2 mutants compared with N2. Results are from four experiments each done in duplicate. B, co-immunoprecipitation experiments demonstrate that GRK-2 interacts with AMX-2, and this interaction is unaffected by G protein activation with aluminum fluoride. No co-immunoprecipitation is observed in the amx-2(ok1235) and grk-2(gk268) mutant strains. C, top panel, AMX-2 was immunoprecipitated from N2, amx-2(ok1235), grk-2(gk268), and grk-2(rt97) lysates and then analyzed by Western blotting using a mouse monoclonal phospho-Thr antibody. The lysates were also analyzed for GRK-2 (middle panel) and AMX-2 (bottom panel) expression. This result is representative of five experiments. All error bars indicate S.E. AU, arbitrary units; IP, immunoprecipitation; IB, immunoblotting.
5-HIAA inhibits egg laying through ser-1 and goa-1
Reduced 5-HT levels play an important role in the egg laying defects observed in grk-2 mutant strains because the defect can be rescued by 1) addition of exogenous 5-HT, 2) inhibition of 5-HT metabolism, or 3) expression of grk-2 in HSNs. However, because the egg laying defect in grk-2 mutants is actually more severe than those seen in tph-1 mutants, which have no 5-HT or 5-HIAA, we also tested whether 5-HIAA might directly contribute to the egg laying defect. Interestingly, wild-type worms treated with 1 mm 5-HIAA had a clear defect in egg laying compared with worms without 5-HIAA, whereas the grk-2 mutant strains were unaffected by 5-HIAA (Fig. 8A). Treatment of the egg laying-constitutive mutants egl-30(ep271), eat-16(ep273), and dgk-1(sy428) with 5-HIAA had an even more pronounced effect and effectively suppressed the constitutive egg laying phenotype (Fig. 8A). In striking contrast, the goa-1(n1134) loss-of-function strain was unaffected by 5-HIAA.
Figure 8.
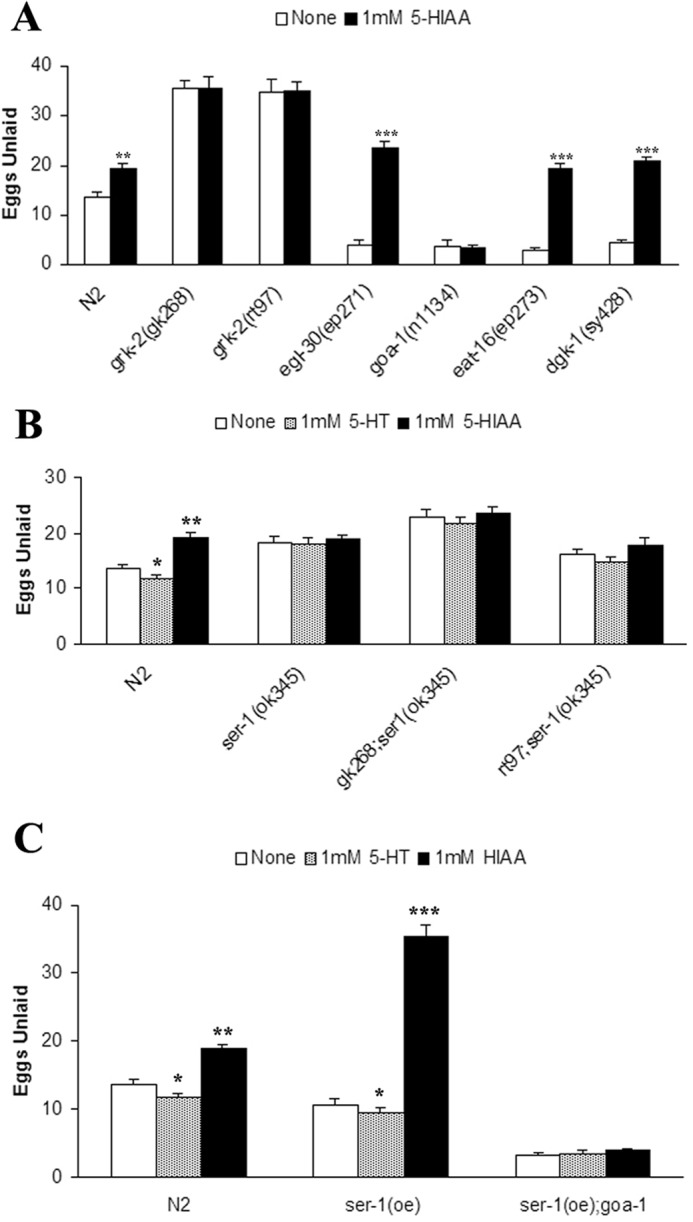
Exogenous 5-HIAA inhibits egg laying behavior via SER-1 and GOA-1. A, 5-HIAA inhibits egg laying behavior in wild type and egg laying-constitutive mutants but has no effect on grk-2(lf) strains (**, p < 0.01 and ***, p < 0.001 compared with None). B, the ser-1(ok345) mutant does not respond to exogenous 5-HT or 5-HIAA and suppresses the egg laying defect of grk-2 mutants (*, p < 0.05; **, p < 0.01; and ***p < 0.001 compared with None). C, overexpression of ser-1 is supersensitive to both 5-HT and 5-HIAA in a goa-1-dependent manner (*, p < 0.05 and **, p < 0.01 compared with None). All error bars indicate S.E.
There are many 5-HT receptors in C. elegans with SER-1, SER-4, SER-5, SER-7, and MOD-1 contributing to 5-HT-responsive egg laying (18, 22, 23). 5-HT synthesized in HSNs plays a critical role in egg laying via its ability to bind to SER-1 and SER-7 on vulval muscle cells and stimulate Ca2+ transients and muscle contraction mediated by the L-type Ca2+ channel EGL-19 (12). In contrast, SER-4 appears to function upstream of HSNs to provide a 5-HT- and GOA-1-dependent negative feedback on egg laying. SER-1 is a 5-HT2-type receptor that activates egg laying through coupling to EGL-30 (22–25), and although the ser-1 loss-of-function strain ser-1(ok345) only has a weak egg laying defect, it is completely unresponsive to exogenous 5-HT (24). Interestingly, we found that ser-1(ok345) is not only unresponsive to 5-HT but is also completely unresponsive to 5-HIAA (Fig. 7B). Moreover, double mutants of ser-1(ok345) with grk-2(gk268) or grk-2(rt97) suppress the strong egg laying defect normally observed by grk-2(lf) and remain unresponsive to exogenous 5-HT and 5-HIAA (Fig. 8B). These results demonstrate that ser-1 is indispensable for the egg laying defect of grk-2 mutants as well as the egg laying inhibition seen with 5-HIAA.
Because SER-1 mediates the effects of 5-HIAA, we also evaluated the effects of ser-1 overexpression and found that it induced a weak egg laying-constitutive phenotype that could be modestly enhanced by exogenous 5-HT (Fig. 8C). Interestingly, 5-HIAA induced a much stronger egg laying defect in ser-1(oe) than seen in N2 (Fig. 8C). The enhanced sensitivity of ser-1(oe) to 5-HIAA was completely lost when crossed with the goa-1 loss-of-function mutant goa-1(n1134) (Fig. 8C). Taken together, these studies demonstrate that 5-HIAA inhibits egg laying in a SER-1- and GOA-1-dependent manner.
Discussion
G protein-coupled receptor kinases play an essential role in regulating G protein signaling, and defects in GRK function have been implicated in a number of diseases including cardiovascular defects, neurological disorders, and cancer (3). To further dissect the links among GRK structure, function, and physiology, we performed a detailed genetic and biochemical analysis to define the role of GRK-2 in C. elegans egg laying responses. Two grk-2 mutant strains were used to dissect GRK-2 function: grk-2(gk268), which contained a deletion of the first three exons, resulting in a complete loss of GRK-2 expression, and grk-2(rt97), which contained a T354I point mutation in GRK-2 that leads to reduced expression and activity (7). Both grk-2 loss-of-function strains had a significant egg laying defect that could be rescued by expression of wild-type GRK-2 but not by catalytically inactive GRK-2. Interestingly, grk-2 mutants were found to have abnormally low levels of 5-HT and high levels of the 5-HT metabolite 5-HIAA, suggesting that 5-HT metabolism was enhanced. Indeed, the egg laying defect in grk-2 mutants could be rescued by addition of exogenous 5-HT or by inhibition of endogenous 5-HT metabolism. It is noteworthy that although the grk-2(rt97) allele could be fully rescued by these various treatments, the grk-2(gk268) allele was only partially rescued, suggesting that there may be additional defects induced by the complete loss of GRK-2. To address this, future studies may require microarray and/or proteomic analysis of N2 and grk-2(gk268) strains to assess differences in mRNA/protein levels.
GRK-2 is broadly expressed in C. elegans including in various sensory neurons, interneurons, and ventral nerve cord motor neurons (7) as well as in vulval muscle, HSNs, and VC neurons (Fig. 2B). The major site of action of GRK-2 in the egg laying process appears to be the HSN because expression of grk-2 in HSNs rescues the egg laying defect in a grk-2 mutant strain. In contrast, no rescue was observed when grk-2 was expressed in VC neurons or vulval muscle cells. However, it is worth noting that expression of GRK-2 in vulval muscle induced a multivulva phenotype in ∼65% of the worms (data not shown). This suggests that GRK-2 may regulate signaling pathways such as EGF receptor, Notch, or Wnt that determine the fate of vulval precursor cells and the development of the vulva (26). Indeed, previous studies have suggested a link between the EGF receptor and GRK2 in mammalian cells (27, 28).
5-HT is produced in a number of neurons including the HSN, and it plays an essential role in regulating egg laying with five distinct 5-HT receptors providing excitatory and inhibitory serotonergic input into this process (18, 22, 23). SER-1, SER-5, and SER-7 are all expressed in vulval muscle cells, and their activation stimulates egg laying, whereas SER-4 and MOD-1 appear to provide a 5-HT-dependent negative feedback on egg laying. SER-1 is a 5-HT2-type receptor that activates egg laying through coupling to EGL-30 (22–25). Although a ser-1 knock-out strain only has a weak egg laying defect, it is completely unresponsive to exogenous 5-HT (24). SER-7 is also important in egg laying and is coupled to Gs to regulate cAMP production and PKA activation, which then regulate muscle contraction that is mediated by the L-type Ca2+ channel EGL-19 (29). A ser-7 knock-out strain also only has a weak egg laying defect and has been reported to be unresponsive to exogenous 5-HT, whereas a double mutant, ser-1;ser-7, has a more pronounced egg laying defect (23). SER-5 is also coupled to Gs to regulate cAMP production and stimulate egg laying, although it has not been as well characterized as SER-1 and SER-7 (23). Because GRK-2 is expressed in vulval muscle cells and overexpression inhibits egg laying, it likely has a role in regulating the function of one or more of these 5-HT receptors.
Although relatively little is currently known about 5-HT metabolism in C. elegans, 5-HT is primarily metabolized by MAO-A and MAO-B in mammals (30). Both of these enzymes are anchored in the outer mitochondrial membrane primarily through their C-terminal domains (31). There appear to be three potential monoamine oxidases in C. elegans: AMX-1, AMX-2, and AMX-3. Each shares 35–40% amino acid similarity with MAO-A and MAO-B, and the protein sequence alignment of human MAOs and C. elegans AMXs suggests that they share many functional and structural properties (Ref. 21 and data not shown). These include the N-terminal FAD binding domain, which is the most highly conserved region across the MAO and AMX families. Although the AMX proteins are related to MAOs, AMX-1 also has 40.7% similarity with the histone demethylase LSD2/KDM1b, and AMX-3 has 40.2% similarity with polyamine oxidase PAO1. Computational modeling of AMX-2 suggests that it has a structure highly related to the MAO family, although it contains N-terminal and C-terminal extensions compared with MAO-A (data not shown). Interestingly, AMX-2 also contains a C-terminal α-helical domain that is similar to the C-terminal domain in MAO-A that mediates mitochondrial membrane localization.
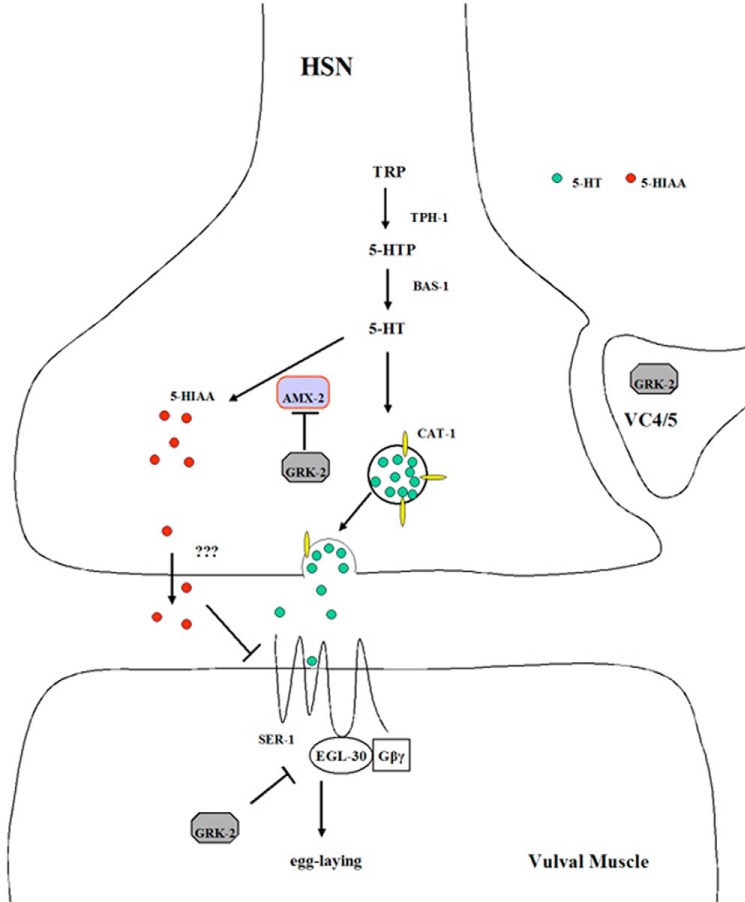
The enhanced metabolism of 5-HT in the grk-2 mutants appears to be due to an increased protein level of AMX-2. This increase does not appear to be due to transcriptional regulation because amx-2 mRNA levels are not significantly different in the grk-2 mutants compared with the wild-type control. GRK-2 regulation of AMX-2 protein function likely involves a post-translational mechanism. This is supported by our findings that the catalytic activity of GRK-2 is essential to rescuing the egg laying defect in grk-2 mutant strains and the fact that GRK-2 can associate with and likely phosphorylate AMX-2. We postulate that GRK-2 interacts with and phosphorylates AMX-2 in the HSN and that it regulates the activity and/or stability of this protein (Fig. 9). GRK-2-mediated regulation of the level of AMX-2 results in concomitant changes in the rate of 5-HT metabolism. This regulates levels of the 5-HT metabolite 5-HIAA, which can inhibit egg laying in a SER-1/GOA-1-dependent manner. GRK-2 is also expressed in vulval muscle where it appears to turn off EGL-30 signaling to inhibit egg laying.
Figure 9.
Model of GRK-2 regulation of egg laying in C. elegans. 5-HT released from the HSN acts on SER-1 expressed in vulval muscle cells to activate EGL-30 and stimulate egg laying. GRK-2 regulates 5-HT metabolism by phosphorylating and inhibiting AMX-2 function, possibly by promoting AMX-2 degradation. AMX-2 oxidizes 5-HT, resulting in increased levels of the 5-HT metabolite 5-HIAA, which can inhibit egg laying in a SER-1-dependent manner. GRK-2 is also expressed in vulval muscle cells where it plays an inhibitory role in egg laying and in VC4/5 neurons where the function is not known. TRP, tryptophan; 5-HTP, 5-hydroxytryptophan.
Another interesting observation of our work was the finding that the primary metabolite of 5-HT, 5-HIAA, appears to effectively inhibit egg laying in a GPCR (SER-1)- and G protein (GOA-1)-dependent manner. Although it is tempting to speculate that 5-HIAA might serve as a biased agonist or allosteric modulator of SER-1 to mediate GOA-1 activation, our attempts to detect 5-HIAA binding to SER-1 have been unsuccessful. For example, 5-HIAA does not compete with 3H-labeled lysergic acid diethylamide binding to SER-1 and does not antagonize the ability of 5-HT to stimulate a Ca2+ flux in HEK 293 cells expressing SER-1 (data not shown). Nevertheless, further investigation of 5-HIAA effects in C. elegans and other animal models is warranted. Indeed, recent studies demonstrate that 5-HIAA can antagonize Ras signaling in C. elegans in a SER-1-dependent manner (21).
Because GRK-2 regulates 5-HT metabolism in worms, this raises the question as to whether GRKs may also regulate 5-HT metabolism in humans. Although this has not been explicitly tested, previous studies have shown a number of changes in GRK2 and GRK3 expression that are associated with mood disorders. For example, membrane-associated GRK2 and GRK3 levels are increased in the prefrontal cortex of depressed suicide victims (32), and this increase is normalized by treatment with antidepressants (33). In addition, GRK2 protein and mRNA levels in mononuclear leukocytes are significantly lower in patients with major depression compared with healthy subjects (34). Interestingly, antidepressant treatment normalized GRK2 levels, and this effect preceded clinical improvement by 1–2 weeks. Genome-wide linkage analysis studies have suggested a susceptibility locus for bipolar disorder near the GRK3 gene (ADRBK2) (35, 36), whereas a polymorphism that enhances GRK3 expression is associated with depression (37, 38). Although these latter studies suggest that increased expression of GRK3 may be associated with depression in humans, a number of animal studies reveal that decreased expression of GRK3 is also associated with brain dysfunction. For example, learned helplessness is associated with reduced GRK3 expression in the locus coeruleus and amygdala in rats (39), whereas increased sensitivity to reward is associated with decreased GRK3 expression in the nucleus accumbens in mice (40). In addition, GRK3 is also involved in mediating stress-induced depression in mice (41). Overall, these studies suggest that GRK2 and GRK3 are associated with mood disorders and that normal brain function appears sensitive to the expression of these important regulatory proteins.
Although our work provides important insights into the regulation of MAO function by GRK-2, future studies need to better define the detailed mechanisms involved in this regulation. For example, what specific AMX-2 residues are phosphorylated by GRK-2, and how does this regulate the expression and/or function of AMX-2? This would have to be determined empirically because there is no clear consensus sequence for GRK phosphorylation, although mammalian GRK2 preferentially phosphorylates Ser/Thr in an acidotropic environment in peptides (42). Similarly, future studies will need to dissect in greater detail the mechanisms involved in GRK2/3 regulation of mood disorders in humans and establish whether changes in expression correlate with changes in MAO expression and 5-HT metabolism as observed in C. elegans. Results from such studies may reveal potential novel drug targets for treating such debilitating disorders.
Experimental procedures
Worm culture
The wild-type N2 and various mutants were maintained at 24.5 °C on NGM plates with seeding of OP50 Escherichia coli (43). To evaluate the effect of various drugs on egg laying behavior, specific compounds were added to the agar solution after it had cooled to 55 °C during preparation of the plates, and the plates were stored subsequently at 4 °C. The mutant strain grk-2(rt97), which results in a T354I point mutation in grk-2, was received from Dr. Anne Hart (Massachusetts General Hospital, Boston, MA) and was confirmed as a loss-of-function mutation as shown previously (7). The knock-out strain grk-2(gk268) was received from the Caenorhabditis Genetics Center and is missing the first three exons of grk-2. This strain was backcrossed with N2 six times. The following mutant strains were received from the Caenorhabditis Genetics Center: I: egl-30(ad806), egl-30(n686), egl-30(ep271), goa-1(n1134), and amx-2(ok1235); III: grk-2(gk268); IV: eat-16(ep273) and tph-1(mg280); and X: dgk-1 (sy428) and ser-1(ok345). We also received the strain GR1366 carrying a tph-1::GFP transgene (mgIs42) from Caenorhabditis Genetics Center.
Plasmid injection and X-ray integration
The grk-2 constructs were made using 3 kb of promoter sequence plus the coding region from the grk-2 gene linked into the plasmid vector. The grk-2(K220R) was made by site-directed mutagenesis and sequenced to confirm that no additional mutations were present. For cell-specific expression of grk-2, we cloned a grk-2 cDNA into plasmids containing cell-specific promoters (HSN:pJM66A, VC:pJT37A, and ELM:pJT36A) that were provided by Dr. Michael Koelle (Yale University, New Haven, CT). The 3-kb promoter of amx-2 was linked with GFP vector pPD117.01 (Addgene) to assess the amx-2 expression pattern.
A PCR product of genomic amx-2 (∼9 kb) was used to rescue amx-2(ok1235). We used the traditional worm injection method with a plasmid DNA concentration of 10 μg/ml and 100 μg/ml pRF4 (rol-6) as the dominant marker. After injection, three to five worms were transferred to a new plate, and after the next generation each individual roller was transferred to a new plate. Transgenic lines carrying an extrachromosomal array were then established with a stable ratio of rollers for each generation. We used 20 grays of X-irradiation to integrate the extrachromosomal array, and after exposure to X-ray five L4 or young adult worms were picked and transferred to individual plates. Two generations later, 12 transgenic worms from each plate were transferred to individual plates. Only the plates with 100% transgenic worms were chosen for further assessments of integration. All of the integrated worms were backcrossed with wild-type worms at least twice.
Isolation of stage-specific nematodes
Nematodes were partially synchronized by bleaching egg-containing adults, and the surviving eggs were spread onto 10-cm plates and stored at 24.5 °C. Egg numbers (∼600–800) were controlled to prevent starvation, and worms were collected at four different stages. After 24 h, the majority of the worms were at the L2 stage, and the worms were at the L4 stage after 48 h. After another 24 h, the adult worms began to lay eggs. At this stage, filter paper (pore size, ∼125 μm) was used to remove the young larva and eggs. To get purified 1st day adults, the worms collected on the filter paper were washed with phosphate-buffered saline (PBS), and a portion of the collected worms was spread onto new plates (∼500–600 worms/plate). The next day, filtration was again used to collect the 2nd day adult worms.
Western blotting
For Western blotting, we followed the method described in the WormBook chapter on immunohistochemistry (44). When collecting the desired worm samples, 10× worm lysis buffer and proteinase inhibitor mixture were added (Roche Applied Science). The worms were quickly frozen in liquid nitrogen and stored at −80 °C until future use. We used glass beads (325–450 μm) to shear the worms in a cell beater, and after ultracentrifugation the supernatant was collected, total protein concentration was measured, and equal protein amounts were analyzed by SDS-PAGE. Proteins were transferred onto nitrocellulose filters, blocked with 5% skim-milk in PBS with 0.1% Tween 20 (PBST), and then incubated with primary antibody (anti-GRK2/3 monoclonal from Millipore, Bedford, MA) overnight at 4 °C. The blots were washed six times with PBST, incubated with HRP-conjugated anti-mouse secondary antibody for 1 h, washed six times, and analyzed using a chemiluminescence detection kit (Thermo Scientific). The Tph1 antibody was a rabbit polyclonal antibody made against the C-terminal domain of mammalian Tph1 (Sigma-Aldrich). A rabbit polyclonal antibody to amino acids 603–698 from AMX-2 (WP:CE40797) was generated and purified by Genomic Antibody TechnologyTM at Strategic Diagnostics, Inc. (SDIX, Newark, DE).
AMX-2 immunoprecipitation and phosphorylation
AMX-2 was immunoprecipitated using a purified rabbit polyclonal AMX-2 antibody from lysates prepared from N2, amx-2(ok1235), grk-2(gk268), and grk-2(rt97) strains. Samples were electrophoresed on a 10% polyacrylamide gel, transferred to nitrocellulose, and blocked with 5% nonfat milk. Blots were incubated overnight at 4 °C with a mouse monoclonal phosphothreonine antibody (Cell Signaling Technology) at a 1:2000 dilution, washed, and then incubated with an HRP-conjugated goat anti-mouse secondary antibody (1:4000 dilution) for 2 h at room temperature. Blots were analyzed by chemiluminescence (Thermo Scientific).
Brood size
For each gene, 10–12 young adult hermaphrodites were picked from a single plate. Each worm was transferred to a new NGM plate with OP50 bacteria every day for 5 consecutive days. Two days later, the total number of hatched progeny on each plate were counted and then divided by the number of adult hermaphrodites. Each experiment was repeated at least three times.
Number of eggs in the uterus
For all egg laying assays, we transferred 25–30 L4 stage worms with crescent vulva induction to a fresh plate. The next day, worms were transferred to new NGM plates with or without drug. After overnight incubation (16 h), we transferred the worms to a glass slide with a 2% agar pad containing M9 buffer and 0.1% azide and then counted the number of eggs in the uterus using a dissecting microscope. At least 20 worms were counted per condition, and at least three independent experiments were conducted.
Egg laying assay
About 25–30 adult worms were moved to a fresh NGM plate. After 2 h, the adults were discarded, and the freshly laid eggs were analyzed for their developmental stage. The eggs were divided into three categories: premature (earlier than nine cells), normal (nine cells to comma stage) and postmature (postcomma stage). Approximately 200–300 eggs were characterized per experiment, and at least three independent experiments were performed.
HPLC-EC analysis
Worms were synchronized on the 2nd day at the adult stage, purified by filtration, washed with PBS, and pelleted by low speed centrifugation. The worms were then frozen in liquid nitrogen and lysed using glass beads. After centrifugation, the supernatant was collected, and an equal volume of 200 mm HClO4 was added. The supernatant was filtered through a 0.22-μm filter (Millipore) and then analyzed using reverse-phase HPLC with EC detection to measure the amount of 5-HT and 5-HIAA. The system consisted of Coulochem III, a model 5300 detector with a model 5014B analytical cell, and a model 5020 guard cell (ESA Inc., Bedford, MA). HPLC-EC analysis was performed with the analytical cell potentials −0.15 and +0.18 mV, and separation was achieved at 0.6 ml/min on a MD C18 150 × 3.2-mm 3-μm column (Thermo Scientific) with a mobile phase containing 90 mm sodium dihydrogen phosphate, 50 mm citric acid, 1.7 mm 1-octane sulfonic acid sodium salt, 50 μm EDTA, and 9.5% acetonitrile (pH 3.0). The identity and amount of each component were determined using appropriate standards (Sigma-Aldrich) and are reported as ng of 5-HT or 5-HIAA/g of washed worm pellet.
Quantitative RT-PCR assay
We measured amx-2 mRNA levels in wild type and grk-2 mutants using quantitative RT-PCR following a method described previously (45). Worm lysates were collected, protein was removed by phenol extraction, and the samples were treated with RNase-free DNase I (Thermo Scientific). The RNA was precipitated and washed with ethanol, and after evaporation all RNA samples were adjusted to a final concentration of 1 mg/ml. The total RNA was converted into cDNA using RevertAid reverse transcriptase according to the manufacturer's protocol (Thermo Scientific). cDNAs were analyzed using a thermocycler and EvaGreen PCR Master Mix (Bio-Rad). The relative mRNA levels were calculated based on the 2−ct method (46) using act-1 for normalization. For each data set, at least four independent experiments were performed.
Protein sequence alignment
The amino acid sequence alignment of human MAO-A and MAO-B and C. elegans AMX-1, AMX-2, and AMX-3 was performed using a ClustalW alignment from Network Protein Sequence Analysis. Because the N-terminal region of AMX-1 is closely related to lysine-specific demethylase, we removed the first 349 amino acids from this alignment.
Statistical analysis
Bar graphs are shown as the means ± S.E. All egg laying assays were performed using at least 20 worms, and p values for all pairwise comparisons were calculated using Student's t test and are denoted as * for p < 0.05, ** for p < 0.01, and *** for p < 0.001. In the HPLC-EC reverse-phase analysis of 5-HT and 5-HIAA, we used ANOVA with Bonferroni pairwise corrections, and each group included seven to eight individual samples.
Author contributions
J. W. and J. L. B designed the overall project. J. W. and J. L. carried out most of the experiments in the laboratory of J. L. B. D.K.A. performed the 5-HT and 5-HIAA measurements in the laboratory of W. C. W. R. N. provided AMX-2 antiserum for Western analysis. All authors contributed to the writing of the manuscript.
Acknowledgments
We thank Dr. Michael Koelle for providing the cell type-specific promoter constructs; Dr. Anne Hart for providing the grk-2(rt97) strain; Drs. Janet Duerr, Denise Ferkey, Bryan Roth, and Konstantin Komolov for helpful comments; the Sidney Kimmel Cancer Center Bioimaging Facility for technical advice and support; and the Caenorhabditis Genetics Center for providing multiple C. elegans strains. The neurochemistry experiments were conducted with equipment purchased with a grant from the North Carolina Biotechnology Center.
This work was supported by National Institutes of Health Grant R01 GM044944 (to J. L. B.) and Cancer Center Support Grant P30 CA056036. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- GPCR
- G protein-coupled receptor
- 5-HT
- serotonin (5-hydroxytryptamine)
- 5-HIAA
- 5-hydroxyindole acetic acid
- GRK
- GPCR kinase
- HSN
- hermaphrodite-specific neuron
- lf
- loss-of-function
- MAO
- monoamine oxidase
- VC
- ventral cord
- EC
- electrochemical
- AMX
- amine oxidase
- NGM
- nematode growth medium
- ANOVA
- analysis of variance.
References
- 1. Reiter E., and Lefkowitz R. J. (2006) GRKs and β-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 17, 159–165 [DOI] [PubMed] [Google Scholar]
- 2. Moore C. A., Milano S. K., and Benovic J. L. (2007) Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 69, 451–482 [DOI] [PubMed] [Google Scholar]
- 3. Gurevich E. V., Tesmer J. J., Mushegian A., and Gurevich V. V. (2012) G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol. Ther. 133, 40–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patikoglou G. A., and Koelle M. R. (2002) An N-terminal region of Caenorhabditis elegans RGS proteins EGL-10 and EAT-16 directs inhibition of Gαo versus Gαq signaling. J. Biol. Chem. 277, 47004–47013 [DOI] [PubMed] [Google Scholar]
- 5. Schafer W. F. (2006) Genetics of egg-laying in worms. Annu. Rev. Genet. 40, 487–509 [DOI] [PubMed] [Google Scholar]
- 6. Bargmann C. I. (1998) Neurobiology of the Caenorhabditis elegans genome. Science 282, 2028–2033 [DOI] [PubMed] [Google Scholar]
- 7. Fukuto H. S., Ferkey D. M., Apicella A. J., Lans H., Sharmeen T., Chen W., Lefkowitz R. J., Jansen G., Schafer W. R., and Hart A. C. (2004) G protein-coupled receptor kinase function is essential for chemosensation in C. elegans. Neuron 42, 581–593 [DOI] [PubMed] [Google Scholar]
- 8. Wani K. A., Catanese M., Normantowicz R., Herd M., Maher K. N., and Chase D. L. (2012) D1 dopamine receptor signaling is modulated by the R7 RGS protein EAT-16 and the R7 binding protein RSBP-1 in Caenorhabditis elegans motor neurons. PLoS One 7, e37831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wood J. F., Wang J., Benovic J. L., and Ferkey D. M. (2012) Structural domains required for C. elegans G protein-coupled receptor kinase 2 (GRK-2) function in vivo. J. Biol. Chem. 287, 12634–12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ezak M. J., Hong E., Chaparro-Garcia A., and Ferkey D. M. (2010) Caenorhabditis elegans TRPV channels function in a modality-specific pathway to regulate response to aberrant sensory signaling. Genetics 185, 233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hukema R. K., Rademakers S., Dekkers M. P., Burghoorn J., and Jansen G. (2006) Antagonistic sensory cues generate gustatory plasticity in Caenorhabditis elegans. EMBO J. 25, 312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shyn S. I., Kerr R., and Schafer W. R. (2003) Serotonin and Go modulate functional states of neurons and muscles controlling C. elegans egg-laying behavior. Curr. Biol. 13, 1910–1915 [DOI] [PubMed] [Google Scholar]
- 13. Bastiani C. A., Gharib S., Simon M. I., and Sternberg P. W. (2003) Caenorhabditis elegans Gαq regulates egg-laying behavior via a PLCβ-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics 165, 1805–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carman C. V., Parent J.-L., Day P. W., Pronin A. N., Sternweis P. M., Wedegaertner P. B., Gilman A. G., Benovic J. L., and Kozasa T. (1999) Selective regulation of Gq/11α by an RGS domain in the G protein-coupled receptor kinase, GRK2. J. Biol. Chem. 274, 34483–34492 [DOI] [PubMed] [Google Scholar]
- 15. Brundage L., Avery L., Katz A., Kim U. J., Mendel J. E., Sternberg P. W., and Simon M. I. (1996) Mutations in a C. elegans Gqα gene disrupt movement, egg-laying, and viability. Neuron 16, 999–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanis J. E., Moresco J. J., Lindquist R. A., and Koelle M. R. (2008) Regulation of serotonin biosynthesis by the G proteins Gαo and Gαq controls serotonin signaling in. Caenorhabditis elegans. Genetics 178, 157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trent C., Tsuing N., and Horvitz H. R. (1983) Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104, 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chase D. L., and Koelle M. R. (2007) Biogenic amine neurotransmitters in C. elegans. WormBook 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy D. L., Karoum F., Pickar D., Cohen R. M., Lipper S., Mellow A. M., Tariot P. N., and Sunderland T. (1998) Differential trace amine alterations in individuals receiving acetylenic inhibitors of MAO-A (clorgyline) or MAO-B (selegiline and pargyline). J. Neural Transm. Suppl. 52, 39–48 [DOI] [PubMed] [Google Scholar]
- 20. Edmondson D. E., Mattevi A., Binda C., Li M., and Hubálek F. (2004) Structure and mechanism of monoamine oxidase. Curr. Med. Chem. 11, 1983–1993 [DOI] [PubMed] [Google Scholar]
- 21. Schmid T., Snoek L. B., Fröhli E., van der Bent M. L., Kammenga J., and Hajnal A. (2015) Systemic regulation of RAS/MAPK signaling by the serotonin metabolite 5-HIAA. PLoS Genet. 11, e1005236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carre-Pierrat M., Baillie D., Johnsen R., Hyde R., Hart A., Granger L., and Ségalat L. (2006) Characterization of the Caenorhabditis elegans G protein-coupled serotonin receptors. Invert. Neurosci. 6, 189–205 [DOI] [PubMed] [Google Scholar]
- 23. Hapiak V. M., Hobson R. J., Hughes L., Smith K., Harris G., Condon C., Komuniecki P., and Komuniecki R. W. (2009) Dual excitatory and inhibitory serotonergic inputs modulate egg laying in Caenorhabditis elegans. Genetics 181, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dempsey C. M., Mackenzie S. M., Gargus A., Blanco G., and Sze J. Y. (2005) Serotonin (5HT), fluoxetine, imipramine and dopamine target distinct 5HT receptor signaling to modulate Caenorhabditis elegans egg-laying behavior. Genetics 169, 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carnell L., Illi J., Hong S. W., and McIntire S. L. (2005) The G-protein-coupled serotonin receptor SER-1 regulates egg laying and male mating behaviors in Caenorhabditis elegans. J. Neurosci. 25, 10671–10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sternberg P. W. (2005) Vulval development. WormBook 1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Y., Long H., Wu Z., Jiang X., and Ma L. (2008) EGF transregulates opioid receptors through EGFR-mediated GRK2 phosphorylation and activation. Mol. Biol. Cell 19, 2973–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. So C. H., Michal A., Komolov K. E., Luo J., and Benovic J. L. (2013) G protein-coupled receptor kinase 2 (GRK2) is localized to centrosomes and mediates epidermal growth factor-promoted centrosomal separation. Mol. Biol. Cell 24, 2795–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hobson R. J., Hapiak V. M., Xiao H., Buehrer K. L., Komuniecki P. R., and Komuniecki R. W. (2006) SER-7, a Caenorhabditis elegans 5-HT7-like receptor, is essential for the 5-HT stimulation of pharyngeal pumping and egg laying. Genetics 172, 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bortolato M., Chen K., and Shih J. C. (2008) Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv. Drug Deliv. Rev. 60, 1527–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rebrin I., Geha R. M., Chen K., and Shih J. C. (2001) Effects of carboxyl-terminal truncations on the activity and solubility of human monoamine oxidase B. J. Biol. Chem. 276, 29499–29506 [DOI] [PubMed] [Google Scholar]
- 32. García-Sevilla J. A., Escribá P. V., Ozaita A., La Harpe R., Walzer C., Eytan A., and Guimón J. (1999) Up-regulation of immunolabeled α2A-adrenoceptors, Gi coupling proteins, and regulatory receptor kinases in the prefrontal cortex of depressed suicides. J. Neurochem. 72, 282–291 [DOI] [PubMed] [Google Scholar]
- 33. Grange-Midroit M., García-Sevilla J. A., Ferrer-Alcón M., La Harpe R., Huguelet P., and Guimón J. (2003) Regulation of GRK 2 and 6, β-arrestin-2 and associated proteins in the prefrontal cortex of drug-free and antidepressant drug-treated subjects with major depression. Brain Res. Mol. Brain Res. 111, 31–41 [DOI] [PubMed] [Google Scholar]
- 34. Matuzany-Ruban A., Golan M., Miroshnik N., Schreiber G., and Avissar S. (2010) Normalization of GRK2 protein and mRNA measures in patients with depression predict response to antidepressants. Int. J. Neuropsychopharmacol. 13, 83–91 [DOI] [PubMed] [Google Scholar]
- 35. Edenberg H. J., Foroud T., Conneally P. M., Sorbel J. J., Carr K., Crose C., Willig C., Zhao J., Miller M., Bowman E., Mayeda A., Rau N. L., Smiley C., Rice J. P., Goate A., et al. (1997) Initial genomic scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 3, 5, 15, 16, 17, and 22. Am. J. Med. Genet. 74, 238–246 [PubMed] [Google Scholar]
- 36. Kelsoe J. R., Spence M. A., Loetscher E., Foguet M., Sadovnick A. D., Remick R. A., Flodman P., Khristich J., Mroczkowski-Parker Z., Brown J. L., Masser D., Ungerleider S., Rapaport M. H., Wishart W. L., and Luebbert H. (2001) A genome survey indicates a possible susceptibility locus for bipolar disorder on chromosome 22. Proc. Natl. Acad. Sci. U.S.A. 98, 585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barrett T. B., Hauger R. L., Kennedy J. L., Sadovnick A. D., Remick R. A., Keck P. E., McElroy S. L., Alexander M., Shaw S. H., and Kelsoe J. R. (2003) Evidence that a single nucleotide polymorphism in the promoter of the G protein receptor kinase 3 gene is associated with bipolar disorder. Mol. Psychiatry 8, 546–557 [DOI] [PubMed] [Google Scholar]
- 38. Zhou X., Barrett T. B., and Kelsoe J. R. (2008) Promoter variant in the GRK3 gene associated with bipolar disorder alters gene expression. Biol. Psychiatry 64, 104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taneja M., Salim S., Saha K., Happe H. K., Qutna N., Petty F., Bylund D. B., and Eikenburg D. C. (2011) Differential Effects of inescapable stress on locus coeruleus GRK3, α2-adrenoceptor and CRF1 receptor levels in learned helpless and non-helpless rats: a potential link to stress resilience. Behav. Brain Res. 221, 25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dinieri J. A., Nemeth C. L., Parsegian A., Carle T., Gurevich V. V., Gurevich E., Neve R. L., Nestler E. J., and Carlezon W. A. Jr. (2009) Altered sensitivity to rewarding and aversive drugs in mice with inducible disruption of cAMP response element-binding protein function within the nucleus accumbens. J. Neurosci. 29, 1855–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bruchas M. R., Land B. B., Aita M., Xu M., Barot S. K., Li S., and Chavkin C. (2007) Stress-induced p38 mitogen-activated protein kinase activation mediates κ-opioid-dependent dysphoria. J. Neurosci. 27, 11614–11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Onorato J. J., Palczewski K., Regan J. W., Caron M. G., Lefkowitz R. J., and Benovic J. L. (1991) Role of acidic amino acids in peptide substrates of the β-adrenergic receptor kinase and rhodopsin kinase. Biochemistry 30, 5118–5125 [DOI] [PubMed] [Google Scholar]
- 43. Brenner S. (1974) The genetics of Caenorhabditis elegans. Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duerr J. S. (2006) Immunohistochemistry. WormBook 1–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han T., Manoharan A. P., Harkins T. T., Bouffard P., Fitzpatrick C., Chu D. S., Thierry-Mieg D., Thierry-Mieg J., and Kim J. K. (2009) 26G endo-siRNAs regulate spermatogenic and zygotic gene expression in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 106, 18674–18679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nolan T., Hands R. E., and Bustin S. A. (2006) Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 1, 1559–1582 [DOI] [PubMed] [Google Scholar]