Abstract
Schistosomiasis is a parasitic worm infection that affects over 260 million individuals worldwide. Women with schistosome infections have been demonstrated to have a 4-fold increase in the odds of human immunodeficiency virus (HIV) infection compared with women without schistosome infections. A relationship between schistosome and HIV infections has not been clearly defined in men. Among 674 men aged 18–50 years living in rural Tanzania, we identified 429 (63.6%) who had a schistosome infection as defined by serum positivity for schistosome circulating anodic antigen, visualization of parasite eggs in urine or stool, or both. HIV infection was identified in 38 (5.6%). The odds of HIV infection was 1.3 [95% confidence interval = 0.6–2.5] (P = 0.53) among men with any schistosome infection (Schistosoma haematobium or Schistosoma mansoni), and it was 1.4 [0.6–3.3] (P = 0.43) among men with S. haematobium infection. Men with S. haematobium infection were significantly more likely to report the symptom of hemospermia than men without S. haematobium infection. We conclude that schistosome infections appear to have little to no association with HIV infection in men.
Introduction
Schistosomiasis is a parasitic infection that affects over 260 million people worldwide, with approximately 85% of cases in Africa.1 We have found an increased likelihood of being human immunodeficiency virus (HIV) infected among women in Tanzania with Schistosoma haematobium infection as compared with women without schistosomiasis.2 Other groups have reported similar results in women, and suggested that parasite-inducted genital tract lesions may permit HIV viral entry.3 Our additional observation that women with Schistosoma mansoni infection also had an increased prevalence of HIV infection4 led us to postulate that chronic systemic inflammation caused by schistosome infection may be an additional mechanism of increased HIV risk, since S. mansoni primarily affects the gastrointestinal tract. Therefore, increased susceptibility to HIV infection in individuals with schistosomiasis may be due to generalized systemic immune alterations, and for this reason may affect men in addition to women.
No epidemiologic studies of HIV and schistosomiasis to date have focused solely on men. Several population-based studies in Tanzania and Uganda, which included both men and women, did not find an increased prevalence of HIV among those with S. mansoni infection.5,6 Neither of these studies stratified data by gender, and both relied on schistosome egg excretion as the primary endpoint, which is less sensitive than schistosome antigen testing7 and may be diminished in the setting of HIV infection.8,9 Of note, in a subset of the Ugandan individuals who underwent urine schistosome antigen testing, the odds ratio (OR) for HIV infection was 1.5 with a P value of 0.19, suggesting that the study may have lacked sufficient power to detect a smaller increased risk.6 One other study that did use schistosome antigen testing in Uganda also did not report effects of S. mansoni infection on men and women separately.10
Therefore, we conducted a large epidemiologic study to determine the relationship between S. haematobium, S. mansoni, and HIV infections among men in regions of Tanzania where schistosome infection is endemic. We predicted that both genders would experience schistosome-induced systemic immunomodulation that would increase the odds of HIV infection. We postulated that the reported ORs of 3–4 in women2–4 were higher than would be observed in men, due to schistosome-induced epithelial breaches in the female genital tract that may facilitate HIV viral entry following sexual exposure, for which there would not be a comparable effect in men. We therefore hypothesized that the odds of HIV infection in schistosome-infected men would be increased 2-fold compared with men without schistosome infection, and we conducted interviews and additional testing to control for other factors known to be associated with HIV infection.
Materials and Methods
Study setting and participant recruitment.
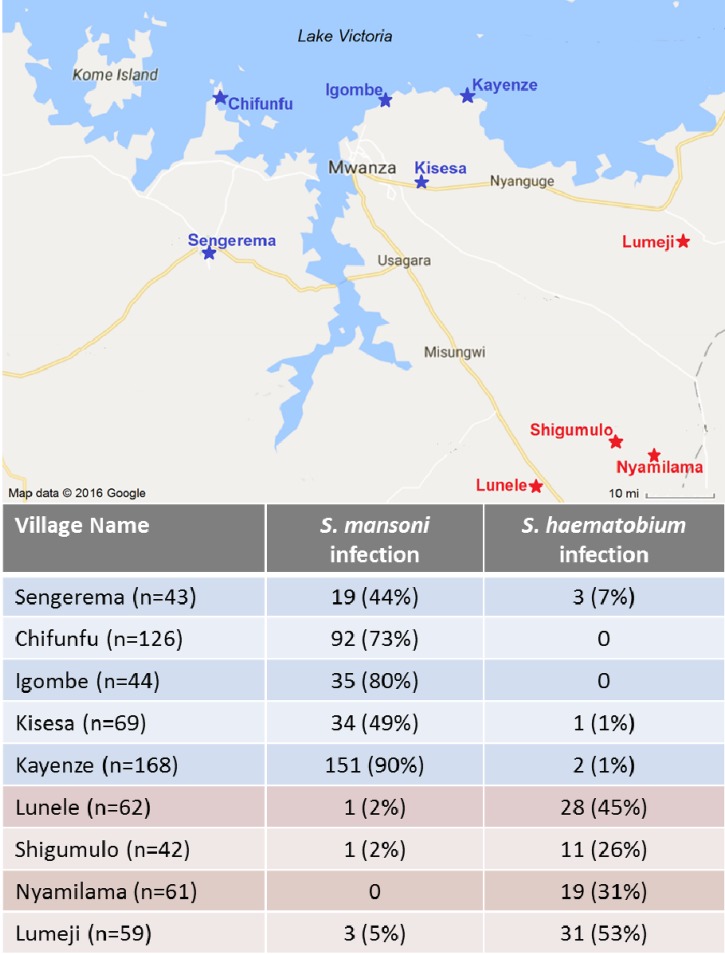
This study was conducted in nine rural villages in the Mwanza region of Tanzania. Five of these were lakeside villages near Lake Victoria where S. mansoni is highly endemic, and four were inland villages where S. haematobium is highly endemic (Figure 1 ).2,4,5
Figure 1.
Map of study sites and prevalence of schistosome infections in the Lake Victoria region of northwest Tanzania. Villages noted in blue are endemic for Schistosoma mansoni, and villages shown in red are endemic for Schistosoma haematobium.
The study team visited each of the health posts for 1–3 days between April 2014 and February 2016. We recruited a community-based sample of healthy outpatient men aged 18–50 years in each study villages.
Data collection.
Each man provided blood, urine, and a single stool sample and completed an oral questionnaire. Men who reported current genital symptoms also underwent genital examination by a physician. Ten milliliters of urine were filtered and samples were read on-site by a trained parasitologist. Five Kato Katz slides were prepared from each stool sample using 41.7 mg of stool per slide, which has been demonstrated to have a sensitivity for diagnosis of S. mansoni that is equivalent to collecting stool on three separate days.11 Stool slides were read by trained parasitologists at the National Institute for Medical Research (NIMR) in Mwanza, Tanzania. All study participants living in these highly endemic areas were treated with praziquantel on the day of study enrollment, in accordance with World Health Organization (WHO) treatment recommendations.12
Interview and examination.
Men participated in a 20-minute structured interview that included demographic information, sexual history, and urogenital symptoms. The interview was administered in Kiswahili in a private setting by a male member of the study team. Men provided information about past and current genital symptoms, sexual partners, and sexual behavior. Those with current genital symptoms underwent examination, and were provided with treatment of themselves and their partners in accordance with the Tanzanian national guidelines.13
Syphilis.
Venous blood was collected and tested for syphilis using a point-of-care Treponema pallidum particle agglutination assay (Crystal TP; Span Diagnostics Ltd., Surat, India). Men who tested positive for syphilis were asked to return to the study site with all of their sexual partners, and all were treated on-site with benzathine penicillin free of charge.
Human immunodeficiency virus.
Men were offered on-site voluntary HIV counseling and testing in Kiswahili by a trained nurse counselor. Rapid tests (SD Bioline; Standard Diagnostics, Inc., Kyonggi-do, South Korea) were used with confirmatory testing for positive samples (Unigold; Trinity Biotech, Bray, Ireland) as per the national testing algorithm. Patients received their results and posttest counseling on the same day. Men who were diagnosed with HIV were referred to the local HIV clinic for free care and treatment.
Laboratory testing.
Schistosome circulating anodic antigen (CAA) is a glycosaminoglycan-like carbohydrate that is produced by gut epithelial cells of schistosome worms and secreted into the host bloodstream during active infection.14 The CAA test does not distinguish S. haematobium from S. mansoni infection. The test usually becomes negative within 1 week of successful anti-schistosomal therapy.15,16
CAA testing was performed at the NIMR laboratory in Mwanza using the upconverting phosphor (UCP) technology lateral flow assay as previously described.17,18 We used the strategy recommended by the WHO/the Special Programme for Research and Training in Tropical Diseases to define a composite reference standard for schistosome infection as the presence of schistosome eggs visualized microscopically and/or a serum CAA value ≥ 30 pg/mL.17,19 We further defined S. mansoni infection as any of the following: 1) S. mansoni ova in stool and/or 2) CAA ≥ 30 pg/mL in an S. mansoni-endemic region in an individual with no S. haematobium ova in urine. We defined S. haematobium infection as any of the following: 1) S. haematobium ova in urine and/or 2) CAA ≥ 30 pg/mL in an S. haematobium-endemic region in an individual with no S. mansoni ova in stool.
Statistical considerations.
We predicted that the prevalence of schistosome infection would be 72% among those with HIV infection, and that the prevalence of schistosome infection would be 60% in those without HIV infection. We therefore calculated that enrolling 670 men would provide 90% power to detect an OR of 2 for HIV infection in those with versus without schistosome infection.
Data were entered into Microsoft Excel (Microsoft Corp., Redmond, WA) and analyzed using Stata/IC version 13 (College Station, TX). Continuous variables were summarized by median and interquartile range (IQR), and categorical variables were summarized by frequency and percentage. For factors associated with HIV infection, we performed univariable followed by multivariable logistic regression to examine factors associated with HIV with a P value of < 0.05. We used backward elimination, sequentially removing the least-significant factor one by one until all remaining factors were significant, to arrive at the final multivariable model. Associations between factors and the endpoint were summarized using ORs with 95% confidence intervals (CIs) and associated P values.
In cases in which an outcome yielded a value less than 5 on the χ2 analysis, we performed Fisher's exact test to determine the strength of an association. We then used Firth logistic regression with backward elimination to construct a multivariable model of factors associated with S. haematobium or S. mansoni infection due to multiple significant factors with a small number of outcomes.
Ethical considerations.
The study was explained to men in a large group and subsequently one on one by a trained study team member fluent in the local language. To participate in the study, men were asked to provide written informed consent or place their mark on the consent form. At the local level, permission was obtained from the District Medical Officers and clinicians stationed at participating dispensaries and health centers. Ethical approvals were granted by the research ethics committee at Bugando Medical Center, the Medical Research Coordinating Committee of NIMR in Tanzania, and the Institutional Review Board at Weill Cornell Medical College.
Results
Study population.
Between April 2014 and February 2016, we invited a total of 702 men aged 18–50 years from nine different villages to participate in this study. Of these, 28 men did not consent to voluntary counseling and testing for HIV. This left a total of 674 men who completed all study procedures and were included in the analysis.
Baseline characteristics of the population are shown in Table 1. The median age was 34 years [interquartile range = 25–42]. Nearly half of the men (328, 48.7%) had had more than one sexual partner in the past 6 months, and the majority of men reported that their sexual partners were typically more than 5 years younger. One-fourth of men (167, 24.8%) had experienced genital symptoms within the past year. Nearly two-thirds of the men (418, 62.1%) had been previously tested for HIV infection, whereas 47.6% (320) had previously received treatment of schistosome infection.
Table 1.
Demographic and behavioral characteristics of 674 rural adult men screened for schistosomiasis and sexually transmitted infections in Tanzania
| Characteristic | Number (%) or median [IQR] |
|---|---|
| Age in years | 34 [25–42] |
| Residence in village endemic for Schistosoma haematobium | 224 (33.2) |
| Lumeji | 59 (8.8) |
| Lunele | 62 (9.2) |
| Shigumulo | 42 (6.2) |
| Nyamilama | 61 (9.1) |
| Residence in village endemic for S. mansoni | 450 (66.8) |
| Chifunfu | 126 (18.7) |
| Igombe | 44 (6.5) |
| Kayenze | 168 (24.9) |
| Kisesa | 69 (10.2) |
| Sengerema | 43 (6.4) |
| Years attended school | |
| 0–2 years | 66 (9.8) |
| 3–5 years | 53 (7.9) |
| 6–8 years | 402 (59.6) |
| > 8 years | 150 (22.3) |
| Number of people living in household | 6 [4–9] |
| Failed to eat an afternoon or evening meal in past month due to food shortage | 147 (22.4) |
| Prior schistosomiasis treatment | |
| Never | 354 (52.5) |
| Within the past year | 38 (5.6) |
| 1 to < 3 years ago | 94 (13.9) |
| 3 to < 5 years ago | 42 (6.2) |
| 5 to < 10 years ago | 62 (9.2) |
| More than 10 years ago | 84 (12.5) |
| Ever been previously tested for HIV | 418 (62.1) |
| Reports being circumcised | 471 (70.3) |
| Age in years at first sexual encounter | 18 [16–20] |
| Number of children | 3 [1–6] |
| Number of sexual partners in the past 6 months | |
| 0–1 | 329 (48.8) |
| 2 | 160 (23.7) |
| 3–4 | 119 (17.7) |
| 5 or more | 49 (7.2) |
| Declined to answer | 17 (2.5) |
| Typical sexual partner ≥ 5 years younger | 311 (51.5) |
| Ever given money/gifts to obtain sex (outside of marriage) | 540 (81.2) |
| Used a condom during most recent sex | 157 (23.8) |
| Ever treated for sexually transmitted infection | 225 (33.6) |
| Genital symptoms in the past year | 167 (24.8) |
| Penile discharge | 64 (9.5) |
| Dyspareunia | 106 (15.7) |
| Hemospermia | 14 (2.1) |
| Painful genital ulcers | 46 (6.8) |
| Painless genital ulcers | 27 (4.0) |
HIV = human immunodeficiency virus; IQR = interquartile range.
Non-missing data were included in all calculations.
Clinical outcomes.
We documented HIV infection in 38/674 men (5.6%) and syphilis in 45 (6.7%, Table 2). Just under two-thirds of the total population (429, 63.6%) had schistosome infections, with the percentage approaching three-fourths among adults living in S. mansoni-endemic villages. Schistosoma mansoni and S. haematobium infections were sharply geographically demarcated in seven of the nine villages, with < 3% of the population in inland S. haematobium-endemic villages having S. mansoni infection, and vice versa (Figure 1).
Table 2.
Clinical infections in 674 adult men in rural Tanzania
| Infection | Schistosoma mansoni-endemic village (N = 450) | Schistosoma haematobium-endemic village (N = 224) |
|---|---|---|
| HIV | 24 (5.3) | 14 (6.3) |
| Syphilis | 29 (6.4) | 16 (7.1) |
| Any schistosome infection (CAA positive or ova positive) | 335 (74.4) | 94 (42.0) |
| S. mansoni infection (by stool ova or CAA with negative urine in endemic region) | 331 (73.6) | 5 (2.2) |
| S. haematobium infection (by urine ova or CAA with negative stool in endemic region) | 6 (1.3)* | 89 (39.7) |
| S. mansoni ova positive | 227 (50.4) | 5 (2.2) |
| S. haematobium ova positive | 6 (1.3) | 31 (13.8) |
CAA = circulating anodic antigen.
Two individuals living in an S. mansoni-endemic village had both S. mansoni and S. haematobium infection confirmed by ova.
Association of HIV with demographic and clinical factors.
On univariable analysis, a number of factors were significantly associated with HIV infection, including fewer years of education, fewer people living in one's household, not being circumcised, more sexual partners in the past 6 months, having sexual partners more than 5 years younger than oneself, having been ever treated for a sexually transmitted infection, genital symptoms (dyspareunia, hemospermia, and painful genital ulcers), and having a positive syphilis test (Table 3s ). Factors that remained significant in the multivariable model were fewer years of education (OR per each additional year in school = 0.86 [0.77–0.96], P = 0.005), more sexual partners in the past 6 months (OR = 1.2 [1.1–1.3] per additional partner, P = 0.001), and dyspareunia (OR = 2.7 [1.3–5.7], P = 0.009). Schistosome infection, neither when analyzed as “any schistosome infection” nor when analyzed by the individual species, was not significantly associated with HIV infection in these men.
Table 3.
Factors associated with HIV infection in rural Tanzanian men
| Factor | HIV infected (N = 38) | HIV uninfected (N = 636) | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|---|---|
| Number (%) or median [IQR] | Number (%) or median [IQR] | Odds ratio [95% CI] | P value | Odds ratio [95% CI] | P value | |
| Age in years | 34 [30–40] | 34 [25–42] | 1.01 [0.98–1.04] | 0.60 | ||
| Residence in village endemic for Schistosoma mansoni | 24 (63.2) | 426 (67.0) | 0.85 [0.43–1.67] | 0.63 | ||
| Years of school completed | 7 [3–7] | 7 [7–7] | 0.84 [0.76–0.93] | 0.001 | 0.86 [0.77–0.96] | 0.005 |
| Number of people living in household | 5 [3–6] | 6 [4–9] | 0.88 [0.79–0.98] | 0.017 | ||
| Food shortage | 8 (21.1) | 139 (22.5) | 0.92 [0.41–2.05] | 0.84 | ||
| Circumcised | 21 (55.3) | 450 (71.2) | 0.50 [0.26–0.97] | 0.040 | ||
| Age in years at first sex | 18 [16–19] | 18 [16–20] | 1.02 [0.93–1.12] | 0.73 | ||
| Children have more than one mother | 13 (34.2) | 163 (25.6) | 1.5 [0.8–3.0] | 0.25 | ||
| Number of sexual partners in the past 6 months | 3 [1–4] | 1 [1–2] | 1.2 [1.1–1.3] | 0.001 | 1.13 [1.05–1.23] | 0.001 |
| Typical sex partners more than 5 years younger | 25 (71.4) | 286 (50.3) | 2.5 [1.2–5.2] | 0.018 | ||
| Ever given money/gifts for sex (outside of marriage) | 34 (91.9) | 506 (80.6) | –* | 0.13 | ||
| Used a condom during most recent sex | 12 (31.6) | 145 (23.4) | 1.5 [0.7–3.1] | 0.25 | ||
| Ever treated for sexually transmitted infection | 19 (50.0) | 206 (32.6) | 2.1 [1.1–4.0] | 0.030 | ||
| Genital symptoms in the past year | ||||||
| Penile discharge | 5 (13.2) | 59 (9.3) | –* | 0.39 | ||
| Dyspareunia | 12 (31.6) | 94 (14.8) | 2.7 [1.3–5.5] | 0.008 | 2.7 [1.3–5.7] | 0.009 |
| Hemospermia | 3 (7.9) | 11 (1.7) | –* | 0.039 | ||
| Painful genital ulcers | 7 (18.4) | 39 (6.1) | 3.5 [1.4–8.3] | 0.006 | ||
| Painless genital ulcers | 3 (8.1) | 24 (3.8) | –* | 0.18 | ||
| Syphilis | 6 (15.8) | 39 (6.1) | 2.9 [1.1–7.3] | 0.026 | ||
| Any schistosome infection (CAA positive or ova positive) | 26 (68.4) | 403 (63.4) | 1.3 [0.6–2.5] | 0.53 | ||
| S. mansoni infection (by ova or CAA in endemic region) | 19 (50.0) | 317 (49.8) | 1.0 [0.5–1.9] | 0.99 | ||
| Schistosoma haematobium infection (by ova or CAA in endemic region) | 7 (18.4) | 88 (13.8) | 1.4 [0.6–3.3] | 0.43 | ||
| S. mansoni ova positive | 11 (29.0) | 221 (34.8) | 0.8 [0.4–1.6] | 0.47 | ||
| S. haematobium ova positive | 2 (5.3) | 35 (5.5) | –* | 1.0 | ||
CAA = circulating anodic antigen; CI = confidence interval; HIV = human immunodeficiency virus; IQR = interquartile range.
Unable to calculate odds ratio due to small numbers of outcomes; P value calculated using Fisher's exact test.
Bold P-values indicate P-values < 0.05 that were included in the multivariable model.
Association of schistosome infections with demographic and clinical factors.
Men with S. haematobium infection, confirmed by visualization of ova in the urine, were younger and reported significantly more hemospermia in the prior year than men without schistosome infection living in the same region (Table 4, OR = 0.96 [0.92–0.996] per year older, P = 0.033 and OR = 4.2 [1.1–16.2], P = 0.038, respectively). Compared with men without S. mansoni infection, men with S. mansoni infection had less education and reported more sexual partners (OR = 0.92 [0.87–0.98] per additional year in school, P = 0.010 and OR = 1.15 [1.03–1.28] per additional sexual partner, P = 0.014, Table 5).
Table 4.
Factors associated with egg-patent urogenital Schistosoma haematobium infection in rural Tanzanian men in S. haematobium-endemic villages
| Factor | S. haematobium egg-positive (N = 31) | S. haematobium egg-negative (N = 188) | Univariable analysis | Multivariable analysis* | ||
|---|---|---|---|---|---|---|
| Number (%) or median [IQR] | Number (%) or median [IQR] | Odds ratio [95% CI] | P value | Odds ratio [95% CI] | P value | |
| Age in years | 24 [20–39] | 33 [24–43] | 0.95 [0.91–0.99] | 0.010 | 0.96 [0.92–0.996] | 0.033 |
| Years in school | 7 [7–7] | 7 [7–7] | 1.0 [0.8–1.1] | 0.53 | ||
| Children have more than one mother | 3 (9.7) | 49 (26.1) | –† | 0.066 | ||
| Number of sexual partners in the past 6 months | 2 [1–3] | 2 [1–3] | 1.0 [0.9–1.1] | 0.58 | ||
| Ever treated for sexually transmitted infection | 4 (13.3) | 52 (28.0) | –† | 0.12 | ||
| Penile discharge in past year | 4 (12.9) | 21 (11.2) | –† | 0.76 | ||
| Dyspareunia in past year | 10 (32.3) | 40 (21.3) | 1.8 [0.8–4.0] | 0.18 | ||
| Hemospermia in past year | 4 (13.3) | 5 (2.7) | –† | 0.023 | 4.2 [1.1–16.2] | 0.038 |
| Painful genital ulcers in past year | 5 (16.1) | 13 (6.9) | 2.6 [0.9–7.9] | 0.093 | ||
| Painless genital ulcers in past year | 0 | 6 (3.2) | –† | 0.60 | ||
| Syphilis | 2 (6.5) | 14 (7.5) | –† | 1.0 | ||
| HIV | 2 (6.5) | 12 (6.4) | –† | 1.0 | ||
CI = confidence interval; HIV = human immunodeficiency virus; IQR = interquartile range.
Calculated using Firth logistic regression with backward elimination due to small sample sizes.
Unable to calculate odds ratio due to small numbers of outcomes; P value calculated using Fisher's exact test.
Bold P-values indicate P-values < 0.05 that were included in the multivariable model.
Table 5.
Factors associated with egg-patent Schistosoma mansoni infection in rural Tanzanian men in S. mansoni-endemic villages
| Factor | S. mansoni egg-positive (N = 225) | S. mansoni egg-negative (N = 219) | Univariable analysis | Multivariable analysis* | ||
|---|---|---|---|---|---|---|
| Number (%) or median [IQR] | Number (%) or median [IQR] | Odds ratio [95% CI] | P value | Odds ratio [95% CI] | P value | |
| Age in years | 33 [26–41] | 35 [25–42] | 0.99 [0.97–1.01] | 0.31 | ||
| Years in school | 7 [7–7] | 7 [7–10] | 0.91 [0.86–0.97] | 0.002 | 0.92 [0.87–0.98] | 0.010 |
| Children have more than one mother | 60 (26.7) | 62 (28.3) | 0.9 [0.6–1.4] | 0.70 | ||
| Number of sexual partners in the past 6 months | 2 [1–3] | 1 [1–2] | 1.2 [1.1–1.3] | 0.004 | 1.15 [1.03–1.28] | 0.014 |
| Ever treated for sexually transmitted infection | 81 (36.0) | 85 (39.0) | 0.9 [0.6–1.3] | 0.52 | ||
| Penile discharge in past year | 21 (9.3) | 17 (7.8) | 1.2 [0.6–2.4] | 0.56 | ||
| Dyspareunia in past year | 27 (12.0) | 25 (11.4) | 1.1 [0.6–1.9] | 0.85 | ||
| Hemospermia in past year | 0 | 5 (2.3) | –† | 0.029 | ||
| Painful genital ulcers in past year | 15 (6.7) | 12 (5.5) | 1.2 [0.6–2.7] | 0.60 | ||
| Painless genital ulcers in past year | 12 (5.4) | 8 (3.7) | 1.5 [0.6–3.7] | 0.39 | ||
| Syphilis | 19 (8.4) | 9 (4.1) | 2.2 [0.95–4.9] | 0.066 | ||
| HIV | 11 (4.9) | 13 (5.9) | 0.8 [0.4–1.9] | 0.63 | ||
CI = confidence interval; HIV = human immunodeficiency virus; IQR = interquartile range.
Calculated using Firth logistic regression with backward elimination due to small sample sizes.
Unable to calculate odds ratio due to small numbers of outcomes; P value calculated using Fisher's exact test.
Bold P-values indicate P-values < 0.05 that were included in the multivariable model.
Discussion
Men with schistosome infection did not have a higher odds of HIV infection than men without schistosome infection, even when we used a more sensitive diagnostic test for schistosome infections than had been used in prior studies. To the best of our knowledge, this was the first study of its kind to investigate this issue in men alone. In nearly 700 men, we found an increased odds of HIV infection of 1.3 [0.6–2.5], with an upper limit of the 95% CI of 2.5 and an upper 80% CI of 2.0. On examination of differential effects by species, S. haematobium-infected men had a slightly higher odds of HIV infection (1.4 [0.6–3.3]), whereas the odds of HIV infection in S. mansoni-infected men was below 1. Given our findings, we can be 82% certain that the true odds of HIV infection in men with S. haematobium infection is less than 2. Therefore, it remains possible that schistosome infections, particularly S. haematobium infection, may be mildly associated with HIV infection in men, but the association appears to be markedly diminished compared with that observed in women.
A recent study reported no difference in innate immune or HIV-1-specific immune responses in the blood of individuals with HIV–S. mansoni coinfection compared with individuals with HIV alone.20 Our epidemiological findings support this study, suggesting that systemic schistosome-induced immunomodulation, which we hypothesized could affect interactions between HIV and schistosomiasis in men as well as women, may not lead to increased susceptibility to HIV infection. Two corollaries follow from these observations. First, our work implicates changes in the genital mucosa of women with schistosome infections as the likely reason that women, but not men, who have schistosome infections have an increased odds of HIV infection. Second, it implies that, if the impaired antiviral control that is induced by schistosomiasis in animals21–23 leads to interactions with HIV, these parasite–virus interactions may occur after HIV infection has been acquired rather at the time of HIV exposure.
Our findings additionally offer helpful clinical clues for improved diagnosis of urogenital schistosomiasis in men, which causes tissue inflammation in the urinary and genital tracts and can be easily misdiagnosed as a sexually transmitted infection.24 Among Tanzanian men in our study, hemospermia was strongly associated with S. haematobium infection. Autopsy studies in men demonstrate that the highest concentrations of S. haematobium ova in tissue occur in the urinary bladders, ureters, seminal vesicles, and prostate.25 Semen of infected men frequently contains S. haematobium ova, together with increased seminal levels of leukocytes and the inflammatory cytokines interleukin (IL)-4, IL-6, IL-10, and tumor necrosis factor alpha (TNF-α).26 These findings and ours support the hypothesis that men with urogenital schistosomiasis may more easily transmit HIV to their sexual partners due to tissue inflammation and bleeding, leading to higher concentrations of HIV in the semen of HIV–schistosome coinfected men than in HIV-infected men without schistosome infection.26,27
A recent prospective study demonstrated that men and women with the helminthic infection lymphatic filariasis had a 2-fold increased risk of incident HIV infection compared with those without filariasis.28 Unlike schistosomiasis, filariasis is not known to have direct effects on the genital tract, suggesting that systemic immune changes induced by filariasis could increase HIV susceptibility. Our prior findings in women with S. mansoni, which preferentially affects the gastrointestinal tract, had led us to a similar hypothesis.4 Now, based on our finding that schistosome-infected men do not appear to have increased odds of HIV infection, an alternate hypothesis seems plausible as well. Schistosomiasis may increase the seminal and systemic HIV viral load, thereby increasing HIV transmission from one schistosome-infected individual to another in schistosome-endemic communities. Could this also be a mechanism underlying the apparent increased HIV incidence in adults with lymphatic filariasis? Further studies to explore these possibilities are urgently needed.
In conclusion, we have used stringent methods for detection of schistosome infections to demonstrate that schistosome-infected men have little to no increased odds of HIV infection. The robustness of our work is supported by the strong associations we found between HIV and other known HIV risk factors including lack of circumcision, number of sexual partners, and syphilis infection. Given these findings, we posit that systemic immune changes provoked by schistosome infection are not likely to exert major effects on HIV susceptibility. Our data provide additional information in the ongoing discussion of the relationship between schistosomiasis and HIV infection, suggesting that post-HIV infection effects in schistosome–HIV coinfected individuals strongly merit further investigation.
ACKNOWLEDGMENTS
This study would not have been possible without the help and support of many collaborators. We thank our study team nurses (Jane Mlingi, Ndalloh Paul, and Inobena Tosiri) and our study team parasitologists (John Igogote and Petro Mnyeshi) for their tireless and dependable work. We thank our colleagues at the laboratory at the National Institute for Medical Research, Mwanza (Pius Ikigo, Peter Lutonja, Eric Lyimo, and Crispin Mukerebe) for receiving and processing samples. We thank our clinical colleagues at the study sites for warmly receiving our team and assisting us in inviting study participants. Finally, we thank study participants themselves for their trust and enthusiastic participation.
Footnotes
Financial support: This study was supported by grants from the National Institutes of Health (K23 AI 110238 to JAD; K24 AI 098627 to DWF).
Authors' addresses: Jennifer A. Downs, Hannah E. Dee, Megan McGeehan, Myung Hee Lee, and Daniel W. Fitzgerald, Department of Medicine, Center for Global Health, Weill Cornell Medicine, New York, NY, E-mail: jna2002@med.cornell.edu, hed2011@med.cornell.edu, mam2167@med.cornell.edu, myl2003@med.cornell.edu, and dfitzgerald@gheskio.org. Claudia J. de Dood and Paul L. A. M. Corstjens, Department of Molecular Cell Biology, Leiden University Medical Center, Leiden, The Netherlands, E-mails: c.j.de_Dood@lumc.nl and p.corstjens@lumc.nl. Hijab Khan and Abena Marenga, Human Ecology, Cornell University, Ithaca, NY, E-mails: hak58@cornell.edu and mam674@cornell.edu. Patrick E. Adel, Edward Faustine, Benson Issarow, Emmanuel F. Kisanga, Godfrey Alfred Kisigo, Salvius Ngahyolerwa, and Frank Zahoro, Department of Medicine, Bugando Medical Centre, Mwanza,Tanzania, E-mails: adel87.pa@gmail.com, eddo1566@gmail.com, issarowjr@gmail.com, kisanga.emmanuel@gmail.com, godkisigo@gmail.com, salvius19@gmail.com, and fredfrank743@gmail.com. Donald Miyaye, Ruth Gideon Magawa, and Julius Mngara, National Institute for Medical Research-Mwanza Research Centre, Mwanza, Tanzania, E-mails: miyaye64@yahoo.com, ruthmagawa@gmail.com, and juliusmngara@yahoo.com. Govert J. van Dam, Department of Parasitology, Leiden University Medical Centre, Leiden, The Netherlands, E-mail: g.j.van_Dam@lumc.nl.
References
- 1.World Health Organization chistosomiasis Fact Sheet No 115. 2016. http://www.who.int/topics/schistosomiasis/en/ Available at. Accessed May 8, 2016.
- 2.Downs JA, Mguta C, Kaatano GM, Mitchell KB, Bang H, Simplice H, Kalluvya SE, Changalucha JM, Johnson WD, Jr, Fitzgerald DW. Urogenital schistosomiasis in women of reproductive age in Tanzania's Lake Victoria region. Am J Trop Med Hyg. 2011;84:364–369. doi: 10.4269/ajtmh.2011.10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, Mason PR, Sandvik L, Friis H, Gundersen SG. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20:593–600. doi: 10.1097/01.aids.0000210614.45212.0a. [DOI] [PubMed] [Google Scholar]
- 4.Downs JA, van Dam GJ, Changalucha JM, Corstjens PL, Peck RN, de Dood CJ, Bang H, Andreasen A, Kalluvya SE, van Lieshout L, Johnson WD, Jr, Fitzgerald DW. Association of schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg. 2012;87:868–873. doi: 10.4269/ajtmh.2012.12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazigo HD, Dunne DW, Wilson S, Kinung'hi SM, Pinot de Moira A, Jones FM, Morona D, Nuwaha F. Co-infection with Schistosoma mansoni and human immunodeficiency virus-1 (HIV-1) among residents of fishing villages of north-western Tanzania. Parasit Vectors. 2014;7:587. doi: 10.1186/s13071-014-0587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanya RE, Muhangi L, Nampijja M, Nannozi V, Nakawungu PK, Abayo E, Webb EL, Elliott AM. LaVIISWA study team Schistosoma mansoni and HIV infection in a Ugandan population with high HIV and helminth prevalence. Trop Med Int Health. 2015;20:1201–1208. doi: 10.1111/tmi.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corstjens PLAM, Nyakundi RK, de Dood CJ, Kariuki TM, Ochola EA, Karanja DM, Mwinzi PN, van Dam GJ. Improved sensitivity of the urine CAA lateral-flow assay for diagnosing active Schistosoma infections by using larger sample volumes. Parasit Vectors. 2015;8:241. doi: 10.1186/s13071-015-0857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya: I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am J Trop Med Hyg. 1997;56:515–521. doi: 10.4269/ajtmh.1997.56.515. [DOI] [PubMed] [Google Scholar]
- 9.Fontanet AL, Woldemichael T, Sahlu T, van Dam GJ, Messele T, Rinke de Wit T, Masho W, Yeneneh H, Coutinho RA, van Lieshout L. Epidemiology of HIV and Schistosoma mansoni infections among sugar-estate residents in Ethiopia. Ann Trop Med Parasitol. 2000;94:145–155. [PubMed] [Google Scholar]
- 10.Ssetaala A, Nakiyingi-Miiro J, Asiki G, Kyakuwa N, Mpendo J, Van Dam GJ, Corstjens PL, Pala P, Nielsen L, Bont J, Pantaleo G, Kiwanuka N, Kaleebu P, Kamali A, Elliott AM. Schistosoma mansoni and HIV acquisition in fishing communities of Lake Victoria, Uganda: a nested case-control study. Trop Med Int Health. 2015;20:1190–1195. doi: 10.1111/tmi.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D, Moore R, Habte E, Redda A, Gebre-Michael T, Gundersen SG. Variations in helminth faecal egg counts in Kato–Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Trop. 2004;92:205–212. doi: 10.1016/j.actatropica.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 12.WHO . Preventative Chemotherapy in Human Heminthiasis. Geneva, Switzerland: WHO Press; 2006. [Google Scholar]
- 13.National Aids Control Programe and Reproductive and Child Health Services . National Guidelines for Management of Sexually Transmitted and Reproductive Tract Infections. Dar es Salaam, the United Republic of Tanzania: The United Republic of Tanzania; Minstry of Health and Social Welfare: 2007. [Google Scholar]
- 14.de Water R, Fransen JAM, Deelder AM. Ultrastructural localization of the circulating anodic antigen in the digestive tract of Schistosoma mansoni using monoclonal antibodies in an immunogold labeling procedure. Am J Trop Med. 1986;35:549–558. doi: 10.4269/ajtmh.1986.35.549. [DOI] [PubMed] [Google Scholar]
- 15.Kremsner PG, Enyong P, Krijger FW, De Jonge N, Zotter GM, Thalhammer F, Mühlschlegel F, Bienzle U, Feldmeier H, Deelder AM. Circulating anodic and cathodic antigen in serum and urine from Schistosoma haematobium-infected Cameroonian children receiving praziquantel: a longitudinal study. Clin Infect Dis. 1994;18:408–413. doi: 10.1093/clinids/18.3.408. [DOI] [PubMed] [Google Scholar]
- 16.de Jonge N, De Caluwé P, Hilberath GW, Krijger FW, Polderman AM, Deelder AM. Circulating anodic antigen levels in serum before and after chemotherapy with praziquantel in schistosomiasis mansoni. Trans R Soc Trop Med Hyg. 1989;83:368–372. doi: 10.1016/0035-9203(89)90507-5. [DOI] [PubMed] [Google Scholar]
- 17.Corstjens PLAM, De Dood CJ, Kornelis D, Fat EM, Wilson RA, Kariuki TM, Nyakundi RK, Loverde PT, Abrams WR, Tanke HJ, Van Lieshout L, Deelder AM, Van Dam GJ. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology. 2014;141:1841–1855. doi: 10.1017/S0031182014000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corstjens PL, van Lieshout L, Zuiderwijk M, Kornelis D, Tanke HJ, Deelder AM, van Dam GJ. Up-converting phosphor technology-based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum. J Clin Microbiol. 2008;46:171–176. doi: 10.1128/JCM.00877-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, Poole F, Smith PG, Sriram N, Wongsrichanalai C, Linke R, O'Brien R, Perkins M, Cunningham J, Matsoso P, Nathanson CM, Olliaro P, Peeling RW, Ramsay A. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol. 2010;8:S17–S29. [PubMed] [Google Scholar]
- 20.Obuku AE, Asiki G, Abaasa A, Ssonko I, Harari A, van Dam GJ, Corstjens PL, Joloba M, Ding S, Mpendo J, Nielsen L, Kamali A, Elliott AM, Pantaleo G, Kaleebu P, Pala P. Effect of Schistosoma mansoni infection on innate and HIV-1-specific T-cell immune responses in HIV-1-infected Ugandan fisher folk. AIDS Res Hum Retroviruses. 2016;32:668–675. doi: 10.1089/aid.2015.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reese TA, Wakeman BS, Choi HS, Hufford MM, Huang SC, Zhang X, Buck MD, Jezewski A, Kambal A, Liu CY, Goel G, Murray PJ, Xavier RJ, Kaplan MH, Renne R, Speck SH, Artyomov MN, Pearce EJ, Virgin HW. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science. 2014;345:573–577. doi: 10.1126/science.1254517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborne LC, Monticelli LA, Nice TJ, Sutherland TE, Siracusa MC, Hepworth MR, Tomov VT, Kobuley D, Tran SV, Bittinger K, Bailey AG, Laughlin AL, Boucher JL, Wherry EJ, Bushman FD, Allen JE, Virgin HW, Artis D. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science. 2014;345:578–582. doi: 10.1126/science.1256942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayash-Rashkovsky M, Chenine A-L, Steele LN, Lee SJ, Song R, Ong H, Rasmussen RA, Hofmann-Lehmann R, Else JG, Augostini P, McClure HM, Secor WE, Ruprecht RM. Coinfection with Schistosoma mansoni reactivates viremia in rhesus macaques with chronic simian-human immunodeficiency virus clade C infection. Infect Immun. 2007;75:1751–1756. doi: 10.1128/IAI.01703-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leutscher PDC, Ramarokoto C-E, Hoffmann S, Jensen JS, Ramaniraka V, Randrianasolo B, Raharisolo C, Migliani R, Christensen N. Coexistence of urogenital schistosomiasis and sexually transmitted infection in women and men living in an area where Schistosoma haematobium is endemic. Clin Infect Dis. 2008;47:775–782. doi: 10.1086/591127. [DOI] [PubMed] [Google Scholar]
- 25.Smith JH, Kamel IA, Elwi A, Von Lichtenberg F. A quantitative post mortem analysis of urinary schistosomiasis in Egypt. I. Pathology and pathogenesis. Am J Trop Med Hyg. 1974;23:1054–1071. doi: 10.4269/ajtmh.1974.23.1054. [DOI] [PubMed] [Google Scholar]
- 26.Leutscher PDC, Pedersen M, Raharisolo C, Jensen JS, Hoffmann S, Lisse I, Ostrowski SR, Reimert CM, Mauclere P, Ullum H. Increased prevalence of leukocytes and elevated cytokine levels in semen from Schistosoma haematobium-infected individuals. J Infect Dis. 2005;191:1639–1647. doi: 10.1086/429334. [DOI] [PubMed] [Google Scholar]
- 27.Mbabazi PS, Andan O, Fitzgerald DW, Chitsulo L, Engels D, Downs JA. Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS Negl Trop Dis. 2011;5:e1396. doi: 10.1371/journal.pntd.0001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroidl I, Saathof E, Maganga L, Makunde WH, Hoerauf A, Geldmacher C, Clowes P, Maboko L, Hoelscher M. Effect of Wuchereria bancrofti infection on HIV incidence in southwest Tanzania (EMINI): a prospective cohort study. Lancet. 2016;388:1912–1920. doi: 10.1016/S0140-6736(16)31252-1. [DOI] [PubMed] [Google Scholar]