Abstract
Pregnant women and their developing fetuses are vulnerable to multiple environmental insults, including exposure to aflatoxin, a mycotoxin that may contaminate as much as 25% of the world food supply. We reviewed and integrated findings from studies of aflatoxin exposure during pregnancy and evaluated potential links to adverse pregnancy outcomes. We identified 27 studies (10 human cross-sectional studies and 17 animal studies) assessing the relationship between aflatoxin exposure and adverse birth outcomes or anemia. Findings suggest that aflatoxin exposure during pregnancy may impair fetal growth. Only one human study investigated aflatoxin exposure and prematurity, and no studies investigated its relationship with pregnancy loss, but animal studies suggest aflatoxin exposure may increase risk for prematurity and pregnancy loss. The fetus could be affected by maternal aflatoxin exposure through direct toxicity as well as indirect toxicity, via maternal systemic inflammation, impaired placental growth, or elevation of placental cytokines. The cytotoxic and systemic effects of aflatoxin could plausibly mediate maternal anemia, intrauterine growth restriction, fetal loss, and preterm birth. Given the widespread exposure to this toxin in developing countries, longitudinal studies in pregnant women are needed to provide stronger evidence for the role of aflatoxin in adverse pregnancy outcomes, and to explore biological mechanisms. Potential pathways for intervention to reduce aflatoxin exposure are urgently needed, and this might reduce the global burden of stillbirth, preterm birth, and low birthweight.
Introduction
Aflatoxins are toxic secondary metabolites of Aspergillus molds that contaminate foods such as maize, rice, and legumes. Around 0.5 billion people, predominantly those living in developing countries, are at significant risk of exposure to dietary aflatoxins, with many people chronically exposed to aflatoxins throughout life.1 In many developing countries, aflatoxins are not effectively controlled in the food system and consumption of high-risk foods, such as maize and groundnuts, is common. Aflatoxin accumulation in food is highly dependent on environmental factors such as moisture, temperature, nitrogen availability, and plant density,2 as well as poor harvest practices and improper grain storage.3–6 Aflatoxins have been most widely studied as causative agents of liver cancer.7 Chronic exposure has also been associated with other adverse human health outcomes, including growth faltering8 and maternal anemia.9 Exposure during pregnancy has been widely documented,10,11 but the effects on the mother and fetus are not well described.
Aflatoxins inhibit protein synthesis, are cytotoxic, teratogenic, and immunotoxic.12,13 Thus, they may affect fetal health both directly during critical periods of development, or indirectly, through their adverse effects on maternal health. Accordingly, aflatoxin exposure during pregnancy may plausibly contribute to adverse pregnancy outcomes, including intrauterine growth restriction, premature delivery, and pregnancy loss.14 If this is the case, the public health implications could be substantial: globally there are 2.65 million stillbirths,15 32 million small-for-gestational-age,16 and 15 million premature deliveries17 every year.
Adverse birth outcomes stem from multiple causes, many of which remain poorly understood. Advanced maternal age, parity, maternal infection, and smoking are well-documented risk factors for adverse birth outcomes, but do not account for a large proportion of cases.18 Maternal anemia during the first and second trimesters has also been associated with preterm birth and low birthweight, although the causal mechanism is not well understood.19–21 Maternal inflammation is the only pathologic process with a clearly defined causal link to preterm birth.22 Notably, aflatoxin exposure in humans and animals has been associated with increases in inflammatory markers,23–25 suggesting a potential mechanism pathway linking aflatoxin exposure and adverse birth outcomes. Anemia of inflammation might also occur, and could mediate prematurity or poor fetal growth.
Recently, documented high prevalence of exposure during pregnancy has directed focus on aflatoxins as a potential harmful exposure during the first 1,000 days23,26—the developmentally sensitive period from conception to 2 years of age. There are no published randomized controlled trials evaluating the effect of aflatoxin exposure during pregnancy. Therefore, we aimed to conduct a narrative review to evaluate the evidence for a potential role of aflatoxin exposure and anemia in intrauterine growth retardation, preterm birth, and pregnancy loss.
Methods
We searched ISI Web of Knowledge and PubMed (with medical subject headings [MeSH] strings) using these search terms: preterm, low birthweight, fetal loss, fetal resorption, hemolysis, anemia, iron deficiency, and miscarriage, with mycotoxin or aflatoxin in the search, between the years 1970 and 2015. We identified additional relevant papers from related review articles identified in the primary search. A total of 27 studies (10 humans, 17 animals) assessing the relationship between aflatoxin exposure and adverse birth outcomes or anemia were identified. A second search was conducted to identify literature evaluating mechanisms that could mediate these adverse health outcomes, using these terms: placenta, cytokines, interleukin-1, interleukin-6 (IL-6), tumor necrosis factor-α, insulin-like growth factor, intestine, inflammation, immune, and organ, with mycotoxin or aflatoxin in the search.
Aflatoxin metabolism and implications for exposure during pregnancy.
Aflatoxins B1 (AFB1), B2, G1, and G2 are produced by several species of Aspergillus; AFB1 exposure is the focus of most research because it occurs most frequently and is most toxic.27 All aflatoxins are readily absorbed and undergo a variety of biotransformation reactions; both the parent aflatoxins and their metabolites are detectable in urine. AFB1 becomes toxic through metabolic activation by various cytochrome P450 enzyme families including CYP1a2, CYP3a4, and CyP3a5, which are mainly found in the liver, but also present in other tissues including the placenta, intestine, and spleen.12 Activation generates two reactive epoxide species, AFB1-8,9-exo-epoxide and AFB1-8,9-endo-epoxide, and several other metabolites including aflatoxin M1 (AFM1). The aflatoxin epoxides and AFM1 are toxic; AFB1 exo-epoxide binds to DNA forming mutagenic lesions.13
The epoxides can be detoxified by glutathione-S transferases (GST) before urinary excretion as aflatoxin mercapturates.28 They may also be enzymatically hydrolyzed, and then detoxified to a dialchohol.29,30 Enzymes involved in both activation and detoxification of aflatoxin are polymorphic, and can therefore influence the toxic insult of exposure.31 GST polymorphisms are relatively common and have been associated with risk of various cancers as well as adverse birth outcomes.32–34
In evaluating the available evidence for a potential role of aflatoxin exposure in adverse pregnancy outcomes, it is important to recognize variations in the balance of activation versus detoxification depending on dose, species, and age. A fetal form of CYP3a4, known as CYP3a7, has been observed in fetal liver within 2 months of conception,35,36 indicating that the fetus may metabolically generate reactive epoxides following transplacental transfer of maternally ingested AFB1.37 Fetal livers catalyze the formation of the epoxide at similar rates to adults but produce fewer GSTs, and thus have a lower capacity to protect against toxicity.38 In addition to age differences and genetic differences in aflatoxin metabolism, there is also considerable interspecies variability in aflatoxin metabolism. A review by Wild and others39 suggested that humans may be relatively sensitive to the effects of aflatoxin, as higher levels of aflatoxin-albumin (AF-alb) were formed per dose of AFB1 in humans compared with many standard laboratory animals.
Human biomarker measurement.
The human studies identified in this review relied on biomarkers to assess aflatoxin exposure; no trials involving experimental manipulations of the diet during pregnancy were identified. Dietary aflatoxins and their metabolites can be detected in blood, urine, and breast milk, but their concentrations are not equally associated with dietary intake. There are three validated biomarkers of aflatoxin exposure: urinary biomarkers reflecting exposure in the prior 24–48 hours (AFM1 and aflatoxin-N7-guanine) and serum biomarkers reflecting cumulative exposure over the prior 2–3 months (AF-alb). These biomarkers are all quantitatively associated with dietary aflatoxin intake.40 Other aflatoxin metabolites (e.g., serum AFM1 or AFG1, urinary AFB1 or AFG1, and milk AFM1 or AFG2) are indicative of exposure, but levels do not correlate with dietary intake and they are therefore termed biomeasures. This difference is important when comparing the strength of epidemiological data that describe relationships between aflatoxin and health outcomes.
Prevalence of aflatoxin exposure during pregnancy.
We identified 12 epidemiologic studies from Africa, Asia, and the Middle East, including a total of more than 2,000 participants, which reported AF exposure in pregnant women and/or infant cord blood. Of these studies, eight measured AF exposure in both cord blood and maternal blood, whereas four measured exposure only in cord blood. Prevalence of exposure ranged from 6% to 100%,23,41–51 suggesting that in utero exposure to aflatoxin is widespread where maternal diets are contaminated. Cord blood samples in Taiwan were found to have AF-DNA adducts,49 confirming both fetal aflatoxin exposure and biotransformation capacity to generate reactive aflatoxin-epoxides.
Aflatoxin and intrauterine growth restriction.
Although exposure is widely documented, there are few human studies examining the relationship between AF exposure and pregnancy outcomes. We found four human studies that relied on aflatoxin biomeasures; three reported a negative association between an aflatoxin biomeasure and birthweight,46,47,51,52 and one reported no association42 (Supplemental Table 1). The presence of AFB1 and AFM1 in cord blood is quite transient, and in the Nigerian study by Maxwell and others,42 only 14.6% of samples were positive for aflatoxins. These studies may have inconsistent findings in part because the indicators of aflatoxin exposure used were biomeasures, which represent transient exposure and are not correlated with dietary intake. We identified two studies that used quantitative biomarkers, and both of these reported significant associations. A study of 785 pregnant Ghanaian women found that those in the highest quartile of AF-alb had significantly greater odds of having a low birthweight infant compared with the lowest quartile, with a linear trend of increasing risk of low birthweight with ascending aflatoxin quartile.14 In a study of 119 Gambian mother-infant pairs, average maternal AF-alb (at 5 and 8 months gestation) was significantly associated with lower weight-for-age (−0.249 z scores, P = 0.012) and lower height-for-age (−0.207 z scores, P = 0.044).48
We identified nine experimental animal studies, which reported consistent significant adverse effects of aflatoxin on fetal growth. Animal studies in rats, mice, hamsters, swine, rabbits, and quail consistently report decreased fetal weight, crown-rump length, and organ weight among exposed animals compared with control animals across a wide range of aflatoxin doses (Supplemental Table 2).
Aflatoxin, fetal loss, and spontaneous preterm birth.
We did not identify any human studies investigating risk of fetal loss, and only one study investigating preterm birth in association with aflatoxin exposure during pregnancy. We only found two studies investigating aflatoxin and stillbirth; both reported high levels of aflatoxin biomeasures in cord blood of three stillborn infants, though neither study was designed to assess a causal relationship nor investigate a mechanism41,51 (summarized in Supplemental Table 1). A study in Ghana in which gestational age was measured by ultrasound or palpation during routine antenatal care found no relationship between preterm birth (< 37 weeks gestation) and AF-alb biomarkers.14 Although these methods are relatively accurate for determining gestational age in the first 20 weeks, in Ghana, 43% of women have their first antenatal care (ANC) visit after the first trimester.53 In addition, the best methods for gestational age dating include an error of ±week that can lead to misclassification of prematurity.54,55
In animals, aflatoxin exposure during pregnancy causes fetal anomalies and decreased live births and litter size. In a study assessing aflatoxin exposure in pregnant rabbits, animals were dosed with 0–100 μg AF/kg body weight (bw)/day.56 Wangikar and others56 reported a decreased percentage of live fetuses and increased resorption (∼5%), impaired organ development (14% reduction in organ weight in 100 μg AF/kg bw/day treatment), and skeletal anomalies (28% of offspring with anomalies in 100 μg AF/kg bw/day). Similarly, Kihara and others57 found that rats treated with aflatoxin (300 μg AF/kg bw/day) on day 15–18 of gestation had a lower proportion of live births/implants (85%) compared with controls (94%). Aflatoxin is a potent teratogen because of its ability to bind DNA and subsequently inhibit protein synthesis. Several studies have reported skeletal anomalies in the offspring of animals treated with aflatoxin during pregnancy.56,58–60 The doses of aflatoxin used in these animal experiments are similar to estimated exposures ranging from 0 to 91 μg AF/kg bw/day in human studies in Zimbabwe and China.61
Aflatoxin and maternal anemia.
Aflatoxin exposure has been associated with anemia in one human study and several animal studies. Shuaib and others9 reported a cross-sectional association between AF-alb and anemia in 785 pregnant Ghanaian women, with those in the highest quartile of aflatoxin exposure having 1.85-fold increased odds of anemia compared with those in the lowest quartile (confidence interval = 1.16–2.85).
In vitro and in vivo studies in dogs, rabbits, catfish, and poultry show that relatively high aflatoxin exposures (range 0.5–1 ppm) are cytotoxic and cause lysis of red blood cells.62–65 This small body of evidence (summarized in Supplemental Table 3) indicates that aflatoxin may cause low hematocrit and hemoglobin,66,67 low iron absorption,68 and microcytic hypochromic anemia,69 all of which are characteristic of iron deficiency.
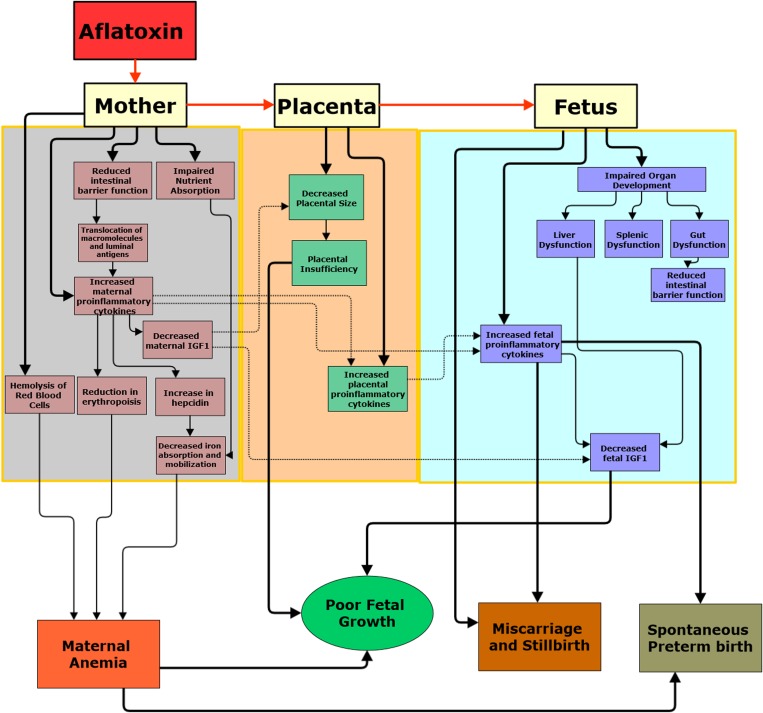
Although in vivo and in vitro studies have focused on a red cell lysis pathway, doses to cause such an effect are likely higher than levels experienced in human populations. It is more probable that chronic aflatoxin exposure could therefore cause anemia through three different mechanisms related to immune activation and enteropathy: a decreased capacity of the intestine to absorb essential nutrients such as iron; a decrease in erythropoiesis arising from chronic inflammation; and reduced availability of iron due to hepcidin upregulation (Figure 1 ). IL-6—which has shown to be upregulated by aflatoxin exposure24—increases hepcidin production, which decreases iron absorption and iron release from macrophages.70 However, further human and animal studies are needed to test these hypotheses. In summary, although it is biologically plausible that aflatoxin exposure causes anemia, the doses used in animal studies are high and the results may not be relevant for human exposure. Although there is evidence that aflatoxin exposure can alter the inflammatory response, none of the studies reviewed explored aflatoxin exposure and inflammation-induced anemia.
Figure 1.
(A) Conceptual framework for the effect of aflatoxin exposure on maternal-fetal health. Maternal exposure to aflatoxin might cause adverse pregnancy outcomes through three primary pathways: induction of environmental enteric dysfunction characterized by intestinal inflammation, impaired barrier function, and systemic immune activation; upregulation of pro-inflammatory cytokines and downregulation of anti-inflammatory cytokines; and potential toxic effects on maternal and fetal organs once absorbed causing systemic immune activation, and impaired placental and fetal development. Red arrows represent the transfer of aflatoxin from mother to placenta to fetus. Solid black arrows represent the hypothesized effects of aflatoxin exposure in the mother, placenta and fetus; dotted black arrows represent the hypothesized indirect effect of aflatoxin-induced maternal inflammation on the placenta and fetus. The few cross-sectional studies conducted in humans suggest that aflatoxin exposure impairs intrauterine growth and may be a cause of pregnancy loss. Although no conclusive studies have been conducted in humans, we have reviewed evidence from in vivo and in vitro studies that aflatoxin exposure may cause anemia, intestinal damage, and elevation of pro-inflammatory cytokines. In addition, animal studies indicate aflatoxin exposure results in impaired organ development. There are no studies investigating the effects of aflatoxin on the placenta, but given that the placenta metabolizes and transports aflatoxin, further investigations into its effects are warranted. This framework provides directions for future research investigating the effects of aflatoxin exposure during pregnancy.
Interactions between aflatoxin exposure and maternal diet.
Pregnant women in developing countries have multiple overlapping risk factors for poor pregnancy outcomes, which may increase vulnerability to even low doses of aflatoxin. For example, when diets are deficient in vitamins A, C, or E, or selenium (all of which protect against the toxic effects of aflatoxin), the detoxifying system for aflatoxin may be impaired, increasing the production of epoxides.71,72 Populations at risk of frequent aflatoxin exposure are often those with poor dietary diversity and thus micronutrient insufficient is common. We did not identify animal studies designed to explore potential interactions between nutritional deficiencies and aflatoxin exposure.
Potential mechanisms linking aflatoxin exposure to adverse pregnancy outcomes.
Mechanistic studies suggest that maternal exposure to aflatoxin might cause adverse pregnancy outcomes through four primary pathways: 1) upregulation of pro-inflammatory cytokines and/or downregulation of anti-inflammatory cytokines23,24,73–76; 2) induction of enteropathy characterized by intestinal inflammation and impaired barrier function, leading to systemic immune activation24,76–78; 3) potential toxic effects on maternal organs causing systemic immune activation and impaired placental and fetal development24,70,79,80; and 4) toxic effects on fetal organs causing fetal inflammation and impaired fetal development56,81–83 (Figure 1).
This framework offers numerous hypotheses suggested by the current literature, with proposed mechanisms drawing heavily on evidence from in vivo animal studies and in vitro studies. We highlight two necessary areas for future research to refine and test these hypotheses. First, there is a need for rigorous human studies on the relationship between maternal aflatoxin exposure and adverse pregnancy outcomes, using validated biomarkers. Although cross-sectional studies at delivery would be informative, stronger designs would investigate the longitudinal relationship of maternal exposure during pregnancy and pregnancy outcomes or experimentally intervene to reduce aflatoxin exposure in maternal diets. Second, research is needed to elucidate the mechanism by which aflatoxin mediates adverse pregnancy outcomes. For translational science in particular, studies are needed to understand the effects of aflatoxin on the placenta and fetus at modest dose and on a background of maternal dietary insufficiency, as this commonly co-occurs with aflatoxin exposure in human populations.
Conclusion
Aflatoxin exposure is common in developing countries, making it an issue of substantial public health importance. Despite this, there are relatively few human studies investigating the effects of aflatoxin during pregnancy on the mother and fetus. Aflatoxin exposure assessment studies have been small and geographically scattered and the majority of human studies have focused on low birthweight. However, animal studies provide biological support for the hypothesis that aflatoxin exposure may mediate adverse pregnancy outcomes.
Given the enormous burden of aflatoxin exposure, anemia, intrauterine growth restriction, and preterm birth in developing countries in the context of co-exposure to multiple environmental insults, further investigations into the effects of aflatoxin are strongly warranted. Research is needed to comprehensively evaluate the relationship and potential mechanisms linking aflatoxin with adverse health outcomes in pregnancy, so that novel pathways for intervention can be defined to mitigate aflatoxin exposure and reduce the global burden of stillbirth, preterm birth, and low birthweight.
Supplementary Material
Footnotes
Authors' addresses: Laura E. Smith, Division of Nutritional Sciences, Cornell University, Ithaca, NY, and Zvitambo Institute for Maternal and Child Health Research, Harare, Zimbabwe, E-mail: les36@cornell.edu. Andrew J. Prendergast, Centre for Pediatrics, Queen Mary University of London, London, United Kingdom, and Zvitambo Institute for Maternal and Child Health Research, Harare, Zimbabwe, E-mail: a.prendergast@qmul.ac.uk. Paul C. Turner, Maryland Institute for Applied Environmental Health, School of Public Health, University of Maryland at College Park, College Park, MD, E-mail: pturner3@umd.edu. Jean H. Humphrey, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, and Zvitambo Institute for Maternal and Child Health Research, Harare, Zimbabwe, E-mail: jhumphrey@zvitambo.co.zw. Rebecca J. Stoltzfus, Division of Nutritional Sciences, Cornell University, Ithaca, NY, E-mail: rjs62@cornell.edu.
References
- 1.Wild CP, Miller DS, Groopman JD. Mycotoxin Control in Low- and Middle-Income Countries. Leone, France: IARC; 2016. [PubMed] [Google Scholar]
- 2.Cotty PJ, Jaime-Garcia R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int J Food Microbiol. 2007;119:109–115. doi: 10.1016/j.ijfoodmicro.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 3.Bruns HA. Controlling aflatoxin and fumonisin in maize by crop management. Toxin Rev. 2003;22:153–173. [Google Scholar]
- 4.Lewis L, Onsongo M, Njapau H, Schurz-Rogers H, Luber G, Kieszak S, Nyamongo J, Backer L, Dahiye AM, Misore A, DeCock K, Rubin C. Kenya Aflatoxicosis Investigation Group Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ Health Perspect. 2005;113:1763–1767. doi: 10.1289/ehp.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutiga SK, Were V, Hoffmann V, Harvey JW, Milgroom MG, Nelson RJ. Extent and drivers of mycotoxin contamination: inferences from a survey of Kenyan maize mills. Phytopathology. 2014;104:1221–1231. doi: 10.1094/PHYTO-01-14-0006-R. [DOI] [PubMed] [Google Scholar]
- 6.Hell K, Cardwell K, Setamou M, Poehling H-M. The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, west Africa. J Stored Prod Res. 2000;36:365–382. doi: 10.1016/s0022-474x(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 2010;118:818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong YY, Hounsa A, Egal S, Turner PC, Sutcliffe AE, Hall AJ, Cardwell K, Wild CP. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, west Africa. Environ Health Perspect. 2004;112:1334–1338. doi: 10.1289/ehp.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shuaib F, Jolly PE, Ehiri JE, Jiang Y, Ellis WO, Stiles JK, Yatich NJ, Funkhouser E, Person SD, Wilson C, Williams JH. Association between anemia and aflatoxin B1 biomarker levels among pregnant women in Kumasi, Ghana. Am J Trop Med Hyg. 2010;83:1077–1083. doi: 10.4269/ajtmh.2010.09-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khlangwiset P, Shephard GS, Wu F. Aflatoxins and growth impairment: a review. Crit Rev Toxicol. 2011;41:740–755. doi: 10.3109/10408444.2011.575766. [DOI] [PubMed] [Google Scholar]
- 11.Shuaib F, Ehiri J, Abdullahi A, Williams J, Jolly P. Reproductive health effects of aflatoxins: a review of the literature. Reprod Toxicol. 2010;29:262–270. doi: 10.1016/j.reprotox.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Larsson P, Tjalve H. Extrahepatic bioactivation of aflatoxin B1 in fetal, infant and adult-rats. Chem Biol Interact. 1995;94:1–19. doi: 10.1016/0009-2797(94)03283-e. [DOI] [PubMed] [Google Scholar]
- 13.Turner PC, Flannery B, Isitt C, Ali M, Pestka J. The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr Res Rev. 2012;25:162–179. doi: 10.1017/S095442241200008X. [DOI] [PubMed] [Google Scholar]
- 14.Shuaib F, Jolly P, Ehiri J, Yatich N, Jiang Y, Funkhouser E, Person S, Wilson C, Ellis W, Wang J, Williams J. Association between birth outcomes and aflatoxin B1 biomarker blood levels in pregnant women in Kumasi, Ghana. Trop Med Int Health. 2010;15:160–167. doi: 10.1111/j.1365-3156.2009.02435.x. [DOI] [PubMed] [Google Scholar]
- 15.Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010;10((Suppl 1)):S1. doi: 10.1186/1471-2393-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R, Maternal and Child Nutrition Study Group Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 17.March of Dimes, PMNCH, Save the Children, WHO . In: Born Too Soon: The Global Action Report on Preterm Birth. Howson CP, Kinney MV, Lawn JE, editors. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 18.Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr. 2003;133:1592S–1596S. doi: 10.1093/jn/133.5.1592S. [DOI] [PubMed] [Google Scholar]
- 19.Scanlon KS, Yip R, Schieve LA, Cogswell ME. High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet Gynecol. 2000;96:741–748. doi: 10.1016/s0029-7844(00)00982-0. [DOI] [PubMed] [Google Scholar]
- 20.Bondevik GT, Lie RT, Ulstein M, Kvale G. Maternal hematological status and risk of low birth weight and preterm delivery in Nepal. Acta Obstet Gynecol Scand. 2001;80:402–408. [PubMed] [Google Scholar]
- 21.Levy A, Fraser D, Katz M, Mazor M, Sheiner E. Maternal anemia during pregnancy is an independent risk factor for low birthweight and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2005;122:182–186. doi: 10.1016/j.ejogrb.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 23.Groopman JD, Egner PA, Schulze KJ, Wu LS, Merrill R, Mehra S, Shamim AA, Ali H, Shaikh S, Gernand A, Khatry SK, LeClerq SC, Jr KP, Christian P. Aflatoxin exposure during the first 1000 days of life in rural south Asia assessed by aflatoxin B-lysine albumin biomarkers. Food Chem Toxicol. 2014;9:00417–7. doi: 10.1016/j.fct.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian GQ, Tang LL, Guo X, Wang F, Massey ME, Su JJ, Guo TL, Williams JH, Phillips TD, Wang JS. Aflatoxin B1 modulates the expression of phenotypic markers and cytokines by splenic lymphocytes of male f344 rats. J Appl Toxicol. 2014;34:241–249. doi: 10.1002/jat.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaytor AC, See MT, Hansen JA, de Souza ALP, Middleton TF, Kim SW. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J Anim Sci. 2011;89:124–135. doi: 10.2527/jas.2010-3005. [DOI] [PubMed] [Google Scholar]
- 26.Etzel RA. Reducing malnutrition: time to consider potential links between stunting and mycotoxin exposure? Pediatrics. 2014;134:4–6. doi: 10.1542/peds.2014-0827. [DOI] [PubMed] [Google Scholar]
- 27.Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guengerich FP, Johnson WW, Shimada T, Ueng YF, Yamazaki H, Langouet S. Activation and detoxication of aflatoxin B1. Mutat Res. 1998;402:121–128. doi: 10.1016/s0027-5107(97)00289-3. [DOI] [PubMed] [Google Scholar]
- 29.Bodreddigari S, Jones LK, Egner PA, Groopman JD, Sutter CH, Roebuck BD, Guengerich FP, Kensler TW, Sutter TR. Protection against aflatoxin B1-induced cytotoxicity by expression of the cloned aflatoxin B1-aldehyde reductases rat akr7a1 and human akr7a3. Chem Res Toxicol. 2008;21:1134–1142. doi: 10.1021/tx7004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guengerich FP, Cai H, McMahon M, Hayes JD, Sutter TR, Groopman JD, Deng Z, Harris TM. Reduction of aflatoxin B1 dialdehyde by rat and human aldo-keto reductases. Chem Res Toxicol. 2001;14:727–737. doi: 10.1021/tx010005p. [DOI] [PubMed] [Google Scholar]
- 31.Eaton DL, Gallagher EP. Mechanism of aflatoxin carcinogenesis. Annu Rev Pharmacol Toxicol. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- 32.Strange RC, Fryer AA. The glutathione s-transferases: influence of polymorphism on cancer susceptibility. IARC Sci Publ. 1999;148:231–249. [PubMed] [Google Scholar]
- 33.Bustamante M, Danileviciute A, Espinosa A, Gonzalez JR, Subirana I, Cordier S, Chevrier C, Chatzi L, Grazuleviciene R, Sunyer J, Ibarluzea J, Ballester F, Villanueva CM, Nieuwenhuijsen M, Estivill X, Kogevinas M. Influence of fetal glutathione s-transferase copy number variants on adverse reproductive outcomes. BJOG. 2012;119:1141–1146. doi: 10.1111/j.1471-0528.2012.03400.x. [DOI] [PubMed] [Google Scholar]
- 34.Sata F, Yamada H, Kondo T, Gong Y, Tozaki S, Kobashi G, Kato EH, Fujimoto S, Kishi R. Glutathione s-transferase m1 and t1 polymorphisms and the risk of recurrent pregnancy loss. Mol Hum Reprod. 2003;9:165–169. doi: 10.1093/molehr/gag021. [DOI] [PubMed] [Google Scholar]
- 35.Kitada M, Kamataki T. Cytochrome p450 in human fetal liver: significance and fetal-specific expression. Drug Metab Rev. 1994;26:305–323. doi: 10.3109/03602539409029800. [DOI] [PubMed] [Google Scholar]
- 36.Lacroix D, Sonnier M, Moncion A, Cheron G, Cresteil T. Expression of cyp3a in the human liver—evidence that the shift between cyp3a7 and cyp3a4 occurs immediately after birth. Eur J Biochem. 1997;247:625–634. doi: 10.1111/j.1432-1033.1997.00625.x. [DOI] [PubMed] [Google Scholar]
- 37.Partanen HA, El-Nezami HS, Leppanen JM, Myllynen PK, Woodhouse HJ, Vahakangas KH. Aflatoxin B1 transfer and metabolism in human placenta. Toxicol Sci. 2010;113:216–225. doi: 10.1093/toxsci/kfp257. [DOI] [PubMed] [Google Scholar]
- 38.Doi AM, Patterson PE, Gallagher EP. Variability in aflatoxin B1-macromolecular binding and relationship to biotransformation enzyme expression in human prenatal and adult liver. Toxicol Appl Pharmacol. 2002;181:48–59. doi: 10.1006/taap.2002.9399. [DOI] [PubMed] [Google Scholar]
- 39.Wild CP, Hasegawa R, Barraud L, Chutimataewin S, Chapot B, Ito N, Montesano R. Aflatoxin-albumin adducts: a basis for comparative carcinogenesis between animals and humans. Cancer Epidemiol Biomarkers Prev. 1996;5:179–189. [PubMed] [Google Scholar]
- 40.Turner PC. The molecular epidemiology of chronic aflatoxin driven impaired child growth. Scientifica (Cairo) 2013;2013:152879. doi: 10.1155/2013/152879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamplugh SM, Hendrickse RG, Apeagyei F, Mwanmut DD. Aflatoxins in breast-milk, neonatal cord blood, and serum of pregnant-women. BMJ. 1988;296:968. doi: 10.1136/bmj.296.6627.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maxwell SM, Familusi JB, Sodeinde O, Chan MCK, Hendrickse RG. Detection of naphthols and aflatoxins in Nigerian cord-blood. Ann Trop Paediatr. 1994;14:3–5. doi: 10.1080/02724936.1994.11747684. [DOI] [PubMed] [Google Scholar]
- 43.Jonsyn FE, Maxwell SM, Hendrickse RG. Human fetal exposure to ochratoxin-A and aflatoxins. Ann Trop Paediatr. 1995;15:3–9. doi: 10.1080/02724936.1995.11747742. [DOI] [PubMed] [Google Scholar]
- 44.Denning DW, Allen R, Wilkinson AP, Morgan MRA. Transplacental transfer of aflatoxin in humans. Carcinogenesis. 1990;11:1033–1035. doi: 10.1093/carcin/11.6.1033. [DOI] [PubMed] [Google Scholar]
- 45.Abulu EO, Uriah N, Aigbefo HS, Oboh PA, Agbonlahor DE. Preliminary investigation on aflatoxin in cord blood of jaundiced neonates. West Afr J Med. 1998;17:184–187. [PubMed] [Google Scholar]
- 46.Abdulrazzaq YM, Osman N, Ibrahim A. Fetal exposure to aflatoxins in the United Arab Emirates. Ann Trop Paediatr. 2002;22:3–9. doi: 10.1179/027249302125000094. [DOI] [PubMed] [Google Scholar]
- 47.Abdulrazzaq YM, Osman N, Yousif ZM, Trad O. Morbidity in neonates of mothers who have ingested aflatoxins. Ann Trop Paediatr. 2004;24:145–151. doi: 10.1179/027249304225013420. [DOI] [PubMed] [Google Scholar]
- 48.Turner PC, Collinson AC, Cheung YB, Gong YY, Hall AJ, Prentice AM, Wild CP. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int J Epidemiol. 2007;36:1119–1125. doi: 10.1093/ije/dym122. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh LL, Hsieh TT. Detection of aflatoxin b(1)-DNA adducts in human placenta and cord blood. Cancer Res. 1993;53:1278–1280. [PubMed] [Google Scholar]
- 50.Wild CP, Rasheed FN, Jawla MFB, Hall AJ, Jansen LAM, Montesano R. In utero exposure to aflatoxin in west Africa. Lancet. 1991;337:1602. doi: 10.1016/0140-6736(91)93295-k. [DOI] [PubMed] [Google Scholar]
- 51.Devries HR, Maxwell SM, Hendrickse RG. Fetal and neonatal exposure to aflatoxins. Acta Paediatr Scand. 1989;78:373–378. doi: 10.1111/j.1651-2227.1989.tb11095.x. [DOI] [PubMed] [Google Scholar]
- 52.De Vries HR, Maxwell SM, Hendrickse RG. Foetal and neonatal exposure to aflatoxins. Acta Paediatr Scand. 1989;78:373–378. doi: 10.1111/j.1651-2227.1989.tb11095.x. [DOI] [PubMed] [Google Scholar]
- 53.Doku D, Neupane S, Doku PN. Factors associated with reproductive health care utilization among Ghanaian women. BMC Int Health Hum Rights. 2012;12:29. doi: 10.1186/1472-698X-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander GR, Tompkins ME, Petersen DJ, Hulsey TC, Mor J. Discordance between LMP-based and clinically estimated gestational age: implications for research, programs, and policy. Public Health Rep. 1995;110:395–402. [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H, Kramer MS, Platt RW, Blondel B, Breart G, Morin I, Wilkins R, Usher R. How does early ultrasound scan estimation of gestational age lead to higher rates of preterm birth? Am J Obstet Gynecol. 2002;186:433–437. doi: 10.1067/mob.2002.120487. [DOI] [PubMed] [Google Scholar]
- 56.Wangikar PB, Dwivedi P, Sinha N, Sharma AK, Telang AG. Effects of aflatoxin B1 on embryo fetal development in rabbits. Food Chem Toxicol. 2005;43:607–615. doi: 10.1016/j.fct.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Kihara T, Matsuo T, Sakamoto M, Yasuda Y, Yamamoto Y, Tanimura T. Effects of prenatal aflatoxin B1 exposure on behaviors of rat offspring. Toxicol Sci. 2000;53:392–399. doi: 10.1093/toxsci/53.2.392. [DOI] [PubMed] [Google Scholar]
- 58.El-Nahla S, Imam H, Moussa E, Ibrahim A, Ghanam A. Teratogenic effects of aflatoxin in rabbits (Oryctolagus cuniculus) J. Vet. Anat. 2013;6:67–85. [Google Scholar]
- 59.Appelgren LE, Arora RG. Distribution studies of 14c-labelled aflatoxin B1 and ochratoxin A in pregnant mice. Vet Res Commun. 1983;7:141–144. doi: 10.1007/BF02228609. [DOI] [PubMed] [Google Scholar]
- 60.Wangikar PB, Dwivedi P, Sinha N. Effect in rats of simultaneous prenatal exposure to ochratoxin A and aflatoxin B1. I. Maternal toxicity and fetal malformations. Birth Defects Res B Dev Reprod Toxicol. 2004;71:343–351. doi: 10.1002/bdrb.20021. [DOI] [PubMed] [Google Scholar]
- 61.IARC Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr Eval Carcinog Risks Hum. 2002;82:1–556. [PMC free article] [PubMed] [Google Scholar]
- 62.Docan A, Cristea V, Dediu L, Grecu I. Hematological parameters as indicators of toxic stress produced by mycotoxin food contamination in the European catfish (Silurus glanis L.) J Environ Prot Ecol. 2011;12:1898–1903. [Google Scholar]
- 63.Kumar R, Balachandran C. Haematological and biochemical alterations in broiler chicken fed aflatoxin and cyclopiazonic acid. Indian Vet J. 2005;82:1255–1257. [Google Scholar]
- 64.Verma RJ, Raval PJ. Cytotoxicity of aflatoxin on red blood corpuscles. Bull Environ Contam Toxicol. 1991;47:428–432. doi: 10.1007/BF01702206. [DOI] [PubMed] [Google Scholar]
- 65.Liggett AD, Colvin BM, Beaver RW, Wilson DM. Canine aflatoxicosis: a continuing problem. Vet Hum Toxicol. 1986;28:428–430. [PubMed] [Google Scholar]
- 66.Andretta I, Kipper M, Lehnen CR, Lovatto PA. Meta-analysis of the relationship of mycotoxins with biochemical and hematological parameters in broilers. Poult Sci. 2012;91:376–382. doi: 10.3382/ps.2011-01813. [DOI] [PubMed] [Google Scholar]
- 67.Yousef MI, Salem MH, Kamel KI, Hassan GA, El-Nouty FD. Influence of ascorbic acid supplementation on the haematological and clinical biochemistry parameters of male rabbits exposed to aflatoxin B1. J Environ Sci Health B. 2003;38:193–209. doi: 10.1081/PFC-120018449. [DOI] [PubMed] [Google Scholar]
- 68.Lanza G, Washburn KW, Wyatt RD. Relationship of iron-absorption to development of aflatoxin related anemia. Poult Sci. 1978;57:1104. [Google Scholar]
- 69.Eisa AMA, Metwally AY. Effect of glucomannan on haematological, coagulation and biochemical parameters in male rabbits fed aflatoxin-contaminated ration. World Mycotoxin J. 2011;4:183–188. [Google Scholar]
- 70.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. Il-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alpsoy L, Yalvac ME. Key roles of vitamins A, C, and E in aflatoxin B1-induced oxidative stress. Vitam Horm. 2011;86:287–305. doi: 10.1016/B978-0-12-386960-9.00012-5. [DOI] [PubMed] [Google Scholar]
- 72.Galvano F, Piva A, Ritieni A, Galvano G. Dietary strategies to counteract the effects of mycotoxins: a review. J Food Prot. 2001;64:120–131. doi: 10.4315/0362-028x-64.1.120. [DOI] [PubMed] [Google Scholar]
- 73.Moon EY, Rhee DK, Pyo S. Inhibition of various functions in murine peritoneal macrophages by aflatoxin B1 exposure in vivo. Int J Immunopharmacol. 1999;21:47–58. doi: 10.1016/s0192-0561(98)00069-1. [DOI] [PubMed] [Google Scholar]
- 74.Dugyala RR, Sharma RP. The effect of aflatoxin B1 on cytokine mRNA and corresponding protein levels in peritoneal macrophages and splenic lymphocytes. Int J Immunopharmacol. 1996;18:599–608. doi: 10.1016/s0192-0561(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 75.Bruneau JC, Stack E, O'Kennedy R, Loscher CE. Aflatoxins B(1), B(2) and G(1) modulate cytokine secretion and cell surface marker expression in J774A.1 murine macrophages. Toxicol In Vitro. 2012;26:686–693. doi: 10.1016/j.tiv.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Watzl B, Neudecker C, Hansch GM, Rechkemmer G, Pool-Zobel BL. Short-term moderate aflatoxin B1 exposure has only minor effects on the gut-associated lymphoid tissue of brown Norway rats. Toxicology. 1999;138:93–102. doi: 10.1016/s0300-483x(99)00088-8. [DOI] [PubMed] [Google Scholar]
- 77.Yunus AW, Ghareeb K, Abd-El-Fattah AAM, Twaruzek M, Boehm J. Gross intestinal adaptations in relation to broiler performance during chronic aflatoxin exposure. Poult Sci. 2011;90:1683–1689. doi: 10.3382/ps.2011-01448. [DOI] [PubMed] [Google Scholar]
- 78.Wan XL, Yang ZB, Yang WR, Jiang SZ, Zhang GG, Johnston SL, Chi F. Toxicity of increasing aflatoxin B1 concentrations from contaminated corn with or without clay adsorbent supplementation in ducklings. Poult Sci. 2013;92:1244–1253. doi: 10.3382/ps.2012-02748. [DOI] [PubMed] [Google Scholar]
- 79.Castelino JM, Routledge MN, Wilson S, Dunne DW, Mwatha JK, Gachuhi K, Wild CP, Gong YY. Aflatoxin exposure is inversely associated with IGF1 and IGFBP3 levels in vitro and in Kenyan schoolchildren. Mol Nutr Food Res. 2014;25:201300619. doi: 10.1002/mnfr.201300619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dudley DJ. Pre-term labor: an intra-uterine inflammatory response syndrome? J Reprod Immunol. 1997;36:93–109. doi: 10.1016/s0165-0378(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 81.Lee JT, Jessen KA, Beltran R, Starkl V, Schatzmayr G, Borutova R, Caldwell DJ. Mycotoxin-contaminated diets and deactivating compound in laying hens: 1. Effects on performance characteristics and relative organ weight. Poult Sci. 2012;91:2089–2095. doi: 10.3382/ps.2012-02136. [DOI] [PubMed] [Google Scholar]
- 82.Hurley DJ, Neiger RD, Higgins KF, Rottinghaus GE, Stahr H. Short-term exposure to subacute doses of aflatoxin-induced depressed mitogen responses in young mallard ducks. Avian Dis. 1999;43:649–655. [PubMed] [Google Scholar]
- 83.Gong YY, Wilson S, Mwatha JK, Routledge MN, Castelino JM, Zhao B, Kimani G, Kariuki HC, Vennervald BJ, Dunne DW, Wild CP. Aflatoxin exposure may contribute to chronic hepatomegaly in Kenyan school children. Environ Health Perspect. 2012;120:893–896. doi: 10.1289/ehp.1104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.