Abstract
Little is known about the burden of Q fever in Thailand. We conducted a serological study to describe the prevalence of anti-Coxiella burnetii antibodies among ruminants and occupationally exposed persons in response to the report of the first two Q fever endocarditis patients in Thailand in 2012. We randomly selected ruminant sera from brucellosis surveillance and examined sera of 661 occupationally exposed subjects from two provinces of Thailand: Chiangmai and Nakornratchasima. Animal and human sera were tested using commercial enzyme-linked immunosorbent assay (ELISA). Environmental samples, vaginal swab, and milk from cows in Chiangmai farms with detectable anti-C. burnetii serum antibodies were tested using polymerase chain reaction (PCR). Among the 1,632 animal sera tested, 64 (3.9%) were seropositive. The prevalence was highest in dairy cattle (4.6%, 45/988), followed by goats (3.5%, 18/516) and sheep (2.1%, 1/48). The prevalence of anti-C. burnetii antibodies in each species varied significantly by province: the prevalence in cattle was higher in Chiangmai (5.5% versus 0%), however, the prevalence in sheep and goats was higher in Nakornratchasima (5.9% versus 1.0%). Four out of 60 milk samples were positive by PCR (6.7%). No environmental samples were positive. Among 661 human samples, 83 (12.6%) were ELISA positive. Seroprevalence was statistically higher in Chiangmai compare with Nakornratchasima (42.8% versus 3.0%). Coxiella burnetii infection exists in Thailand, but the prevalence varies by geographic distribution and animal reservoirs. Further studies focusing on the burden and risk factors of C.burnetii infection among high-risk groups should be conducted.
Introduction
Q fever is a zoonotic disease, caused by Coxiella burnetii, a gram-negative intracellular bacteria.1 Ruminants, including sheep, goats, and cattle, are the most common reservoirs of the disease.1 Q fever can be transmitted through inhalation of dust contaminated with infected animal birth products or secretions, such as the placenta.1 A study in the United States showed that C. burnetii can persist in the environment for over 1 year after an outbreak in animals.2 Other routes of transmission, such as food borne and human-to-human transmission, have been reported, but they are rare.1 Coxiella burnetii infection in humans can cause flu-like symptoms, with some patients developing more severe symptoms, such as pneumonia and hepatitis.3 Additionally, it can cause serious chronic infections, including endocarditis, which can be fatal if not diagnosed and treated promptly.3
Coxiella burnetii can infect people of all ages; previous studies showed that seroprevalence is highest among people aged 30–60 years.4,5 The prevalence of anti-C. burnetii antibodies in humans increases among those who work closely with ruminants, underlining the significance of animal–human transmission.3,6,7 Q fever outbreaks have occurred in both humans and animals, with a negative impact on the economy.8 The largest reported outbreak of Q fever was in the Netherlands during 2007–2010, with approximately 4,000 human cases. More than 50,000 dairy goats were euthanized for outbreak control purposes.8
In Thailand, Q fever was first reported in 1966,9 but few studies have been conducted to understand the burden of Q fever.10,11 Laboratory capacity to diagnose Q fever was not established before 2012. In 2012, a collaborative research between Khon Kaen University in Khon Kaen Province, Thailand, the Emerging Tropical Infections Research Unit in Marseille, France, and the U.S. Centers for Disease Control and Prevention (CDC) Division of Vector-Borne Diseases in Fort Collins, CO, identified the first two clinical and laboratory-confirmed Q fever endocarditis cases in Thailand.12 This raised concerns of the Thailand Ministry of Public Health (MoPH), which held a consultative meeting in June 2012 with the National Institutes of Health (NIH), National Institute of Animal Health (NIAH), World Health Organization, and CDC to identify key strategic objectives for Q fever collaboration and research.
As a result of this collaboration, laboratory capacities for Q fever diagnosis have been strengthened to include the capacity for immunofluorescence assay and polymerase chain reaction (PCR). This capacity has allowed further investigation and understanding of the burden of Q fever in Thailand and identification of appropriate prevention measures. In this study, we analyzed blood samples from ruminants and humans occupationally exposed to ruminants to determine the level of exposure to C. burnetii and explored the presence of C. burnetii in the dairy farm environment.
Materials and Methods
Study area.
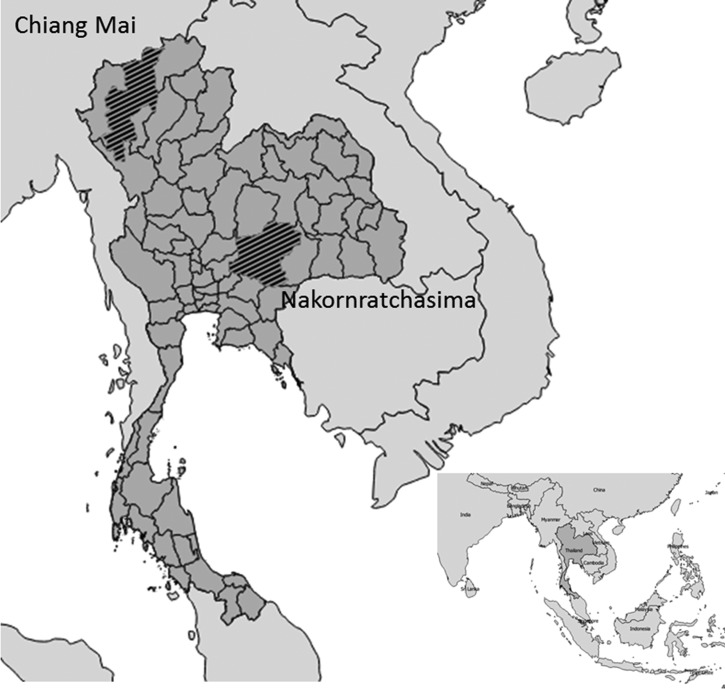
We selected two provinces from two regions of Thailand based on the number of ruminants: Chiangmai and Nakornratchasima are among the top five provinces with ruminants in the north and northeast, respectively.13 The majority of people in these provinces work in agriculture14 (Figure 1 ).
Figure 1.
Study areas where samples for Q fever were collected, Thailand 2012–2013.
Study samples.
Samples came from various sources. Ruminant sera from both Chiangmai and Nakornratchasima came from samples collected for annual brucellosis surveillance. The ruminant serum samples were collected by the authorities from the Bureau of Disease Control and Veterinary Services from January 2012 to September 2013. Eight animal samples from all 204 cattle farms in the two provinces were selected through this routine surveillance to test for antibodies to C. burnetii. For Chiangmai, human sera were collected from dairy farmers, livestock officers, and wildlife caretakers in response to the results of seroprevalence in animals. In addition, six farms in the area with seropositive results in Chiangmai were selected for the environmental study conducted in September 2013. Five samples of environmental swabs from each farm were taken from the birthing areas and stalls of dairy cattle, sheep, and goats. Moreover, serum samples, vaginal swabs, and milk from all the ruminants in these six farms were collected. Human serum samples from Nakornratchasima Province came from a brucellosis investigation.
Laboratory analysis.
Animal serum samples from the annual brucellosis surveillance in animals were tested using commercial enzyme-linked immunosorbent assay (ELISA) (IDEXX©, Westbrook, ME) for the presence of IgG antibody to Q fever. The assays were performed by the Serology Section, NIAH, Department of Livestock Development, following the manufacturer's instructions. Sample per positive percentages (S/P%) was calculated using optical density (OD) values as 100 × (ODsample − ODnegative control)/(ODpositive control − ODnegative control). S/P% ≥ 40% was defined as positive, and S/P% < 40% was defined as nonpositive.
For animal samples from the investigation in six farms in Chiangmai, milk, vaginal swab samples, and swabs from the environment were tested for the presence of C. burnetii DNA using real-time PCR to identify the insertion element IS1111. The forward primer used was 5′-ccgatcatttgggcgct-3′ and the reverse primer is 5′-cggcggtgtttaggc-3′. Hybridization probe method was used in real-time PCR, and the probe sequence was 5′-ttaacacgccaagaaacgtatcgctgtg-3′.15 All laboratory samples from animals and environment were tested at the Veterinary Research and Development Center in Lampang Province. Human serum samples were tested by commercial ELISA (Verion-Serion) for the presence of Q fever antibody by staff from Thailand NIH. Serology results were divided into positive, equivocal, and negative according to the manufacturer's criteria. The outcome of interest was seropositive versus nonpositive test results.
Data analysis.
Data were entered in Microsoft excel, and analyzed using Stata, version 11 (STATA Corp, College Station, TX) statistical software. Proportions were calculated to describe the distribution of Q fever in animals and humans. χ2 and Fisher's exact tests were used to determine the significant difference between proportions. Univariate analysis was applied to estimate prevalence ratios and 95% confidence intervals to assess the association between the covariates and seropositivity.
Ethical considerations.
This study was conducted as part of the outbreak investigation by the bureau of epidemiology team. Thus, it is exempted from the ethical review by the Ethical Committee of Thailand MoPH.
Results
Animal testing of cows, sheep, and goats in Chiangmai Province was performed on serum samples, vaginal swabs, and milk. Serologic testing was performed on 780 cattle serum samples and on 112 sheep and goat serum samples. For cattle, 5.5% (N = 43) of serum samples were positive by Q fever ELISA, and serum samples from both sheep and goats were all ELISA negative. Vaginal swabs were tested from 74 dairy cattle, 19 sheep, and two goats and all were found to be negative by PCR. Sixty cow's milk samples were tested and four (6.7%) were positive by PCR. None of the environmental samples from the six ruminant farms in Chiangmai were positive.
Animal testing in Nakornratchasima was only done on serum samples. Sera from 208 cows were tested by ELISA, with 1% (N = 2) positive. There were also 272 sheep and goats tested in Nakornratchasima with 5.9% (N = 16) positive. The seroprevalence among cattle in Chiangmai (5.5%) was significantly different than seroprevalence among cattle in Nakornratchasima (1%) (Table 1).
Table 1.
Human seroprevalence to Coxiella burnetii in Chiangmai and Nakornratchasima provinces, Thailand 2012–2013
| Province | Characteristics | N | Seropositive N (%) | Prevalence ratio (95% CI) | P value |
|---|---|---|---|---|---|
| Chiangmai | Total | 159 | 68 (42.80) | – | – |
| Gender | |||||
| Men | 126 | 53 (42.06) | 0.93 (0.60–1.42) | 0.7215 | |
| Women | 33 | 15 (45.50) | Reference | – | |
| Occupation | |||||
| Farmer | 48 | 33 (68.75) | 2.23 (1.58–3.16) | < 0.0001 | |
| Livestock officer | 3 | 1 (33.33) | 1.08 (0.21–5.50) | 0.9231 | |
| Unknown | 4 | 2 (50.00) | 1.63 (0.59–4.50) | 0.3516 | |
| Zoo worker | 104 | 32 (30.77) | Reference | – | |
| Chiangmai | Total | 159 | 68 (42.80) | 14.31 (8.43–24.3) | < 0.0001 |
| Nakornratchasima | Total | 502 | 15 (2.99) | Reference | – |
CI = confidence interval.
Human seroprevalence was determined for samples from Chiangmai and Nakornratchasima provinces. Seroprevalence of the subjects from two provinces combined was 12.6% (83/661). Most of the people in the study had an occupation that would put them in contact with livestock and other animals. In Chiangmai, 42.8% (N = 68) of 159 subjects were seropositive by ELISA. The majority of those tested were men (79.2%), but proportions of seropositivity were similar between men (42.1%) and women (45.5%) (Table 1). For 155/159 subjects, an occupation was identified. The occupations were farmer, zoo worker, and livestock officer. The prevalence ratio showed a significantly higher seroprevalence for farmers compared with zoo workers. Seropositivity for the three livestock officers tested was 33.3% and this did not significantly differ from zoo workers.
Testing of samples from Nakornratchasima showed a lower seroprevalence. Of the 502 human sera, 15 samples (3.0%) were positive by ELISA. An additional 23 samples were scored as equivocal by the ELISA test. Of the 502 samples, 468 were from farmers, and all the 15 positive samples were farmers. Occupation data were missing for the other 34 persons, all with a negative ELISA result. The difference in seroprevalence between Chiangmai (42.8%) and Nakornratchasima (3.0%) provinces was statistically significant (P < 0.0001).
Discussion
We analyzed blood samples from ruminants and humans occupationally exposed to ruminants to determine the magnitude of C. burnetii exposure and explored the presence of C. burnetii in the dairy farm environment. In ruminants, the overall seroprevalence was 4.5%, with dairy cattle (4.6%) having a slightly higher seroprevalence than sheep and goats (4.2%). The prevalence of anti-C. burnetii serum antibodies among people occupationally exposed to ruminants was 12.6%. Significant differences were observed between the two provinces analyzed. Chiangmai had significantly higher seroprevalence in dairy cattle and humans, whereas Nakornratchasima had higher seroprevalence among sheep and goats but much lower for humans. A limitation of the study is that different sampling strategies in the two provinces (routine surveillance for abortion versus an outbreak setting) could influence the results. Further studies are needed in Thailand to explore how environmental factors, farm practices, and the density of animal reservoirs in each area impact exposure to C. burnetii.
The prevalence of anti-C. burnetii serum antibodies among ruminants in our study (4.5%) was lower compared with a study performed in Thailand in 1967 where the seroprevalence among cattle, sheep, and goats combined was 5.4%.9 Furthermore, the seroprevalence among cattle in our study (4.6%) was lower compared with another study in Thailand published in 2014 where the prevalence of Q fever among cattle in Bangkok was 6.9%.16 All of the three studies used different serological tests, making direct comparisons difficult, but together provide information regarding the existence of Q fever in Thailand. Our findings show that different ruminants in various regions could be the potential source of C. burnetii infection in humans. However, reservoir species might differ geographically. Dairy cattle could be a potential reservoir for C. burnetii in Chiangmai, while sheep and goats could be the possible major animal reservoir in Nakornratchasima. Individual characteristics of the ruminants, for example age, as well as the environmental factors such as the presence of other animals in each particular area might also influence the maintenance of C. burnetii in ruminants.17
The presence of the antibody to C. burnetii in each particular herd may not indicate bacterial shedding from the animals. A previous study showed that the animals that shed the bacteria might not have antibody and seroconverted animals might not shed the bacteria.18 Identification of shedding animals is critical for reducing the source of Q fever infection. Our study found the evidence of C. burnetii DNA in milk, but not in the vaginal swabs of the same cow, indicating that milk could be a potential source of infection apart from placenta.19
Similar to other studies, our study found that the prevalence of C. burnetii serum antibodies among people who were exposed to animals, such as farmers, tended to be higher than that observed in other studies of the general population.10,20,21 This suggests that farmers might get more exposed to the bacteria as consistent with known routes of C. burnetii transmission. The seroprevalence among farmers, livestock, zoo, and wildlife workers with close contact to ruminants in this study (6.4%) was lower compared with other studies. The prevalence of anti-C. burnetii serum antibodies among farmers and veterinarians in Malaysia during a Q fever outbreak in 2011 was 43%.22 In previous studies, the seroprevalence among veterinarians in Japan was 35%,21 Taiwan 26%,23 and in the United States 22%.24 However, comparing the seroprevalence between studies can be challenging due to the discrepancies in serological methods and the cut-off value used, as well as the discrepancies in times when samples were collected. In addition, other environmental factors such as type and density of animal reservoirs might vary geographically.
Although some studies reported that men are more likely to have anti-C. burnetii antibodies than women,23,25–27 our study did not find a statistically significant difference between the seroprevalence of men versus women in Chiangmai Province. The result is similar to that of studies in Spain28 and in the Netherlands.29 The role of gender in C. burnetii exposure in Thailand might need further investigation if other routes of transmission or other animal reservoirs are found to influence the occurrence in men and women.
Following the identification of the first two confirmed cases of Q fever endocarditis in 2012,12 the Thailand Bureau of Policy and Strategy, Ministry of Public Health reported approximately 4,000–5,500 people had been diagnosed with endocarditis (6–8/100,000) during 2012 and 2013.30 Unfortunately, the etiologies of endocarditis were not identified. A multinational multicenter study in America, Europe, and Asia focused on the etiology of infective endocarditis showed that Q fever was positive in approximately 1% of patients presenting with acute endocarditis.31 Thus, it is likely that Q fever endocarditis is underreported in Thailand.
The main limitation of our study was missing data as we reanalyzed existing samples and no information related to C. burnetii exposures such as age or job description were available. Therefore, it was a challenge in exploring and determining possible risk factors for seropositivity in this study. Human sera from the two provinces came from different sampling strategies; hence, it also limited our ability to compare sampled human populations between regions. Nevertheless, our study has confirmed the presence of Q fever in Thailand.
In conclusion, antibodies to C. burnetii were prevalent in ruminants and humans in selected provinces of Thailand. It is likely that people who are exposed to ruminants might be at risk of becoming seropositive. Thus, health education to the high-risk population, and increasing awareness of Q fever among physicians, should be implemented to prevent the occurrence of the disease and to improve Q fever diagnosis. There are gaps in knowledge of both acute and chronic Q fever in Thailand. Therefore, future systematic epidemiological studies are needed to understand the risk factors for Q fever in animals and humans, and to identify the potential reservoirs for Q fever in Thailand.
ACKNOWLEDGMENTS
We are grateful to the public health and animal health officers who worked in the field and provided us the samples and data for the analyses. We also thank Dorothy L Southern for her scientific writing support, including the critical reviews during the development of this article.
Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Pawinee Doung-ngern and Teerasak Chuxnum, Bureau of Epidemiology, Department of Diseases Control, Ministry of Public Health, Nonthaburi, Thailand, E-mails: pawind@gmail.com and tchuxnum@yahoo.com. Decha Pangjai, National Institute of Health, Department of Medical Science, Ministry of Public Health, Nonthaburi, Thailand, E-mail: decha.p@dmsc.mail.go.th. Pattarin Opaschaitat, National Institute of Animal Health, Department of Livestock Development, Bangkok, Thailand, E-mail: pattarin.o@gmail.com. Nattinee Kittiwan, National Institute of Animal Health, Department of Livestock Development, Lampang Branch, Thailand, E-mail: sieben_sins@hotmail.com. Pranee Rodtian, The Fifth Regional Livestock Office (Chiangmai), Department of Livestock Development, Chiangmai, Thailand, E-mail: pranee.rodtian@gmail.com. Noppawan Buameetoop, Bureau of Disease Control and Veterinary Services, Department of Livestock Development, Bangkok, Thailand, E-mail: fonmaiya@yahoo.com. Gilbert J. Kersh, Rickettsial Zoonoses Branch, National Center for Emerging Zoonoses and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: hws7@cdc.gov. Pawin Padungtod, Global Disease Detection Regional Center, Thai MOPH – U.S. CDC Collaboration, Ministry of Public Health, Thailand and Global Disease Detection Branch, Division of Global Health Protection, Center for Global Health, Nonthaburi, Thailand, E-mail: pawin.padungtod@fao.org.
References
- 1.Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006;367:679–688. doi: 10.1016/S0140-6736(06)68266-4. [DOI] [PubMed] [Google Scholar]
- 2.Kersh GJ, Fitzpatrick KA, Self JS, Priestley RA, Kelly AJ, Lash RR, Marsden-Haug N, Nett RJ, Bjork A, Massung RF, Anderson AD. Presence and persistence of Coxiella burnetii in the environments of goat farms associated with a Q fever outbreak. Appl Environ Microbiol. 2013;79:1697–1703. doi: 10.1128/AEM.03472-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrie TJ, Raoult D. Coxiella burnetii (Q fever) In: Mandell GL, E. Bennett J, editors. Principles and Practice of Infectious Diseases. Philadelphia, PA: Churchill Livingstone; 2012. pp. 2511–2517. [Google Scholar]
- 4.Dahlgren FS, McQuiston JH, Massung RF, Anderson AD. Q fever in the United States: summary of case reports from two national surveillance systems, 2000–2012. Am J Trop Med Hyg. 2014;92:247–255. doi: 10.4269/ajtmh.14-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowbridge CP, Tobin S, Seale H, Ferson MJ. EpiReview: notifications of Q fever in NSW, 2001–2010. N S W Public Health Bull. 2012;23:31–35. doi: 10.1071/NB11037. [DOI] [PubMed] [Google Scholar]
- 6.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen SY, Molbak K, Nybo Andersen AM, Brink Henriksen T, Kantso B, Krogfelt KA, Hjollund NH. Prevalence of Coxiella burnetii in women exposed to livestock animals, Denmark, 1996 to 2002. Euro Surveill. 2013;18:20528. doi: 10.2807/1560-7917.es2013.18.28.20528. [DOI] [PubMed] [Google Scholar]
- 8.van Asseldonk MA, Prins J, Bergevoet RH. Prev Vet Med. 2013. Economic assessment of Q fever in the Netherlands. [DOI] [PubMed] [Google Scholar]
- 9.Sangkasuwan V, Pongpradit P, Bodhidatta P. Annual Research Progress Report. USArmy-SEATO Medical Research Unit; Bangkok, Thailand: 1967. SEATO Medical research study on rickettsial diseases in Thailand; pp. 499–504. [Google Scholar]
- 10.Suputtamongkol Y, Rolain JM, Losuwanaruk K, Niwatayakul K, Suttinont C, Chierakul W, Pimda K, Raoult D. Q fever in Thailand. Emerg Infect Dis. 2003;9:1186–1187. doi: 10.3201/eid0909.030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, Silpasakorn S, Suwancharoen D, Panlar P, Saisongkorh W, Rolain JM, Raoult D, Suputtamongkol Y. Causes of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational study. Ann Trop Med Parasitol. 2006;100:363–370. doi: 10.1179/136485906X112158. [DOI] [PubMed] [Google Scholar]
- 12.Pachirat O, Fournier P-E, Pussadhamma B, Taksinachanekij S, Lulitanond V, Baggett HC, Thamthitiwat S, Watt G, Raoult D, Maloney SA. The first reported cases of Q fever endocarditis in Thailand. Infect Dis Rep. 2012;4:17–18. doi: 10.4081/idr.2012.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunphet S. The Number of Dairy Cattle in Thailand by Province in 2012 (in Thai) 2013. http://www.dld.go.th/th/index.php/th/ Available at. Accessed November 8, 2013.
- 14.Department of Provincial Administration General Information of Districts in Thailand (In Thai) 2010. http://www.amphoe.com/menu.php Available at. Accessed July 25, 2014.
- 15.Kersh GJ, Lambourn DM, Self JS, Akmajian AM, Stanton JB, Baszler TV, Raverty SA, Massung RF. Coxiella burnetii infection of a Steller sea lion (Eumetopias jubatus) found in Washington State. J Clin Microbiol. 2010;48:3428–3431. doi: 10.1128/JCM.00758-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muramatsu Y, Usaki N, Thongchai C, Kramomtong I, Kriengsak P, Tamura Y. Seroepidemiologic survey in Thailand of Coxiella burnetii infection in cattle and chickens and presence in ticks attached to dairy cattle. Southeast Asian J Trop Med Public Health. 2014;45:1167–1172. [PubMed] [Google Scholar]
- 17.Taurel AF, Guatteo R, Joly A, Seegers H, Beaudeau F. Seroprevalence of Q fever in naturally infected dairy cattle herds. Prev Vet Med. 2011;101:51–57. doi: 10.1016/j.prevetmed.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Guatteo R, Beaudeau F, Berri M, Rodolakis A, Joly A, Seegers H. Shedding routes of Coxiella burnetii in dairy cows: implications for detection and control. Vet Res. 2006;37:827–833. doi: 10.1051/vetres:2006038. [DOI] [PubMed] [Google Scholar]
- 19.Yingst SL, Opaschaitat P, Kanitpun R, Thammasart S, Ekgatat M, Jirathanawat V, Wongwicharn P. Q fever surveillance in ruminants, Thailand, 2012. Emerg Infect Dis. 2013;19:2056–2058. doi: 10.3201/eid1912.130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georgiev M, Afonso A, Neubauer H, Needham H, Thiery R, Rodolakis A, Roest H, Stark K, Stegeman J, Vellema P, van der Hoek W, More S. Q fever in humans and farm animals in four European countries, 1982 to 2010. Euro Surveill. 2013;18:20407. [PubMed] [Google Scholar]
- 21.Porter SR, Czaplicki G, Mainil J, Horii Y, Misawa N, Saegerman C. Q fever in Japan: an update review. Vet Microbiol. 2011;149:298–306. doi: 10.1016/j.vetmic.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Bina RS, Kamaludin F, Chow TS, Yoon CK. First documented zoonotic case of Q fever in Penang, Malaysia. OSIR. 2011;4:1–4. [Google Scholar]
- 23.Chang CC, Lin PS, Hou MY, Lin CC, Hung MN, Wu TM, Shu PY, Shih WY, Lin JH, Chen WC, Wu HS, Lin LJ. Identification of risk factors of Coxiella burnetii (Q fever) infection in veterinary-associated populations in southern Taiwan. Zoonoses Public Health. 2010;57:e95–e101. doi: 10.1111/j.1863-2378.2009.01290.x. [DOI] [PubMed] [Google Scholar]
- 24.de Rooij MM, Schimmer B, Versteeg B, Schneeberger P, Berends BR, Heederik D, van der Hoek W, Wouters IM. Risk factors of Coxiella burnetii (Q fever) seropositivity in veterinary medicine students. PLoS One. 2012;7:e32108. doi: 10.1371/journal.pone.0032108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marrie TJ, Pollak PT. Seroepidemiology of Q fever in Nova Scotia: evidence for age dependent cohorts and geographical distribution. Eur J Epidemiol. 1995;11:47–54. doi: 10.1007/BF01719945. [DOI] [PubMed] [Google Scholar]
- 26.Pascual-Velasco F, Montes M, Marimon JM, Cilla G. High seroprevalence of Coxiella burnetii infection in Eastern Cantabria (Spain) Int J Epidemiol. 1998;27:142–145. doi: 10.1093/ije/27.1.142. [DOI] [PubMed] [Google Scholar]
- 27.Whitney EA, Massung RF, Candee AJ, Ailes EC, Myers LM, Patterson NE, Berkelman RL. Seroepidemiologic and occupational risk survey for Coxiella burnetii antibodies among US veterinarians. Clin Infect Dis. 2009;48:550–557. doi: 10.1086/596705. [DOI] [PubMed] [Google Scholar]
- 28.Cardenosa N, Sanfeliu I, Font B, Munoz T, Nogueras MM, Segura F. Short report: seroprevalence of human infection by Coxiella burnetii in Barcelona (northeast of Spain) Am J Trop Med Hyg. 2006;75:33–35. [PubMed] [Google Scholar]
- 29.Schimmer B, Lenferink A, Schneeberger P, Aangenend H, Vellema P, Hautvast J, van Duynhoven Y. Seroprevalence and risk factors for Coxiella burnetii (Q fever) seropositivity in dairy goat farmers' households in The Netherlands, 2009–2010. PLoS One. 2012;7:e42364. doi: 10.1371/journal.pone.0042364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bureau of Policy and Strategy Ministry of Public Health Thailand . ICD-10 Data of Patients Diagnosis with Endocarditis in Thailand 2012–2013 (ICD10 - I33, I33.9, I39.8) Bureau of Policy and Strategy Ministry of Public Health Thailand; Nonthaburi, Thailand: 2014. [Google Scholar]
- 31.Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG, Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falco V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH. International Collaboration on Endocarditis-Prospective Cohort Study Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]