Abstract
Background
GABAergic inhibition and effects of intracellular chloride ions on calcium channel activity have been proposed to regulate neurotransmission from photoreceptors. To assess the impact of these and other chloride-dependent mechanisms on release from cones, the chloride equilibrium potential (ECl) was determined in red-sensitive, large single cones from the tiger salamander retinal slice.
Results
Whole cell recordings were done using gramicidin perforated patch techniques to maintain endogenous Cl- levels. Membrane potentials were corrected for liquid junction potentials. Cone resting potentials were found to average -46 mV. To measure ECl, we applied long depolarizing steps to activate the calcium-activated chloride current (ICl(Ca)) and then determined the reversal potential for the current component that was inhibited by the Cl- channel blocker, niflumic acid. With this method, ECl was found to average -46 mV. In a complementary approach, we used a Cl-sensitive dye, MEQ, to measure the Cl- flux produced by depolarization with elevated concentrations of K+. The membrane potentials produced by the various high K+ solutions were measured in separate current clamp experiments. Consistent with electrophysiological experiments, MEQ fluorescence measurements indicated that ECl was below -36 mV.
Conclusions
The results of this study indicate that ECl is close to the dark resting potential. This will minimize the impact of chloride-dependent presynaptic mechanisms in cone terminals involving GABAa receptors, glutamate transporters and ICl(Ca).
Background
Regulation of intracellular chloride levels results in a chloride equilibrium potential (ECl) that is hyperpolarized with respect to the resting potential in many nerve cells, but depolarized in others [1-5]. For example, ECl in salamander rod photoreceptors is 25 mV more positive than the dark resting potential [6]. The resting potential of cone photoreceptors in darkness is around -42 to -47 mV and estimates of ECl in cones have ranged from -65 mV to -36 mV [7-11]. Cone photoreceptors possess a number of Cl- conductances that help to shape their responses and synaptic output. As discussed below, the value of ECl in cones is an important parameter for determining the strength and polarity of these effects.
It has been suggested GABAa receptors in the terminals of cones may mediate inhibitory synaptic feedback from horizontal cells to cones [8]. Under this hypothesis, the light-evoked hyperpolarization of horizontal cells causes a cessation of GABA release and this disinhibition leads to a "feedback depolarization" in cones. There is evidence both for [e.g., [8]] and against [e.g., [12,13]; see review in ref. [14]]) this hypothesis. However, one prediction of the hypothesis is that the Cl- equilibrium potential (ECl) must be negative to the resting potential in order for GABA disinhibition to depolarize a cone.
Cones possess prominent Ca2+-activated Cl- currents (ICl(Ca)) [15-17] activated by the influx of Ca2+ through voltage-gated Ca2+ channels as well as by release of Ca2+ from intracellular stores [16]. Cl- flux through ICl(Ca) can be substantial: during a 1.4 sec depolarizing step, the charge movement accompanying activation of ICl(Ca) is estimated to be 8.5 times that produced by activation of ICa alone [16]. These large membrane currents can strongly influence photoreceptor responses, but the nature of these effects depends on the value of ECl. If ECl is positive to the resting potential, activation of ICl(Ca) can boost depolarizing feedback responses from horizontal cells onto cones and produce prolonged, regenerative depolarizing responses lasting many seconds [9,18,19]. On the other hand, if ECl is negative to the resting potential, activation of ICl(Ca) can operate as a negative feedback mechanism to limit regenerative activation of Ca2+ channels [15,17]. In addition to altering membrane potential, depletion of intracellular Cl- can directly inhibit the open channel probability of single Ca2+ channels, presumably by modifying an anion binding site on the intracellular surface of the channel [11]. In rods, where ECl is positive to the resting potential, there is evidence for a negative feedback pathway between ICa and ICl(Ca) in which activation of ICa stimulates ICl(Ca) leading to a Cl- efflux that in turn inhibits Ca2+ channel activation [6,20]. If, however, ECl in cones is negative to the membrane potential, then activation of ICl(Ca) would stimulate an influx of Cl- that would be expected to enhance Ca2+ channel open probability [11].
Cone photoreceptors have presynaptic glutamate transporters that are coupled to Cl- channels [21-23]. The transporters in cones have been shown to respond to glutamate released from their own terminals [24]. Whether synaptically released glutamate causes cones to hyperpolarize or depolarize depends on ECl. Furthermore, analogous to the negative feedback from ICl(Ca) onto ICa described above, the chloride current produced by activation of glutamate transporters in rods can cause a Cl- efflux that inhibits ICa [25]. As with the feedback between ICl(Ca) and ICa, the strength and polarity of this potential interaction in cones depends on ECl.
Given the importance of ECl in determining the impact of various feedback mechanisms in the photoreceptor terminal, we determined ECl in cone photoreceptors of the salamander retina using a combination of imaging with a chloride-sensitive dye and electrophysiological approaches.
Results
In control superfusate, dark resting potentials of cones from slices prepared under visible light averaged -46.0 ± 2.00 mV (n = 9) after correcting for the liquid junction potential. This is nearly identical to the dark resting potentials of salamander cones prepared under infrared illumination (-46.8 ± 2.03 mV, n = 18).
To measure ECl, ICl(Ca) was recorded using gramicidin perforated patch whole cell recordings and activated by applying a 500 ms step from -78 to -8 mV. This depolarizing step typically evoked a sustained inward tail current arising largely from activation of ICl(Ca) [19]. Only cells that exhibited an inward tail current were used for analysis. As shown in the example of Fig. 1A, the current/voltage relationship of a cone cell was assessed during the tail current by using a ramp voltage protocol (1 mV/ms from -98 to +52 mV) begun 25 ms after the end of the depolarizing step. The same protocol was then repeated after applying niflumic acid (0.1 mM; Fig. 1B). At this concentration, niflumic acid is a selective inhibitor of ICl(Ca) in cones [[19]; niflumic acid may not be as selective in rods: [20,26]]. Subtracting the control ramp-evoked current from that obtained in the presence of niflumic acid yields the current/voltage profile for ICl(Ca) (Fig. 1C). In the example shown in Fig. 1C, the difference current reversed around -46 mV. The reversal potential of the niflumic acid-sensitive difference current determined from 8 cones averaged -45.5 ± 2.5 mV. As a control for the possible perturbation of intracellular Cl- by possible patch rupture, we repeated the same experiment using a pipette solution with only 3.5 mM Cl-. ECl was not significantly different when measured using the low Cl- pipette solution (-50.4 mV ± 3.4 mV; n = 7; p = 0.49, unpaired t-test). If patch rupture had occurred, ECl would be expected to attain -89 mV with the low Cl- pipette solution and -20 mV with the original pipette solution.
Figure 1.
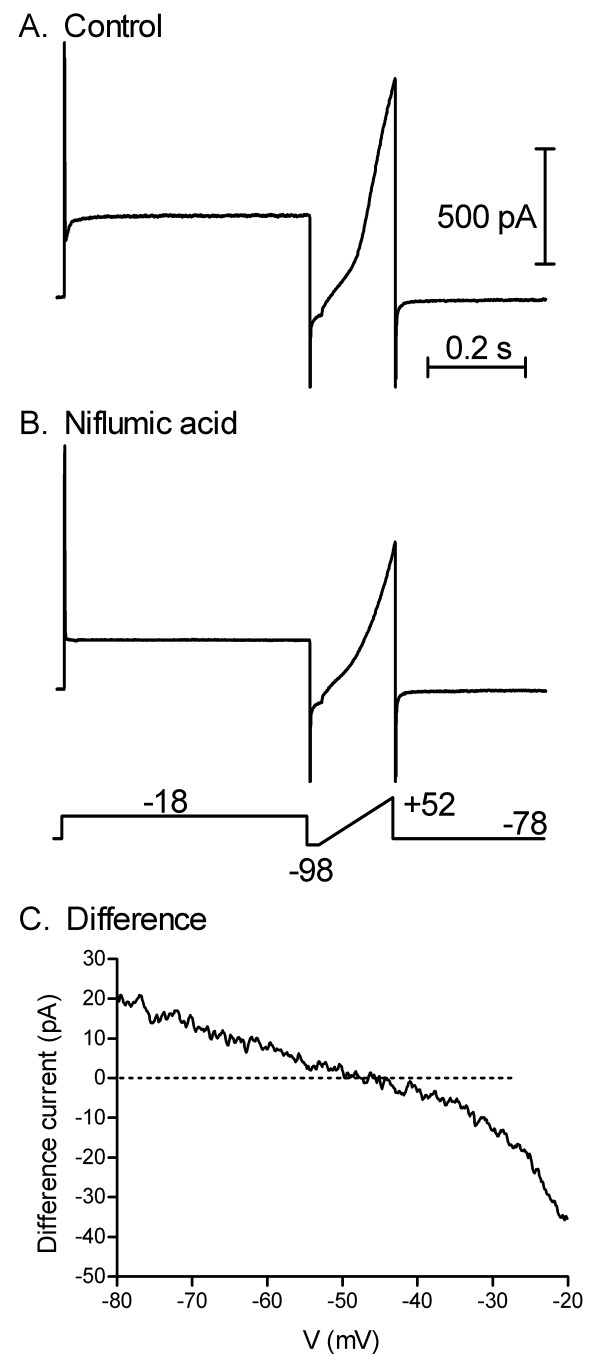
Cone ECl estimated from the reversal of ICl(Ca). A) ICl(Ca) tail current was activated by applying a 500 ms step from -78 to -18 mV during a gramicidin perforated patch whole cell recording from a rod. A ramp voltage protocol (-98 to +52 mV, 1 mV/ms) was applied during the tail current and begun 25 ms after termination of the step. B. The same protocol was then repeated in the presence of niflumic acid (0.1 mM) to inhibit ICl(Ca). C. The ramp current/voltage relationship obtained in control medium (A) was subtracted from that obtained in the presence of niflumic acid (B) to yield a niflumic acid-sensitive difference current that reversed in this cell around -46 mV (after correcting for a liquid junction potential of -8 mV).
Bath application of GABA evoked small reversible inward currents that averaged -3.4 ± 0.3 pA at the holding potential of -78 mV (not shown). The small size of these currents may be due to receptor desensitization [27]. Consistent with results obtained from measurements of ICl(Ca), difference currents calculated from ramps applied before and during GABA application indicate that the GABA-evoked current reversed at -46.4 ± 2.7 mV (n = 9).
In a complementary approach for measuring ECl, we used a Cl-sensitive dye, MEQ, to examine the Cl- flux that accompanied cone depolarization evoked by bath application of various high K+ solutions (12, 22, 31, 41, 50 and 70 mM K+). In a separate set of experiments, we used gramicidin-perforated patch recording methods to measure the membrane potentials produced in cones by application of the different high K+ solutions. Slices used for MEQ experiments and for measurement of membrane potentials in different solutions were prepared using similar techniques under visible illumination; control experiments showed that the fluorescent illumination used during MEQ experiments did not produce any further changes in the cone resting membrane potential (n = 3). An example of a retinal slice loaded with MEQ is shown in Fig. 2A. Measurements of MEQ fluorescence were made from the cone soma (circle, Fig. 2A). For a single wavelength dye such as MEQ, the change in fluorescence relative to basal fluorescence (ΔF/F) can be used as a measure of the change in ion concentration [28]. In the cone in Fig. 2B, bath application of 12 mM K+, which depolarized cones to -36 mV, produced a 1.2% decrease in MEQ fluorescence. Since MEQ fluorescence is quenched by Cl- ions this indicates that depolarization to -36 mV stimulated an influx of Cl- ions. Application of a solution with 70 mM K+, which depolarizes cones to -7 mV, produced a greater influx of Cl- as evidenced by the 10% decrease in MEQ fluorescence seen in a different cone (Fig. 2C). Fig. 2D shows the average change in ΔF/F (x100) plotted as a function of the membrane potential evoked by the different high K+ solutions. The finding that 12 mM K+ consistently stimulated an influx of Cl- indicates that the reversal potential must be below -36 mV.
Figure 2.
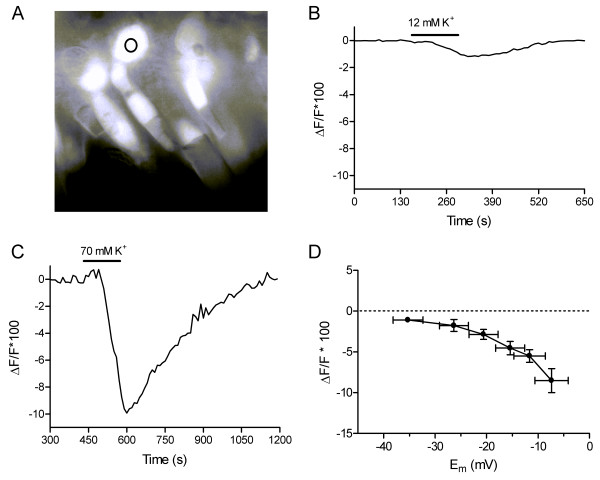
Intracellular chloride measurements. A. Example of a retinal slice fluorescently stained with the Cl- sensitive dye MEQ. The circle indicates the somatic region of a cone in which MEQ measurements were made. B. An example of the reduction in MEQ fluorescence, indicating an increase in intracellular [Cl-], produced by application of 12.1 mM K+ to a cone. C. A greater decrease in MEQ fluorescence was produced by application of 69.9 mM K+. Different cone from panel B. D. MEQ fluorescence changes produced by bath application of superfusate containing 12.1, 21.6, 31.2, 40.7, 50.3, or 69.9 mM [K+]. After correcting for liquid junction potentials, these high K+ solutions depolarized cones to -35.3 ± 2.90, -26.3 ± 2.82, -20.6 ± 2.82, -15.4 ± 2.84, -11.6 ± 3.05, and -7.4 ± 3.25 mV (n = 9), respectively, determined in current clamp recordings using gramicidin-perforated patch recording techniques. The change in MEQ fluorescence relative to basal fluorescence (ΔF/F*100) is plotted against the cone membrane potential determined with each high K+ solution.
Discussion
The main finding of this study is that ECl in salamander cones is close to the dark resting potential (~-46 mV). ECl was found to be -46 mV from block of ICl(Ca) by niflumic acid; small GABA-evoked currents reversed around the same potential. MEQ fluorescence changes produced by depolarization support these electrophysiological measurements by indicating that ECl is below -36 mV.
There can be local variations of ECl within cells [4]. Large single cones in the salamander retina do not have a distinct axon and terminal; synaptic proteins are instead located at the base of the soma [29]. MEQ measurements were made in the cell soma from a region adjacent to the synaptic ending (see Fig. 2). ICl(Ca) is localized to the terminal region in rods (30) and these channels are probably also localized to the terminals of cones. Thus, the measurements in the present study are likely to provide estimates of ECl in the synaptic terminal and adjacent regions of the cone cell. Measurements of intracellular Cl- levels suggest that ECl in the inner segment is not significantly different from that measured in the soma [11].
The finding that the Cl- equilibrium potential is close to the resting potential does not necessarily mean that Cl- is passively distributed. Electrophysiological experiments required that cells be voltage clamped at -70 mV for many minutes. Nonetheless, the value of ECl determined from these electrophysiological experiments in which cells were voltage clamped at -70 mV was similar to the value estimated from MEQ studies in which cells were not voltage clamped and thus at their resting membrane potential. Results from experiments on the prolonged depolarization in cones also suggest that ECl can be maintained indefinitely at a value above the membrane potential. The plateau phase of the prolonged depolarization, which largely reflects ICl(Ca) activation [9,19], could remain above the membrane potential established by an adapting background for hours [9]. The ability of cones to maintain ECl above the membrane potential may arise from activity of the Na/KCl cotransporter as shown in rods (20) as well as from other mechanisms (e.g., CLC-2) [2,31].
Comparisons with other studies
ECl in cones has been estimated in a number of previous studies. The most positive value for ECl of -36 mV comes from calibration of MEQ fluorescence levels to determine the resting intracellular Cl- concentrations in cones isolated from the salamander retina (11). However, these measurements showed a large variability (range of S.E.M.: -26.5 to -46.6 mV). The most negative estimate of ECl comes from a study by Attwell et al [7] showing that the sign-reversing pathway from rods to cones reversed around -65 mV. Based on the presumption that this pathway involved disinhibition of GABAergic inputs into cones, this study has been interpreted as suggesting that ECl is around -65 mV. However, more recent evidence questions whether the horizontal cell to cone feedback pathway thought to underlie this sign-reversing pathway from rods to cones is truly GABAergic [9,13,14]. Other studies have arrived at values for ECl similar to those found in the present study. 1) By examining the polarity of GABA-evoked currents after patch rupture with either 12 or 24 mM Cl- in the recording pipette, Kaneko and Tachibana [8] estimated ECl to be around -47 mV in isolated turtle cones. 2) Based on the membrane potential attained by the plateau phase of the prolonged depolarization in turtle cones from the eyecup slice preparation, ECl was estimated to be at or slightly above the dark resting potential of -42 mV [after correction for a liquid junction potential of -2 mV; ref. [9]]. 3) In a single recording from a salamander cone obtained with a Cl-sensitive electrode, Miller and Dacheux [32] found that ECl was 2 mV more positive than the dark resting potential. 4) A slightly more negative value for ECl was found in ruptured patch recordings from goldfish cones by examining the voltage dependence of the ICl(Ca) tail current [10]. By extrapolating measurements back to the time of patch rupture, Kraaij et al [10] concluded that ECl was ~-55 mV.
Functional implications
ICa in cones, like that of rods, can be inhibited by lowering extracellular Cl- [33]. The inhibition of ICa produced by lowering extracellular Cl- appears to result from a reduction in intracellular Cl- which in turn causes a reduction in the open probability of single Ca2+ channels [11]. In rods, where ECl is positive to the resting potential, activation of Cl- channels leads to a Cl- efflux thereby producing an inhibition of Ca2+ channels [6,11,20]. The present results indicate that activation of Cl- channels when the cell is at its resting potential would produce minimal changes in intracellular Cl- in cones. Therefore, the feedback between ICa and ICl(Ca) postulated for rod photoreceptors [6,20] would be expected to be minimal in cones in darkness.
Another implication of the finding that ECl is close to the dark resting potential is that the stimulation of Cl- channels associated with glutamate transporters by glutamate released from cone terminals [24] would tend to stabilize the cell membrane potential near the dark potential. In rods, the Cl- efflux accompanying activation of glutamate transporters appears to contribute to a glutamate-mediated inhibition of ICa [25]. As with the feedback between ICa and ICl(Ca) considered in the previous paragraph, the finding that ECl is near the resting potential leads to the prediction that in darkness there would be no Cl- efflux accompanying glutamate transporter activation and therefore glutamate would not be expected to inhibit ICa.
Cones hyperpolarize to light, although with prolonged illumination the membrane potential recovers to near the dark resting potential. The impact of chloride-dependent negative feedback between ICl(Ca) and ICa or the glutamate transporter chloride current and ICa would be expected to increase as a cone hyperpolarizes in response to light. By reducing glutamate release, these chloride-dependent negative feedback mechanisms might thus contribute to making post-synaptic responses more transient.
The finding that ECl is near the resting potential of cones indicates that GABAergic disinhibition near the dark potential should produce little membrane potential change. This result is inconsistent with the postulated role for GABA in generating the feedback depolarization [8] and supports other studies suggesting that GABA is not directly responsible for horizontal to cone feedback [9,13,14].
Conclusions
Electrophysiological measurements, supported by experiments using chloride-sensitive dyes, indicate that ECl in salamander cones is close to the dark resting membrane potential. By minimizing the trans-membrane flux of chloride, this will minimize the presynaptic impact of GABAa receptors, ICl(Ca), and glutamate transporter chloride channels.
Methods
Tissue preparation
ECl is positive to the resting potential of many neurons in the immature brain [5]. Based on their size, the neotenous tiger salamanders (Ambystoma tigrinum, 15–25 cm) used in these experiments are thought to be 2–7 years old out of a life span of ~12 years (34).
Salamanders were handled humanely in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center. Chilled salamanders were rapidly decapitated, an eye was enucleated, and the front of the eye was removed. The resulting eyecup was cut into three or four pieces and a single piece was placed vitreal surface down onto a piece of filter paper (2 × 5 mm, Millipore type AAWP, 0.8 μm pores). After adhering to the filter paper, the retina was isolated under chilled amphibian superfusate and cut into 125 μm slices using a razor blade tissue chopper (Stoelting Co., Wood Dale, IL). The slices were rotated 90° to view the retinal layers when placed under a water immersion objective (60X, 1.0 NA) on an upright fixed stage microscope (EF 600, Nikon Inc., USA). Slices were prepared under visible light but recordings were performed in darkness. All experiments were done using red-sensitive large single cones selected by anatomical criteria [35].
Solutions and perfusion
Solutions were applied with a single-pass, gravity-feed perfusion system (1 ml/min). The normal amphibian superfusate contained (in mM): 111 NaCl, 2.5 KCl, 1.8 CaCl2, 0.5 MgCl2, 10 N-2-hydroxyethylpiperazine-N' 2-ethanesulfonic acid (HEPES), and 5 glucose (pH 7.8). The osmolarity was measured with a vapor pressure osmometer (Wescor, Logan, UT) and adjusted, if necessary, to 242 ± 5 mOsm. For high K+ solutions, various quantities of NaCl were replaced with equimolar KCl. Niflumic acid was diluted (1:10,000) from DMSO stock solutions. Unless otherwise specified, chemicals were obtained from Sigma/Aldrich/RBI (St. Louis, MO).
Electrophysiology
Patch pipettes were pulled on a PP-830 vertical puller (Narishige USA, New York) from borosilicate glass pipettes (1.2 mm O.D., 0.95 mm I.D., with internal filament) and had tips of ~1 μm outer diameter with resistances of 10 to 15 MΩ. To maintain endogenous levels of intracellular Cl-, we obtained perforated patch whole cell recordings using the cation channel, gramicidin [36]. Gramicidin was dissolved in ethanol (5 mg/ml) and then added to the pipette electrolyte solution to achieve a final concentration of 5 μg/ml. For current clamp measurements of membrane potentials, the pipette electrolyte solution contained (in mM): 54 KCl, 61.5 KCH3SO4 (Pfaltz and Bauer, Waterbury, CT), 3.5 NaCH3SO4, 10 HEPES. The pH was adjusted to 7.2 with KOH. The liquid junction potential (LJP) of this solution was estimated to be -7 mV using the junction potential calculator of PClamp (Axon Instruments). Membrane potential values reported throughout this manuscript were corrected for the LJP. For experiments with niflumic acid or GABA, pipettes were typically filled with a solution containing (in mM): 54 CsCl, 61.5 CsCH3SO3, 3.5 NaCH3SO4, 10 HEPES (LJP = -8 mV). In some experiments, a low Cl- pipette solution was used containing: 115.5 mM CsCH3SO3, 3.5 NaCl, 10 HEPES (LJP = -10 mV). The pH of both solutions was adjusted to 7.2 with CsOH. The osmolarity of pipette solutions were also adjusted, if necessary, to 242 ± 5 mOsm. Recordings were made using an Axopatch 200B amplifier (Axon Instruments Inc., Union City, CA) and PClamp 8 software (Axon Instruments). Cell input resistance calculated using a step from -70 to -90 mV averaged 695 ± 111 MΩ. Access resistance estimated from the peak of the capacitative transient averaged 30.7 ± 4.8 MΩ (n = 24).
Imaging experiments
Digital fluorescent images were obtained with a cooled CCD camera (SensiCam, Cooke Corp., Auburn Hills, MI). Axon Imaging Workbench (AIW 2.2, Axon Instruments Inc., Union City, CA) was used to control the camera, filter wheel, and image acquisition. Pixel binning (2 × 2) of the images was used to decrease acquisition time to ≤1 s. Images were acquired at 5 to 10 s intervals during experimental trials.
For measurements of [Cl-]i we used the dye, 6-methoxy-N-ethylquinolinium iodide (MEQ, Molecular Probes, Eugene, OR) [37]. MEQ was loaded into cells after reducing it to DiH-MEQ by adding 30 μM sodium borohydride (100 μl) to MEQ (5 mg) under a continuous stream of nitrogen gas [38]. DiHMEQ enters cells during the incubation period (15 min) where it is oxidized and retained in the form of MEQ. Fluorescence emission decreases as Cl- quenches MEQ. The slow exponential decay in MEQ fluorescence due to dye leakage and bleaching was determined from a 3 min. series of control measurements prior to drug application and subtracted before analysis [11,20].
Variance is reported as ± S.E.M.
Authors' contributions
WT conceived the study and drafted the manuscript. WT and EB participated in all aspects of the experiments but most recordings were performed by EB. Both authors have read and approved the manuscript.
Acknowledgments
Acknowledgments
Supported by the National Eye Institute (EY10542), Research to Prevent Blindness, Inc., the Gifford Foundation, and the Nebraska Lions Foundation. The authors thank Dr. Dwight Burkhardt for his helpful comments on the manuscript.
Contributor Information
Wallace B Thoreson, Email: wbthores@unmc.edu.
Eric J Bryson, Email: ebryson@unmc.edu.
References
- Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley K, Smith R, Schaack J, Wilcox C, Jentsch TJ. Alteration of GABAA receptor function following gene transfer of the CLC-2 chloride channel. Neuron. 1996;17:543–551. doi: 10.1016/S0896-6273(00)80186-5. [DOI] [PubMed] [Google Scholar]
- Reuter D, Zierold K, Schroder WH, Frings S. A depolarizing chloride current contributes to chemoelectrical transduction in olfactory sensory neurons in situ. J Neurosci. 1998;18:6623–6630. doi: 10.1523/JNEUROSCI.18-17-06623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Zhang LL, Payne JA, Sterling P. Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. J Neurosci. 2000;20:7657–7663. doi: 10.1523/JNEUROSCI.20-20-07657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Stella SL, Jr, Bryson EJ, Clements J, Witkovsky P. D2-like dopamine receptors promote interactions between calcium and chloride channels that diminish rod synaptic transfer in the salamander retina. Vis Neurosci. 2002;19:235–247. doi: 10.1017/S0952523802192017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Werblin FS, Wilson M, Wu SM. A sign-reversing pathway from rods to double and single cones in the retina of the tiger salamander. J Physiol. 1983;336:313–333. doi: 10.1113/jphysiol.1983.sp014583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Tachibana M. Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J Physiol. 1986;373:443–461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Burkhardt DA. Ionic influences on the prolonged depolarization of turtle cones in situ. J Neurophysiol. 1991;65:96–110. doi: 10.1152/jn.1991.65.1.96. [DOI] [PubMed] [Google Scholar]
- Kraaij DA, Spekreijse H, Kamermans M. The nature of surround-induced depolarizing responses in goldfish cones. J Gen Physiol. 2000;115:3–16. doi: 10.1085/jgp.115.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, Miller RF. Chloride efflux inhibits single calcium channel open probability in vertebrate photoreceptors: chloride imaging and cell-attached patch-clamp recordings. Vis Neurosci. 2000;17:197–206. doi: 10.1017/S0952523800172025. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Burkhardt DA. Effects of synaptic blocking agents on the depolarizing responses of turtle cones evoked by surround illumination. Vis Neurosci. 1990;5:571–583. doi: 10.1017/s0952523800000730. [DOI] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–5393. doi: 10.1016/S0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Spekreijse H. The feedback pathway from horizontal cells to cones. A mini review with a look ahead. Vision Res. 1999;39:2449–2468. doi: 10.1016/S0042-6989(99)00043-7. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Korenbrot JI. Calcium and calcium-dependent chloride currents generate action potentials in solitary cone photoreceptors. Neuron. 1988;1:503–515. doi: 10.1016/0896-6273(88)90181-X. [DOI] [PubMed] [Google Scholar]
- Barnes S, Hille B. Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol. 1989;94:719–743. doi: 10.1085/jgp.94.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, Macleish PR. Ionic conductances of monkey solitary cone inner segments. J Neurophysiol. 1994;71:656–665. doi: 10.1152/jn.1994.71.2.656. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA, Gottesman J, Thoreson WB. Prolonged depolarization in turtle cones evoked by current injection and stimulation of the receptive field surround. J Physiol. 1988;407:329–348. doi: 10.1113/jphysiol.1988.sp017418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Deschenes MC. Contribution of Ca and Ca-activated Cl channels to regenerative depolarization and membrane bistability of cone photoreceptors. J Neurophysiol. 1992;68:745–755. doi: 10.1152/jn.1992.68.3.745. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Bryson EJ, Rabl K. Reciprocal interactions between calcium and chloride in rod photoreceptors. J Neurophysiol. 2003;90:1747–1753. doi: 10.1152/jn.00932.2002. [DOI] [PubMed] [Google Scholar]
- Eliasof S, Arriza JL, Leighton BH, Kavanaugh MP, Amara SG. Excitatory amino acid transporters of the salamander retina: identification, localization, and function. J Neurosci. 1998;18:698–712. doi: 10.1523/JNEUROSCI.18-02-00698.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasof S, Werblin F. Characterization of the glutamate transporter in retinal cones of the tiger salamander. J Neurosci. 1993;13:402–411. doi: 10.1523/JNEUROSCI.13-01-00402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picaud SA, Larsson HP, Grant GB, Lecar H, Werblin F. Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J Neurophysiol. 1995;74:1760–1771. doi: 10.1152/jn.1995.74.4.1760. [DOI] [PubMed] [Google Scholar]
- Picaud S, Larsson HP, Wellis DP, Lecar H, Werblin F. Cone photoreceptors respond to their own glutamate release in the tiger salamander. Proc Natl Acad Sci USA. 1995;92:9417–9421. doi: 10.1073/pnas.92.20.9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rábl K, Bryson EJ, Thoreson WB. Activation of glutamate transporters in rods inhibits presynaptic calcium currents. Vis Neurosci. 2003;20:557–566. doi: 10.1017/S0952523803205095. [DOI] [PubMed] [Google Scholar]
- Satoh TO, Yamada M. Niflumic acid reduces the hyperpolarization-activated current (I(h)) in rod photoreceptor cells. Neurosci Res. 2001;40:375–381. doi: 10.1016/S0168-0102(01)00252-8. [DOI] [PubMed] [Google Scholar]
- Epstein R, Grundfest H. Desensitization of gamma aminobutyric acid (GABA) receptors in muscle fibers of the crab Cancer borealis. J Gen Physiol. 1970;56:33–45. doi: 10.1085/jgp.56.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F. Calibration of fluorescent calcium indicators. In: Yuste R, Lanni F, Konnerth A, editor. Imaging Neurons: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 2000. pp. 32.1–32.9. [Google Scholar]
- Sherry DM, Yang H, Standifer KM. Vesicle-associated membrane protein isoforms in the tiger salamander retina. J Comp Neurol. 2001;431:424–436. doi: 10.1002/1096-9861(20010319)431:4<424::AID-CNE1080>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Macleish PR, Nurse CA. Ion channel compartments in photoreceptors [abstract] Invest Ophthalm Vis Sci. 2000;41:S494. [Google Scholar]
- Bösl MR, Stein V, Hubner C, Zdebik AA, Jordt SE, Mukhopadhyay AK, Davidoff MS, Holstein AF, Jentsch TJ. Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl- channel disruption. EMBO J. 2001;20:1289–1299. doi: 10.1093/emboj/20.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RF, Dacheux RF. Intracellular chloride in retinal neurons: measurement and meaning. Vision Res. 1983;23:399–411. doi: 10.1016/0042-6989(83)90087-1. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, Miller RF. Reducing extracellular Cl- suppresses dihydropyridine-sensitive Ca2+ currents and synaptic transmission in amphibian photoreceptors. J Neurophysiol. 1997;77:2175–2190. doi: 10.1152/jn.1997.77.4.2175. [DOI] [PubMed] [Google Scholar]
- Townes-Anderson E, Colantonio A, St Jules RS. Age-related changes in the tiger salamander retina. Exp Eye Res. 1998;66:653–667. doi: 10.1006/exer.1998.0472. [DOI] [PubMed] [Google Scholar]
- Stella SL, Jr, Thoreson WB. Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. Eur J Neurosci. 2000;12:3537–3548. doi: 10.1046/j.1460-9568.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-X. [DOI] [PubMed] [Google Scholar]
- Biwersi J, Verkman AS. Cell-permeable fluorescent indicator for cytosolic chloride. Biochemistry. 1991;30:7879–7883. doi: 10.1021/bi00246a001. [DOI] [PubMed] [Google Scholar]
- Woll E, Gschwentner M, Furst J, Hofer S, Buemberger G, Jungwirth A, Frick J, Deetjen P, Paulmichl M. Fluorescence-optical measurements of chloride movements in cells using the membrane-permeable dye diH-MEQ. Pflugers Arch. 1996;432:486–493. doi: 10.1007/s004240050160. [DOI] [PubMed] [Google Scholar]


