Abstract
Vitamin A and zinc are important for immune function and may improve host defense against malaria and reduce the risk of adverse pregnancy outcomes. Our objective was to determine whether daily oral supplementation with either or both nutrients starting in the first trimester reduces the risk of placental malaria and adverse pregnancy outcomes. We undertook a randomized, double-blind placebo-controlled trial with a factorial design among 2,500 human immunodeficiency virus–negative primigravid or secundigravid pregnant women in their first trimester of pregnancy in Dar es Salaam, Tanzania. We randomly allocated equal numbers of participants to 2,500 IU of vitamin A, 25 mg of zinc, both 2,500 IU of vitamin A and 25 mg of zinc, or a placebo until delivery. A total of 625 participants were allocated to each treatment group. Our primary outcome, placental malaria infection (past or current), was assessed in all randomized participants for whom placental samples were obtained at delivery (N = 1,404), which represents 56% of total participants and 62% of all pregnancies lasting 28 weeks or longer (N = 2,266). Birth outcomes were obtained for 2,434 of the 2,500 randomized participants. Secondary outcomes included small for gestational age (SGA) births and prematurity. All analyses were intent to treat. Those who received zinc had a lower risk of histopathology-positive placental malaria compared with those who did not receive zinc (risk ratio = 0.64, 95% confidence interval = 0.44, 0.91), but neither nutrient had an effect on polymerase chain reaction–positive malaria, SGA, or prematurity. No safety concerns were identified. We recommend additional studies in other geographic locations to confirm these findings.
Introduction
Malaria causes nearly 200 million clinical cases and approximately half a million deaths each year, primarily in sub-Saharan Africa.1 The risk of malaria increases during pregnancy,2 a period during which its prevention is especially important. Not only do pregnant women experience greater severity of illness compared with nonpregnant women,2 but studies have shown strong associations between prenatal malaria and maternal anemia,2 fetal loss, low birthweight, and infant mortality.2 Improving preventive measures that specifically target malaria in pregnancy is a global health priority.3
The role of nutritional status in susceptibility to and severity of malaria has long been recognized.4 Laboratory and observational studies have suggested a protective role against malaria for vitamin A and zinc,5–9 two micronutrients central to immune function, though some have been limited in their ability to distinguish nutritional deficiency from depletion that occurs during the acute-phase response to infection. Clinical trials conducted among children have inconsistently demonstrated a clinical benefit of both nutrients10–16 in preventing malaria and related outcomes. Two small clinical trials have been conducted thus far among pregnant women and have shown no clear evidence of benefit against malaria from either nutrient.17,18
Both zinc and vitamin A have been examined in relation to pregnancy complications, including preterm birth and reduced fetal growth. Meta-analyses of existing evidence suggest zinc supplementation may moderately reduce preterm birth though neither zinc nor vitamin A supplementation improve fetal growth.19,20 However, the quality of available evidence is variable, and few studies have evaluated the impact of supplementation in early pregnancy.
Using a factorial design, we therefore conducted a randomized, double-blind, placebo-controlled trial of vitamin A and zinc to determine whether daily oral administration of either or both of these nutrients starting in the first trimester reduces the risk of placental malaria and adverse pregnancy outcomes among pregnant women in Dar es Salaam, Tanzania.
Methods
Study design and participants.
This randomized, double-blind, placebo-controlled trial was implemented at 8 antenatal care clinics in the urban Temeke and Ilala districts of Dar es Salaam, Tanzania. The trial was registered at ClinicalTrials.gov on April 30, 2010, as NCT0111478. This report has been prepared in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Eligible participants were in their first trimester of pregnancy, primigravida or secundigravida, human immunodeficiency virus (HIV) uninfected, and intending to stay in Dar es Salaam for at least 6 weeks after delivery. Participants were recruited at their first antenatal care visit or through a demographic surveillance system (DSS) that was established in the Temeke District to identify early pregnancies in the population. The DSS consisted of two types of operations: 1) a cross-sectional census that enumerated the age and gender of the residents of all households within the geographic location and 2) repeated visits every 2 months to the households of all nulli- and primiparous reproductive-aged women and ascertainment of pregnancy status through a urine test. All first trimester pregnancies identified though the DSS were referred for enrollment at the nearest study clinic. Although initially only women who were less than 8 weeks' gestation were considered eligible, the gestational cutoff for eligibility was raised to 13 weeks in December 2010 because of low enrollment due to the challenge of identifying early pregnancies. Gestational age was estimated using the first date of last menstrual period (LMP). Between July 12, 2010, and September 17, 2013, 101,184 women were screened for eligibility. Follow-up was completed in June 2014.
HIV testing was performed at the first clinic visit using two rapid assays (Alere Determine Walnut Creek, CA, followed by Uni-Gold HIV 1/2; Trinity Biotech; Wicklow, Ireland). Discrepant results were confirmed using enzyme-linked immunosorbent assay (Enzygnost HIV Integral II; Siemens, Marburg, Germany) at the Muhimbili University of Health and Allied Sciences (MUHAS) clinical research laboratory. All women received pre- and posttest counseling. Those who tested positive were referred for standard prenatal care services including antiretroviral therapy. Women who tested HIV negative and provided written informed consent for participation in the trial were enrolled the same day.
Randomization and masking.
Participants were individually randomized in equal numbers to receive single tablets containing one of the following regimens: 1) 2,500 IU vitamin A, 2) 25 mg of zinc (as zinc sulfate), 3) both 2,500 IU vitamin A and 25 mg zinc, or 4) placebo and instructed to take the regimen orally each day until delivery. The tablets were indistinguishable from one another in appearance and taste. Allocation to treatment groups was performed according to a computer-generated randomization sequence using blocks of size 20 by a scientist not involved in the data collection. Neither the participants nor the study personnel had access to the masking information. Each study clinic was issued regimen bottles that were prelabeled according to this sequence by study pharmacists who had no contact with participants. At enrollment, each participant was assigned to the next numbered bottle at their site. The study remained blinded until all trial assessments and database cleaning were completed, at which time study staff analyzing the data were given access to treatment assignments.
Procedures.
Enrolled participants completed background and food frequency questionnaires, underwent a full clinical examination, provided a blood sample that was tested for a complete blood count (CBC; Ac⋅T 5diff AL [Beckman Coulter, Miami FL]) at the MUHAS clinical research laboratory, and were given a 45-day supply of study regimen. In accordance with Tanzanian standard of care, participants were also provided with iron (60 mg daily) and folic acid (5 mg daily) supplements and vouchers for insecticide-treated bednets issued through a government program. Participants attended monthly visits at study clinics until delivery. At each visit, participants were administered a health questionnaire, given an obstetric examination, and provided with a 45-day supply of regimen. Study staff collected used pill bottles at each visit and counted remaining pills. At 20 and 30 weeks' gestation, all participants were provided with intermittent preventive treatment in pregnancy malaria prophylaxis in the form of 1,500 mg sulfadoxine and 75 mg pyrimethamine per standard of care in Tanzania. Women were screened by clinic staff for malaria symptoms and tested for malaria parasites as needed. Incident cases were managed according to the Tanzanian Ministry of Health and Social Welfare guidelines.21
Considerable effort was made to reduce loss to follow-up including limiting enrollment to women who intended to deliver within the study area, and close follow-up of all enrolled women, especially in the last weeks of pregnancy. Nonetheless, some participants later left Dar es Salaam for cultural reasons (e.g., at the request of their mothers or in-laws) or delivered at a non-study facility. Birth outcomes were obtained for 2,434 of the 2,500 randomized participants. On-call midwives documented birth outcomes, obtained blood samples for a CBC, and collected, examined, and sampled placentas following a standardized protocol after onsite training by a placental pathologist. Participants also attended a 6-week postpartum clinic visit during which they were administered questionnaires pertaining to their health and their infants' health and both underwent a physical examination.
Outcomes.
Placental malaria was evaluated using both histopathology and polymerase chain reaction (PCR). Tissue from fresh sampled placentas was divided for both uses. Histopathologic infections were defined as the presence of malaria pigment or parasitized erythrocytes on the slides. Any evidence of placental malaria, whether acute, chronic, or past, was scored as positive. For histopathologic examination, tissue from a central parenchymal section of the placenta was formalin fixed, embedded, sectioned, and stained using hematoxylin and eosin stain. Slides were examined using light microscopy and under polarized light for the presence of hemozoin and parasitized erythrocytes. A single placental histopathologist (Drucilla Roberts), who was masked to treatment groups and clinical history, classified all slides. Diagnoses were externally confirmed in a subset of 100 slides. Samples from 1,361 of the 1,404 obtained placentas (97%) underwent histopathologic examination. For the nucleic acid studies, tissue was stabilized in RNAlater (Qiagen, Hilden, Germany) and homogenized. Genomic DNA was extracted using DNeasy (Qiagen). Taqman® qRT-PCR (Thermo Fisher Scientific, Waltham, MA) was used for amplification using published primer and probe sets (Plasmodium falciparum-specific22 and general Plasmodium 18S rRNA genes23). The tissue was then tested for PCR inhibitors. Positive and negative controls were included on each plate for quality assurance. PCR-detected infections were defined as the detection of Plasmodium DNA through PCR amplification. Samples from 1,158 of the 1,404 obtained placentas (82%) underwent nucleic acid studies.
A number of secondary outcomes were also assessed. These included maternal hemoglobin, infant birthweight, low birthweight (< 2,500 g), very low birthweight (< 2,000 g), small for gestational age (SGA; below 10th percentile for gestational age births, based on the Alexander growth standard24 and the INTERGROWTH-21st standard),25 placental weight, maternal hospitalizations during pregnancy, maternal death, fetal loss (miscarriage and stillbirth), preterm birth (< 37 and < 34 weeks gestational age), perinatal death (> 28 weeks of gestation to 7 days after birth), child death (delivery until 6 weeks postpartum), and prevalence of maternal anemia (hemoglobin [Hb] < 11g/dL) and severe maternal anemia (Hb < 8.5 g/dL).
Statistical analysis.
A sample size of 2,500 was determined to provide at least 80% power at a 5% significance level to detect a 35% effect of each intervention on placental malaria risk assuming at least a 12% prevalence of placental malaria, a 30% effect of the other intervention, and a 10% loss to follow-up. All analyses followed the intent-to-treat principle. χ2 tests of independence or Fisher's exact tests and Wilcoxon rank-sum test were used to compare study outcomes between those who received vitamin A or placebo and zinc or placebo. Twin pregnancies were analyzed as a single outcome. The final birthweight for twin pregnancies was the average of the two twin birthweights. If either of the twins was stillborn, low birthweight, or SGA, the pregnancy was classified as such. Exclusion of twins from the birthweight analyses in a post hoc sensitivity analysis did not change our findings. The presence of interaction between vitamin A and zinc was evaluated by comparing nested log-binomial models with and without cross product terms for the two nutrients using the likelihood ratio test. Potential selection bias due to incomplete placenta collection was evaluated using marginal structural models (MSMs) weighted by the inverse probability of having a placental sample collected.26 Weights were calculated as the inverse of predicted values from a logistic regression model of inclusion in the primary endpoint analyses containing the following variables: treatment arm, baseline maternal characteristics, household characteristics, compliance with the regimen, and pairwise interactions between treatment and all maternal and household characteristics. The effect of supplementation was then estimated using weighted log-binomial models with empirical standard errors.27 All P values were two-sided. SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for all analyses.
The Harvard TH Chan School of Public Health Human Subjects Committee, the MUHAS Senate Research and Publications Committee, and Tanzania's National Institute for Medical Research granted institutional review board approval for the study. The Tanzania Food and Drugs Authority approved the use of the supplements. Study progress was monitored by a Data safety monitoring board annually.
Results
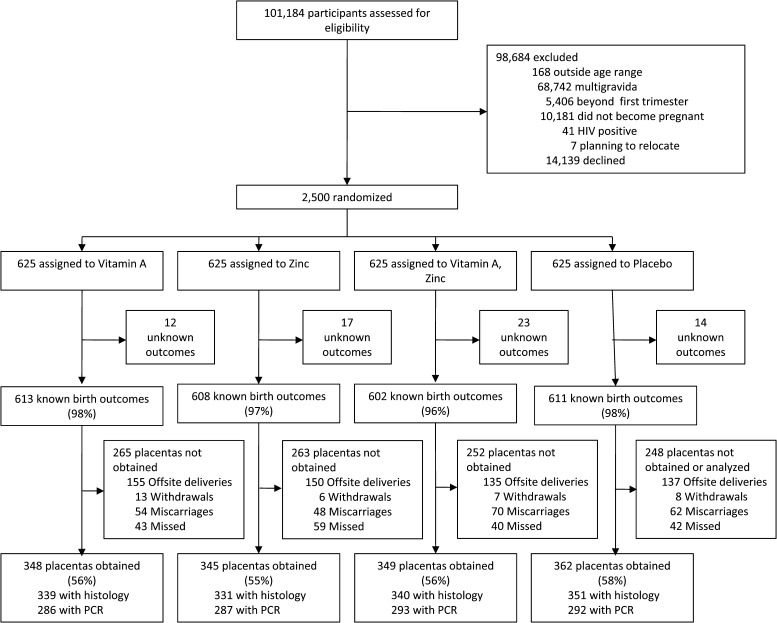
A total of 2,500 screened participants were enrolled in the trial. The trial profile is shown in Figure 1 . It was not possible to collect placentas from 875 participants for the following reasons: miscarriages (fetal loss before 28 weeks of gestation) (N = 234), delivery outside of Dar es Salaam or at a non-study hospital (N = 577), or withdrawal from the study (N = 34). Of the remaining 1,589 women, 1,404 placental samples were obtained (88%); histology results were available for 1,361 participants. PCR results were available for 1,158 participants, and 1,404 participants had either histology or PCR results available.
Figure 1.
Study enrollment, randomization, and pregnancy outcomes.
Baseline characteristics of the 1,404 participants with placental malaria results and the 1,096 participants without these results are shown in Table 1. A higher proportion of participants excluded from the placental malaria analyses due to missing data owned their housing compared with those included in the analysis (26% versus 21%). No other differences in baseline characteristics were apparent between those included in and excluded from the primary outcome analyses.
Table 1.
Baseline characteristics of participants included and excluded from the PM analyses at baseline by randomization arm
| Characteristic | Included in analysis of PM (N = 1,404) | Excluded from analysis of PM (N = 1,096) |
|---|---|---|
| Age (years) | 22.9 (4.4) | 22.5 (4.4) |
| Primigravida | 48% | 48% |
| Gestational age at randomization (weeks) | 10.0 (2.3) | 9.8 (2.5) |
| Education (years) | ||
| 0 to < 5 | 8% | 9% |
| 5 to < 8 | 64% | 62% |
| 8 to < 12 | 23% | 25% |
| ≥ 12 | 5% | 5% |
| Marital status | ||
| Married/cohabitating | 90% | 91% |
| Other | 10% | 9% |
| Employment status | ||
| Skilled | 6% | 5% |
| Unskilled or informal | 24% | 25% |
| Housewife/unemployed | 55% | 57% |
| Other | 15% | 13% |
| Housing type | ||
| Own | 21% | 26% |
| Rent | 72% | 66% |
| Other | 7% | 9% |
| Roof | ||
| Metal | 99% | 98% |
| Other | 1% | 2% |
| Floor | ||
| Concrete | 97% | 97% |
| Tile | 2% | 2% |
| Dirt or wood | 1% | 1% |
| Malaria interventions | ||
| Uses spray, coils, or bednet at night | 94% | 94% |
| Has bednet | 93% | 92% |
| Regularly uses bednet | 92% | 90% |
| Body mass index (kg/m2) | 23.3 (4.4) | 23.1 (4.3) |
| Body mass index category | ||
| < 18.5 | 11% | 10% |
| 18.5–24.9 | 60% | 63% |
| ≥ 25 | 29% | 27% |
| Baseline mid-upper arm circumference (mm) | 26.2 (3.5) | 26.3 (3.4) |
| Baseline hemoglobin (g/dL) | 11.5 (1.5) | 11.6 (1.5) |
PM = placental malaria.
Table 2 displays the baseline characteristics by treatment arm among the participants included in the primary outcome analyses. No differences in baseline characteristics were seen between those who did and did not receive vitamin A and those who did and did not receive zinc. Use of all malaria interventions exceeded 90% in all groups. The mean gestational age at randomization was 10 weeks and was comparable across treatment groups. The average compliance rate (number of tablets absent from returned regimen bottles divided by the number of days the participant had the bottle) was 84.5% (median 97.6%). Average compliance rate was similar between groups.
Table 2.
Baseline characteristics by treatment arm among the participants included in the primary outcome analyses (Mean SD or %)
| Characteristic | Vitamin A (N = 697) | No vitamin A (N = 707) | Zinc (N = 694) | No zinc (N = 710) |
|---|---|---|---|---|
| Age (years) | 23.0 (5.0) | 22.7 (3.7) | 23.0 (4.8) | 22.7 (4.0) |
| Primigravida | 46% | 49% | 48% | 47% |
| Gestational age at randomization (weeks) | 10.0 (2.4) | 10.0 (2.3) | 10.1 (2.4) | 10.0 (2.3) |
| Education (years) | ||||
| 0 to < 5 | 9% | 6% | 7% | 8% |
| 5 to < 8 | 63% | 66% | 65% | 63% |
| 8 to < 12 | 21% | 23% | 21% | 24% |
| ≥ 12 | 7% | 4% | 6% | 5% |
| Marital status | ||||
| Married/cohabitating | 89% | 90% | 91% | 89% |
| Other | 11% | 10% | 11% | 9% |
| Employment status | ||||
| Skilled | 6% | 5% | 6% | 5% |
| Unskilled or informal | 24% | 23% | 24% | 24% |
| Housewife/unemployed | 53% | 57% | 55% | 55% |
| Other | 17% | 14% | 15% | 16% |
| Housing type | ||||
| Own | 26% | 25% | 25% | 27% |
| Rent | 65% | 67% | 66% | 65% |
| Other | 9% | 8% | 9% | 8% |
| Roof | ||||
| Metal | 99% | 98% | 98% | 99% |
| Other | 1% | 2% | 1% | 1% |
| Floor | ||||
| Concrete | 97% | 97% | 97% | 98% |
| Tile | 1% | 2% | 2% | 1% |
| Dirt or wood | 1% | 1% | 1% | 1% |
| Malaria interventions | ||||
| Uses spray, coils, or bednet at night | 93% | 95% | 94% | 94% |
| Has bednet | 92% | 94% | 94% | 93% |
| Regularly uses bednet | 90% | 93% | 92% | 91% |
| Body mass index (kg/m2) | 23.3 (4.5) | 23.4 (4.3) | 23.3 (4.3) | 23.4 (4.5) |
| Body mass index category (kg/m2) | ||||
| 18.5 | 11% | 10% | 10% | 11% |
| 18.5–24.9 | 61% | 60% | 61% | 60% |
| ≥ 25 | 28% | 30% | 29% | 28% |
| Baseline mid-upper arm circumference (mm) | 26.1 (3.5) | 26.3 (3.6) | 26.3 (3.5) | 26.2 (3.5) |
| Baseline hemoglobin (g/dL) | 11.4 (1.4) | 11.5 (1.5) | 11.4 (1.5) | 11.5 (1.4) |
The prevalence of placental malaria detected through histopathology was 8.5% (115/1,361), whereas the prevalence detected by PCR was 14.8% (171/1,158). The Spearman correlation between the two diagnostic methods was 0.11. Those who received zinc had a lower risk of histopathology-positive placental malaria than those who did not receive zinc in unweighted models (risk ratio [RR] = 0.64, 95% confidence interval [CI] = 0.44, 0.91; Table 3); results from MSMs were virtually identical (RR = 0.62, 95% CI = 0.42, 0.89). However, vitamin A administration had no impact on histopathology-positive infection, and neither nutrient affected PCR-positive placental malaria. All infections detected by PCR and histopathology were P. falciparum.
Table 3.
Effect of vitamin A and zinc supplementation on placental malaria*
| Outcome | Vitamin A | Zinc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | N | No | N | RR (95% CI) | P | Yes | N | No | N | RR (95% CI) | P | |
| Unweighted model† | ||||||||||||
| Histopathology positive | 57 (8) | 679 | 58 (9) | 682 | 0.99 (0.70, 1.40) | 0.94 | 44 (7) | 671 | 71 (10) | 690 | 0.64 (0.44, 0.91) | 0.01 |
| PCR positive | 88 (15) | 579 | 83 (14) | 579 | 1.06 (0.80, 1.40) | 0.68 | 90 (16) | 580 | 81 (14) | 578 | 1.11 (0.84, 1.46) | 0.47 |
| Marginal structural models | ||||||||||||
| Histopathology positive | 1.03 (0.82, 1.29) | 0.62 (0.42, 0.89) | ||||||||||
| PCR positive | 1.00 (0.70, 1.45) | 0.89 (0.71, 1.12) | ||||||||||
CI = confidence interval; PCR = polymerase chain reaction; RR = risk ratio.
Mean (standard deviation) for continuous variables and N (%) for dichotomous variables.
Twenty-five participants had both histopathology- and PCR-positive infections.
Effects of vitamin A and zinc supplementation on secondary outcomes are presented in Table 4 . Although neither supplement affected birthweight among the 2,056 participants with birthweight data available, there was a nonsignificant tendency for higher placental weight who received vitamin A compared with those who did not (442 ± 119 g versus 430 ± 111 g; P = 0.08) and among those who received zinc than those who did not (443 ± 116 g versus 431 ± 114 g; P = 0.05) (Table 2). Those who received vitamin A had an increased risk of severe anemia (RR = 1.36, 95% CI = 1.05, 1.76) compared with those who did not. In addition, we observed a statistically nonsignificant trend toward a lower risk of very preterm birth among those who received vitamin A compared with those who did not (RR = 1.34, 95% CI = 0.95, 1.89). Neither vitamin A nor zinc supplementation was associated with fetal loss, perinatal death, infant mortality, low birthweight, SGA, or any other outcomes measured. No interaction between vitamin A and zinc was observed for any outcome (all P > 0.05). No safety concerns were identified for either intervention.
Table 4.
RR (95% CI) or mean difference (SD) for the effect of vitamin A and zinc supplementation on secondary outcomes
| Outcome | Vitamin A | Zinc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | N | No | N | RR (95% CI) | P | Yes | N | No | N | RR (95% CI) | P | |
| Maternal death | 4 (0) | 1,250 | 3 (0) | 1,250 | 1.33 (0.30, 5.95) | 0.71 | 4 (0) | 1,250 | 3 (0) | 1,250 | 1.33 (0.30, 5.95) | 0.71 |
| Fetal loss* | 158 (13.0) | 1,215 | 150 (12.3) | 1,219 | 1.06 (0.86, 1.30) | 0.60 | 160 (13.2) | 1,210 | 148 (12.0) | 1,224 | 1.09 (0.89, 1.35) | 0.40 |
| Perinatal death† | 73 (5.8) | 1,250 | 63 (5.0) | 1,250 | 1.16 (0.83, 1.61) | 0.38 | 66 (5.3) | 1,250 | 70 (5.6) | 1,250 | 0.94 (0.68, 1.31) | 0.72 |
| Infant mortality at 6 weeks‡ | 46 (4.5) | 1,024 | 36 (3.5) | 1,036 | 1.29 (0.84, 1.98) | 0.24 | 37 (3.7) | 1,014 | 45 (4.3) | 1,046 | 0.85 (0.55, 1.30) | 0.45 |
| Placental weight (g) | 442 (119) | 449 | 430 (111) | 466 | 0.08 | 443 (116) | 440 | 430 (114) | 475 | 0.05 | ||
| Birthweight (g) | 3,058 (546)* | 1,021 | 3,066 (535) | 1,035 | 0.93 | 3,075 (551)* | 1,010 | 3,049 (530) | 1,046 | 0.17 | ||
| < 2,500 | 80 (7.8) | 1,021 | 80 (7.7) | 1,035 | 1.01 (0.75, 1.37) | 0.93 | 75 (7.4) | 1,010 | 85 (8.1) | 1,046 | 0.91 (0.68, 1.23) | 0.55 |
| < 2,000 | 29 (2.8) | 1,021 | 23 (2.2) | 1,035 | 1.28 (0.74, 2.19) | 0.37 | 26 (2.6) | 1,010 | 26 (2.5) | 1,046 | 1.04 (0.61, 1.77) | 0.90 |
| Gestational age at delivery (weeks) | 38.6 (2.9) | 1,007 | 38.8 (2.7) | 1,028 | 0.52 | 38.6 (2.9) | 999 | 38.7 (2.7) | 1,036 | 0.83 | ||
| Preterm birth (weeks) | ||||||||||||
| < 37 | 243 (24.1) | 1,007 | 225 (21.9) | 1,028 | 1.10 (0.94, 1.29) | 0.23 | 236 (23.6) | 999 | 232 (22.4) | 1,036 | 1.05 (0.90, 1.24) | 0.51 |
| < 34 | 71 (7.0) | 1,007 | 54 (5.3) | 1,028 | 1.34 (0.95, 1.89) | 0.09 | 62 (6.2) | 999 | 63 (6.1) | 1,036 | 1.02 (0.73, 1.43) | 0.91 |
| SGA (Alexander)§ | 196 (19.2) | 1,021 | 179 (17.3) | 1,035 | 1.11 (0.93, 1.33) | 0.26 | 173 (17.1) | 1,010 | 202 (19.3) | 1,046 | 0.89 (0.74, 1.07) | 0.20 |
| SGA (INTERGROWTH-21st)¶ | 149 (15.9) | 937 | 142 (14.7) | 966 | 1.08 (0.88, 1.34) | 0.47 | 140 (15.0) | 929 | 151 (15.5) | 974 | 0.97 (0.79, 1.20) | 0.79 |
| Hemoglobin (g/dL) | 10.4 (2.3) | 662 | 10.7 (2.3) | 673 | 0.21 | 10.4 (2.4) | 664 | 10.7 (2.3) | 671 | 0.08 | ||
| Anemia∥ | 380 (57.4) | 662 | 385 (57.2) | 673 | 1.00 (0.91, 1.10) | 0.94 | 391 (58.9) | 664 | 374 (55.7) | 671 | 1.06 (0.96, 1.16) | 0.25 |
| Severe anemia** | 115 (17.4) | 662 | 86 (12.8) | 673 | 1.36 (1.05, 1.76) | 0.02 | 104 (15.7) | 664 | 97 (14.5) | 671 | 1.08 (0.83, 1.40) | 0.54 |
CI = confidence interval; RR = risk ratio; SD = standard deviation; SGA = small for gestational age. Mean (SD) presented for continuous variables and N (%) presented for dichotomous variables.
Stillbirth and miscarriage.
All deaths from 28 weeks' gestation to the first 7 days of life.
Among live births only.
SGA was defined as birthweight below the 10th percentile for gestational age using the Alexander standard.
SGA was defined as birthweight below the 10th percentile for gestational age using the INTERGROWTH-21st standard, restricting to births 33–42 weeks' gestation.
Defined as hemoglobin < 11.0 g/dL.
Defined as hemoglobin < 8.5 g/dL.
Discussion
This study is the first to examine the impact of vitamin A and zinc supplementation starting in early pregnancy on placental malaria. We observed that supplementation with 25 mg zinc per day from the first trimester until delivery was associated with a 36% (95% CI = 9–56%) reduced risk of histopathology-positive placental infection, but not PCR-positive infection. Vitamin A supplementation had no impact on placental malaria, but was associated with an increased risk for severe anemia.
Some caution is warranted in the interpretation of these observations. Because placental malaria results were unavailable from a large proportion (46%) of participants, the possibility of selection bias stemming from potential nonrandomness of treatment allocation among those with results available must be considered. Although Table 1 indicates that those included in and excluded from the placental malaria analyses had comparable covariate profiles, we attempted to correct for this potential bias through the use of MSMs. This technique uses weights to create a statistical pseudopopulation in which the distribution of baseline characteristics across treatment assignment reflects the distribution that would have been observed had no attrition occurred. The similarity of the results between the weighted and unweighted models suggests that selection bias did not likely affect our findings. At the same time, this proportion of subjects lost to follow-up was higher than assumed in the power calculations and may have reduced the precision of our estimates.
Another potential source of bias in this study is the use of date of LMP to determine gestational age, which was necessary due to the unavailability of ultrasound in this setting. Inaccurate recollection of this date may have introduced some misclassification of preterm birth and SGA. Because LMP dates were ascertained before these outcomes occurred, this misclassification is likely nondifferential and would bias our results toward the null. In a comparable Nigerian population,28 91% of participants' estimated gestational ages based on their date of LMP were within 2 weeks of their ultrasound-confirmed gestational ages. However, we expect that recall accuracy may be slightly higher in our study, because women were enrolled earlier in pregnancy. Our study has several strengths, including its factorial design, long duration of supplementation, and excellent rate of compliance and overall follow-up.
We observed a lack of agreement between histopathology and PCR-based diagnoses. Because the sensitivity and specificity differ between these two diagnostic methods, some disagreement is expected. A study performed among 272 Mozambican pregnant women found that placental histology had a sensitivity of 41.8% and a specificity of 97.1% when compared with PCR.29 Consistent with their findings, the prevalence of placental malaria was higher when determined by PCR, but we observed a much larger proportion of histology-positive and PCR-negative diagnoses. Placental histology may miss positive cases or lead to false-positive diagnoses due to the difficulty in distinguishing hemozoin and other artefactual pigment, but it is nonetheless still considered the “gold standard” for accuracy. It is unclear why zinc supplementation was associated with a reduction in malaria detected through histopathology and not PCR, and the discrepancy suggests additional caution in interpreting our results.
Previous trials to evaluating the effect of zinc supplementation on malaria outcomes among pregnant women and children have provided similarly mixed results. A trial from Ghana involving 364 pregnant women reported a small absolute reduction (3.2%) in malaria parasitemia among those who received iron and zinc supplementation compared with those who received iron supplementation alone.18 In Papua New Guinea, zinc supplementation was associated with a 38% (95% CI = 3–60; P = 0.037) reduction in P. falciparum health center–based episodes in a trial conducted among 274 preschool children.13 Combined supplementation with zinc and vitamin A reduced malaria incidence by 30% in children in Burkina Faso, but the design of this study precludes attribution of the effect to either nutrient alone.15 Other studies conducted among children have found no evidence that zinc supplementation prevents malaria.14,16
Zinc deficiency is common in sub-Saharan Africa, and pregnant women may be especially vulnerable to this condition. Diets in some African settings are predominantly cereal based and rich in phytates that impair zinc absorption; additionally there is often poor intake of flesh food, a good source of available zinc.30 Using national data on stunting rates and the adequacy of zinc in the national food supply, the International Zinc Nutrition Consultative Group estimates that 44% of Tanzanians are at risk of inadequate zinc intake.31 Zinc deficiency is detrimental to several aspects of adaptive and innate immunity, as reviewed by Shankar and Prasad.32 While our results require replication, it is plausible that improving zinc status through supplementation among pregnant women could lead to increased immunity against histopathologically detectable infection. Though we did not observe a corresponding decrease in adverse birth outcomes among zinc-supplemented participants, other factors such as poor maternal nutritional status and multiple micronutrient deficiencies may have also contributed to the development of these outcomes in this resource-constrained setting.33
Our observation that vitamin A was associated with increased risk of severe anemia is unexpected. A recent meta-analysis shows that vitamin A supplementation in pregnancy improves hemoglobin levels and reduces anemia risk; however, vitamin A supplementation was initiated later in pregnancy than in our study.20 While it is possible that our finding may represent an adverse effect of prolonged vitamin A exposure, little observational or experimental evidence exists to support this, and our finding may therefore be attributable to chance. We saw no difference in overall anemia risk associated with vitamin A supplementation.
We observed no other differences in adverse birth outcomes associated with either supplement. These findings largely confirm those of meta-analyses19,20 and are notable for showing that these supplements may provide no benefit to pregnancy maintenance or fetal growth even when initiated during the first trimester. While our findings do not support the 14% reduction in preterm birth associated with zinc reported in a Cochrane review that pooled 16 supplementation trials involving 7,637 women,19 a much larger trial would be necessary to detect a significant effect of this modest size.
Our results may be generalizable to settings where malaria is well controlled as in Dar es Salaam where preventive interventions include high bednet coverage, systematic spraying of insecticides, and provision of intermittent preventive therapy to pregnant women as standard of care.34 For this reason, trials in other geographic locations are needed. In conclusion, our trial has provided modest support of a potential benefit of zinc supplementation to prevent histopathologically detectable placental malaria. If this finding is confirmed in additional studies, zinc supplementation could be considered as a low-cost addition to malaria control measures aimed at reducing the risk of malaria in pregnant women.
ACKNOWLEDGMENTS
We thank the study participants and the field teams, including study coordinators, doctors, nurses, midwives, supervisors, and the laboratory, administrative, and data staff at MUHAS and the clinic sites for their contributions to the study. Particular appreciation to the dedication offered by the field team including Vera Juma (deceased), Jeremy Kane, Juliana Mghamba, Fee Msafiri, Mwanaidi Said, and Kristina Lugangira. We also thank Paul Ng'walali and Amos Mwakigonja at MUHAS for their technical assistance in placental histopathology; Jaume Ordi and Atis Muehlenbachs for external review of the placental histopathology; and Michael Waisberg for malarial PCR on representative cases, as well as Manoj Duraisingh and Katy Shaw-Saliba at the Harvard TH Chan School of Public Health, and Ambroise Ahouidi at Le Dantec Hospital, Senegal, for their expertise in tissue homogenization, DNA extraction, and qPCR methods for diagnosing placental malaria. Finally, we thank DSMB members Jeffrey Griffiths, Mohamed Bakari, Henrik Friis, David Wypij, and Hassan Mshinda.
Footnotes
Financial support: This study was supported by grants from the National Institute of Child Health and Human Development (NICHD R01 HD057941-01 and K24 HD 058795 [Christopher Duggan]).
Authors' addresses: Anne Marie Darling, Analee J. Etheredge, Nilupa S. Gunaratna, Ajibola Ibraheem Abioye, and Wafaie W. Fawzi, Department of Global Health and Population, Harvard TH Chan School of Public Health, Boston, MA, E-mails: adarling@hsph.harvard.edu, aetheredge@gmail.com, ngunarat@hsph.harvard.edu, drabioye@gmail.com, and mina@hsph.harvard.edu. Ferdinand M. Mugusi, Department of Internal Medicine, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania, E-mail: fm.mugusi@gmail.com. Said Aboud, Department of Microbiology and Immunology, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania, E-mail: aboudsaid@yahoo.com. Christopher Duggan, Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital, Harvard Medical School, Boston, MA, E-mail: christopher.duggan@childrens.harvard.edu. Robert Mongi, Department of Parasitology/Medical Entomology, School of Public Health and Social Sciences, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania, E-mail: rmongi@hotmail.com. Donna Spiegelman, Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, E-mail: stdls@hsph.harvard.edu. Drucilla Roberts, Department of Pathology, Massachusetts General Hospital, Boston, MA, E-mail: djroberts@mgh.harvard.edu. Davidson H. Hamer, Department of Global Health, Boston University School of Public Health, Boston, MA, E-mail: dhamer@bu.edu. Kevin C. Kain, Department of Medicine, University of Toronto, Toronto, Canada, E-mail: kevin.kain@uhn.ca.
References
- 1.World Health Organization World Malaria Report 2014. 2014. http://www.who.int/malaria/publications/world_malaria_report_2014/report/en/ Available at. Accessed July 18, 2016.
- 2.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 3.Menéndez C, Ferenchick E, Roman E, Bardají A, Mangiaterra V. Malaria in pregnancy: challenges for control and the need for urgent action. Lancet Glob Health. 2015;3:e433–e434. doi: 10.1016/S2214-109X(15)00041-8. [DOI] [PubMed] [Google Scholar]
- 4.Shankar AH. Nutritional modulation of malaria morbidity and mortality. J Infect Dis. 2000;1:S37–S53. doi: 10.1086/315906. [DOI] [PubMed] [Google Scholar]
- 5.Davis TM, Skinner-Adams TS, Beilby J. In vitro growth inhibition of Plasmodium falciparum by retinol at concentrations present in normal human serum. Acta Trop. 1998;69:111–119. doi: 10.1016/s0001-706x(97)00129-0. [DOI] [PubMed] [Google Scholar]
- 6.Hamzah J, Davis TM, Skinner-Adams TS, Beilby J. Characterization of the effect of retinol on Plasmodium falciparum in vitro. Exp Parasitol. 2004;107:136–144. doi: 10.1016/j.exppara.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Stürchler D, Tanner M, Hanck A, Betschart B, Gautschi K, Weiss N, Burnier E, Del Guidice G, Degremont A. A longitudinal study on relations of retinol with parasitic infections and the immune response in children of Kikwawila village, Tanzania. Acta Trop. 1987;44:213–227. [PubMed] [Google Scholar]
- 8.Das BS, Thurnham DI, Das DB. Plasma alpha-tocopherol, retinol, and carotenoids in children with falciparum malaria. Am J Clin Nutr. 1996;64:94–100. doi: 10.1093/ajcn/64.1.94. [DOI] [PubMed] [Google Scholar]
- 9.Chevion M, Chuang L, Golenser J. Effects of zinc-desferrioxamine on Plasmodium falciparum in culture. Antimicrob Agents Chemother. 1995;39:1902–1905. doi: 10.1128/aac.39.8.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Tielsch JM, Alpers MP, West KP., Jr Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomized trial. Lancet. 1999;354:203–209. doi: 10.1016/S0140-6736(98)08293-2. [DOI] [PubMed] [Google Scholar]
- 11.Nankabirwa V, Tylleskar T, Nankunda J, Engebretsen IM, Sommerfelt H, Tumwine JK. PROMISE EBF Research Consortium Malaria parasitaemia among infants and its association with breastfeeding peer counselling and vitamin A supplementation: a secondary analysis of a cluster randomized trial. PLoS One. 2011;6:e21862. doi: 10.1371/journal.pone.0021862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owusu-Agyei S, Newton S, Mahama E. Impact of vitamin A with zinc supplementation on malaria morbidity in Ghana. Nutr J. 2013;12:131. doi: 10.1186/1475-2891-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankar AH, Genton B, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Bannon D, Tielsch JM, West KP, Jr, Alpers MP. The influence of zinc supplementation on morbidity due to Plasmodium falciparum: a randomized trial in preschool children in Papua New Guinea. Am J Trop Med Hyg. 2000;62:663–669. doi: 10.4269/ajtmh.2000.62.663. [DOI] [PubMed] [Google Scholar]
- 14.Müller O, Becher H, van Zweeden AB, Ye Y, Diallo DA, Konate AT, Gbangou A, Kouyate B, Garenne M. Effect of zinc supplementation on malaria and other causes of morbidity in west African children: randomized double blind placebo controlled trial. BMJ. 2001;322:1567. doi: 10.1136/bmj.322.7302.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeba AN, Sorgho H, Rouamba N, Zongo I, Rouamba J, Guiguemdé RT, Hamer DH, Mokhtar N, Ouedraogo JB. Major reduction of malaria morbidity with combined vitamin A and zinc supplementation in young children in Burkina Faso: a randomized double blind trial. Nutr J. 2008;7:7. doi: 10.1186/1475-2891-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veenemans J, Milligan P, Prentice AM, Schouten LR, Inja N, van der Heijden AC, de Boer LC, Jansen EJ, Koopmans AE, Enthoven WT, Kraaijenhagen RJ, Demir AY, Uges DR, Mbugi EV, Savelkoul HF, Verhoef H. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med. 2011;8:e1001125. doi: 10.1371/journal.pmed.1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox SE, Staalsoe T, Arthur P, Bulmer JN, Tagbor H, Hviid L, Frost C, Riley EM, Kirkwood BR. Maternal vitamin A supplementation and immunity to malaria in pregnancy in Ghanaian primigravids. Trop Med Int Health. 2005;10:1286–1297. doi: 10.1111/j.1365-3156.2005.01515.x. [DOI] [PubMed] [Google Scholar]
- 18.Saaka M, Oosthuizen J, Beatty S. Effect of joint iron and zinc supplementation on malarial infection and anaemia. East Afr J Public Health. 2009;6:55–62. doi: 10.4314/eajph.v6i1.45748. [DOI] [PubMed] [Google Scholar]
- 19.Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015;2:CD000230. doi: 10.1002/14651858.CD000230.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne-Lyman AL, Fawzi W. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26:36–54. doi: 10.1111/j.1365-3016.2012.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The United Republic of Tanzania Ministry of Health and Social Welfare Standard Treatment Guidelines (STG) and the National Essential Medicines List (NEMLIT) for Mainland Tanzania. 2007. http://www.who.int/medicines/areas/coordination/tanzania_stgs_eml.pdf Available at. Accessed July 18, 2016.
- 22.Rosanas-Urgell A, Mueller D, Betuela I, Barnadas C, Iga J, Zimmerman PA, del Portillo HA, Siba P, Mueller I, Felger I. Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malar J. 2010;9:361. doi: 10.1186/1475-2875-9-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamau E, Tolbert LS, Kortepeter L, Pratt M, Nyakoe N, Muringo L, Oguto B, Waitumbi JN, Ockenhouse CF. Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of Plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J Clin Microbiol. 2011;49:2946–2953. doi: 10.1128/JCM.00276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 25.Villar J, Cheikh Ismail L, Victoria CG, Ohuma EO, Bertino E, Altman DG, Lambert A, Papageorghiou AT, Carvalho M, Jaffer YA, Gravett MG, Purwar M, Frederick IO, Noble AJ, Pang R, Barros FC, Chumlea C, Bhutta ZA, Kennedy SH. International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 26.Robins JM, Hernán MÁ, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 28.Ogbe AE, Ekwempu CC, Musa J, Anzaku AS. A comparison of the accuracy of the use of last menstrual period and symphisio-fundal height for gestational age determination among Nigerian women. Int J Trop Dis Health. 2015;7:32–39. [Google Scholar]
- 29.Mayor A, Moro L, Aguilar R, Bardají A, Cisteró P, Serra-Casas E, Sigaúque B, Alonso PL, Ordi J, Menéndez C. How hidden can malaria be in pregnant women? Diagnosis by microscopy, placental histology, polymerase chain reaction and detection of histidine-rich protein 2 in plasma. Clin Infect Dis. 2012;54:1561–1568. doi: 10.1093/cid/cis236. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson EL, Gadowsky SL, Huddle JM, Cullinan TR, Lehrfeld J, Gibson RS. An interactive 24-h recall technique for assessing the adequacy of trace mineral intakes of rural Malawian women; its advantages and limitations. Eur J Clin Nutr. 1995;49:565–578. [PubMed] [Google Scholar]
- 31.Rwebembera AA, Munubhi EK, Manji KP, Mpembeni R, Philip J. Relationship between infant birth weight ≤2000 g and maternal zinc levels at Muhimbili National Hospital, Dar es Salaam, Tanzania. J Trop Pediatr. 2006;52:118–125. doi: 10.1093/tropej/fmi077. [DOI] [PubMed] [Google Scholar]
- 32.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 33.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R, Maternal and Child Nutrition Study Group Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 34.Roll Back Malaria Partnership Focus on Mainland Tanzania. 2012. http://www.rollbackmalaria.org/microsites/ProgressImpactSeries/docs/report10-en.pdf Available at. Accessed April 15, 2012.