Abstract
A modification of Koga agar plate culture was performed, consisting of a 2 × 2-cm cellophane paper centered on the agar plate to prevent bacterial contamination of the agar and daily dish examinations (days 2–5). Between January 2000 and July 2005, we examined 1,708 infection-suspected patients, of which 147 (8.6%) harbored S. stercoralis. Single modified agar plate cultures exhibited superior sensitivity (93.2%), compared with different three-sample screening methods (sensitivity—Baermann: 76.6%, formalin-ethyl acetate: 22%, and direct smear: 15.3%). Agar plate cultures stand out as helpful alternatives for improved detection and therapy monitoring in poor countries and endemic areas. Combined with Baermann methods, they provide increased probability for S. stercoralis detection.
Strongyloides stercoralis, a worldwide endemic intestinal nematode, is prevalent in southeast Asia, Latin America, and sub-Saharan Africa, infecting approximately 370 million humans.1,2 Coexisting immunosuppressive illnesses predispose to dissemination and/or fatal hyperinfections.3 Globally growing morbidity and mortality stimulated the development of improved recognition procedures.
Visualization of S. stercoralis rhabditiform larvae is feasible on iodine-saline fecal smears, formalin-ethyl acetate (FEA) method, filter paper cultures, or various Baermann (BM) techniques.4,5 In agar plate cultures (APCs), filariform larvae appear to crawl, thus shaping typically serpiginous grooves.6,7
Venezuelan underdiagnosed prevalence and morbidity is imputable to uneven excretion of rhabditiform larvae and to widespread search only on iodine-saline fecal smears.4,8 Standard FEA and BM techniques recognize fecal larvae, whereas agar cultures proffer a yield advantage extendable to sputum, body fluids, and bronchial and duodenal secretions.9–12 This study compares a modified Koga agar plate culture (mAPC) with conventional procedures for the diagnosis of S. stercoralis.
All referred patients were examined between January 2000 and July 2005 at our laboratory. Informed consents and approval by the José María Vargas School of Medicine Bioethics Committee were requested. Three fresh stool samples were collected from 1,708 patients. All were processed with three standard procedures: direct smears (DS), BM, and FEA, the latter being unavailable in the first 316 cases. Only the first sample of each patient was placed in mAPC.6
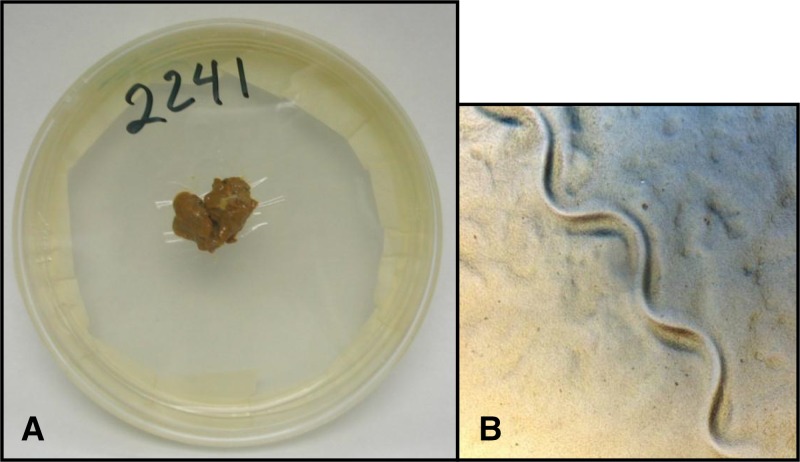
mAPC containing 1.5% agar, 0.5% meat extract, 1.0% peptone, and 0.5% NaCl were autoclaved, and 8.5–9.0 mL aliquots were distributed in sterilized plastic dishes (9-cm diameter × 1.5-cm depth). To solidify, mAPCs were covered for 20 minutes at room temperature and turned over to remove surface water, and then were kept inverted to permit water evaporation at 4°C until time of usage. A 2 × 2-cm cellophane paper was placed in the plate center, ensuring adhesion of the edges to the agar, before depositing 2–4 g fresh stool sample on it. mAPCs were sealed with white adhesive tape (2′), incubated at room temperature (26–32°C) for 120 hours, and screened with a stereoscopic magnifier (10.5−45×) at 48, 72, 96, and 120 hours, since larvae survival in agar lasts 8–21 days (Figure 1 ). Surfaces of all larvae-positive dishes were rinsed with saline solution and stained with lugol for differentiation of larvae. Protection of all laboratory personnel included biosafety double gloves. Cellophane paper to prevent agar–feces contact was the main difference from the original Koga APC.
Figure 1.
Modified agar plate culture and Strongyloides stercoralis larvae groove. (A) The 2 × 2-cm cellophane paper centered on the agar plate to prevent bacterial contamination and (B) the larvae groove.
Presence of one or more larvae in at least one of the four methods was considered positive.
McNemar's calculations performed on Windows SPSS 12.0 (SPSS Inc., Chicago, IL) were used to analyze statistical data. A P value < 0.05 was deemed significant and the combined results of the different techniques served as diagnostic gold standard.
Strongyloides stercoralis larvae were found in 147 of 1,708 subjects (8.6%) by mAPC plus BM; 62 females (42.2%) and 85 males (57.8%). Mean age distribution of positive cases was 45.8 ± 1.69 years of age (range 3–84).
mAPC and BM detected 137 and 115 S. stercoralis infections, respectively (Table 1). The prevalence of S. stercoralis as estimated by a single mAPC alone was 8.0% with a sensitivity of 93.2%, whereas by serial BM alone, the prevalence was 6.7% with a sensitivity of 76.6%. FEA and DS revealed much lower prevalence and sensitivity (Table 2).
Table 1.
Diagnostic methods for Strongyloides stercoralis detection during 2000–2005
| S. stercoralis positive n (%) | S. stercoralis negative n (%) | Total n (%) | |
|---|---|---|---|
| mAPC | |||
| Positive | 137 (8.0) | 0 (0.0) | 137 (8.0) |
| Negative | 10 (0.6)* | 1,561 (91.4) | 1,571 (92.0) |
| Total | 147 (8.6) | 1,561 (91.4) | 1,708 (100) |
| BM | |||
| Positive | 115 (6.7) | 0 (0.0) | 115 (6.7) |
| Negative | 32 (1.9)† | 1,561 (91.4) | 1,593 (93.3) |
| Total | 147 (8.6) | 1,561 (91.4) | 1,708 (100) |
| FEA | |||
| Positive | 22 (1.6) | 0 (0.0) | 22 (1.6) |
| Negative | 69 (5.0) | 1,301 (93.5) | 1,370 (98.4) |
| Total | 91 (6.5) | 1,301 (93.5) | 1,392 (100) |
| DS | |||
| Positive | 20 (1.2) | 0 (0.0) | 20 (1.2) |
| Negative | 127 (7.4) | 1,561 (91.4) | 1,688 (98.8) |
| Total | 147 (8.6) | 1,561 (91.4) | 1,708 (100) |
BM = Baermann method; DS = direct smear; FEA = formalin-ethyl acetate; mAPC = modified agar plate culture.
Negative by mAPC but positive by BM.
Negative by BM but positive by mAPC.
Table 2.
Diagnostic methods sensitivity for Strongyloides stercoralis detection during 2000–2005
| Diagnostic method | Sensitivity (%) | 95% confidence interval | P value |
|---|---|---|---|
| Single mAPC | 93.2 | 87.5–96.5 | |
| Serial BM | 76.6 | 68.5–83.3 | 0.001* |
| Serial FEA | 22.0 | 14.3–32.1 | < 0.001† |
| Serial DS | 15.3 | 10.0–22.7 | < 0.001‡ |
BM = Baermann method; DS = direct smear; FEA = formalin-ethyl acetate; mAPC = modified agar plate culture.
BM vs. mAPC.
FEA vs. mAPC.
DS vs. mAPC.
When comparing one-sample mAPC sensitivity to three-sample serial testing by alternate methods, it surpassed 1.2 times the BM (P = 0.001), 4.5 times the FEA (P < 0.001), and 6.2 times the DS detection (P < 0.001).
Some differing experiences reflect a better performance of serial BM against single APC. In Cambodian schoolchildren (N = 458), dwelling in high-prevalence settings (24.4%), Khieu and others attained a single agar culture sensitivity of 71.3%, whereas for serial BM, the sensitivity was 97.3%.13 In lower prevalence Chinese areas (11.7%), Steinmann and others emphasize a higher yield of the triple BM versus single APC (85.6% versus 63.4%).14 In further divergence, Becker and others in Ivory Coast, encountered more false negatives in single APC (N = 20/37) than in single BM (N = 11/37), and a notable sensitivity difference of 46.0% versus 70.3%, respectively.15
Lacking a gold standard, most researchers achieve optimal results by performance of serial stool examinations, combining BM and APC.13–16 In south-central Ivory Coast, among Léléblé families, Becker and others reported a prevalence of 5.8% with single APC, and 8.9% with single BM, whereas combining both methods, it increased to 12.7%. In Chinese Yumman families, Steinman and others showed that BM sensitivity increased from 47.5% with single sample to 85.6% with three samples, and APC from 63.4% to 95.1%, and overall sensitivity increased even more (from 76.0% to 98.6%) when both methods were combined. Additionally, in Cambodian children in a high-prevalence zone (24.4%), Khieu and others reported augmenting serial BM sensitivity from 69.9% to 97.3% and serial APC from 71.3% to 97.7%, and maximal ascent from 76.0% to 98.6% combining both methods.
The higher positivity of APC versus BM in the current study may be explained by technical modifications such as the use of cellophane paper to prevent bacterial contamination. Absence of bacterial colonies on agar deters macroscopic dish evaluation, thus forcing microscopic plate observation for diagnostic accuracy. Furthermore, daily dish examinations (days 2–5) increases detection chances, particularly in cases of low parasitic loads. Nevertheless, definite proof of yield superiority with these modifications of the original Koga APC would require comparative studies of both techniques. Additionally, several factors such as immune status, clinical severity, and the degree of parasitic prevalence in different populations may affect each method's sensitivity. Further comparative methodological studies could clarify their respective influence.
In chronic infections, conventional microscopy-based search for rhabditiform larvae remains unsatisfactory. Although APC screening requires a long time and fresh fecal samples, and may have risk of contamination, both BM and APC offer reliable results. Rhabditis hominis occurrence, described with the original Koga APC,6 has never appeared during our 16-year experience.
Serology tests are inadequate prevalence predictors in large population studies as they may share immunological cross-reactivity with other nematodes, do not differentiate recent from past infections, and have an inconveniently high cost.4 Recent availability of DNA molecular techniques are highly specific and sensitive, but their expenses markedly exceeded those microscopically based.17,18
Consequently, the APC emerged as a feasible cost-effective alternative for population screening in endemic areas of poor countries and close therapy monitoring. Based on our data and published studies, three samples tested with BM plus APC seem recommendable as the best practice.
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Authors' addresses: Leonor A. Pocaterra, Rosaura Peñaranda, Elsy Rojas, Gladymar Pérez-Chacón, Aurora Hernán, Gabriela Certad, and Luz Núñez, Cátedra de Parasitología, “Escuela de Medicina Jose Maria Vargas,” Universidad Central de Venezuela, Caracas, Venezuela, E-mails: leopoca@gmail.com, rosaura_penaranda@yahoo.com.br, elsyrjs@hotmail.com, gladymar@gmail.com, aurorahrnn@hotmail.com, gabrielacertad@hotmail.com, and nenagold@gmail.com. Giuseppe Ferrara, Laboratorio de Parasitosis Intestinales, Cátedra de Parasitología, Escuela de Medicina “José María Vargas,” Universidad Central de Venezuela, Caracas, Venezuela, E-mail: gferrara1971@gmail.com. Carlos Goldstein, Medicina Interna-Hematología, Centro Médico de Caracas, Caracas, Venezuela, E-mail: carlosgolds@gmail.com.
References
- 1.Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7:e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buonfrate D, Mena MA, Angheben A, Requena-Mendez A, Muñoz J, Gobbi F, Albonico M, Gotuzzo E, Bisoffi Z. COHEMI Project Study Group Prevalence of strongyloidiasis in Latin America: a systematic review of the literature. Epidemiol Infect. 2015;143:452–460. doi: 10.1017/S0950268814001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisoffi Z, Buonfrate D, Montresor A, Requena-Méndez A, Muñoz J, Krolewiecki AJ, Gotuzzo E, Mena MA, Chiodini PL, Anselmi M, Moreira J, Albonico M. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7:e2214. doi: 10.1371/journal.pntd.0002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7:e2002. doi: 10.1371/journal.pntd.0002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernández-Chavarría F, Avendaño L. A simple modification of the Baermann method for diagnosis of strongyloidiasis. Mem Inst Oswaldo Cruz. 2001;96:805–807. doi: 10.1590/s0074-02762001000600011. [DOI] [PubMed] [Google Scholar]
- 6.Arakaki T, Iwanaga M, Kinjo F, Saito A, Asato R, Ikeshiro T. Efficacy of agar-plate culture in detection of Strongyloides stercoralis infection. J Parasitol. 1990;76:425–428. [PubMed] [Google Scholar]
- 7.Koga K, Kasuya S, Khamboonruang C, Sukhavat K, Ieda M, Takatsuka N, Kita K, Ohtomo H. A modified agar plate method for detection of Strongyloides stercoralis. Am J Trop Med Hyg. 1991;45:518–521. doi: 10.4269/ajtmh.1991.45.518. [DOI] [PubMed] [Google Scholar]
- 8.Uparanukraw P, Phongsri S, Morakote N. Fluctuations of larval excretion in Strongyloides stercoralis infection. Am J Trop Med Hyg. 1999;60:967–973. doi: 10.4269/ajtmh.1999.60.967. [DOI] [PubMed] [Google Scholar]
- 9.Ribero Z, Chourio G, Díaz I, Padilla L, Zárate M, Ferrer J. Comparación de cuatro técnicas de laboratorio para el diagnóstico de estrongiloidiasis. Rev Soc Ven Microbiol. 2002;22:68–73. [Google Scholar]
- 10.Salazar SA, Gutierrez C, Berk SL. Value of the agar plate method for the diagnosis of intestinal strongyloidiasis. Diagn Microbiol Infect Dis. 1995;23:141–145. doi: 10.1016/0732-8893(95)00247-2. [DOI] [PubMed] [Google Scholar]
- 11.Campo-Polanco L, Gutiérrez LA, Cardona-Arias J. Infección por Strongyloides stercoralis: metanálisis sobre evaluación de métodos diagnósticos convencionales (1980–2013) Rev Esp Salud Publica. 2014;88:581–600. doi: 10.4321/S1135-57272014000500004. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed NH. Cultivation of parasites. Trop Parasitol. 2014;4:80–89. doi: 10.4103/2229-5070.138534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khieu V, Schär F, Marti H, Sayasone S, Duong S, Muth S, Odermatt P. Diagnosis, treatment and risk factors of Strongyloides stercoralis in schoolchildren in Cambodia. PLoS Negl Trop Dis. 2013;7:e2035. doi: 10.1371/journal.pntd.0002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinmann P, Zhou XN, Du ZW, Jiang JY, Wang LB, Wang XZ, Li LH, Marti H, Utzinger J. Occurrence of Strongyloides stercoralis in Yunnan Province, China, and comparison of diagnostic methods. PLoS Negl Trop Dis. 2007;1:e75. doi: 10.1371/journal.pntd.0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker SL, Sieto B, Silué KD, Adjossan L, Koné S, Hatz C, Kern WV, N'Goran EK, Utzinger J. Diagnosis, clinical features, and self-reported morbidity of Strongyloides stercoralis and hookworm infection in a co-endemic setting. PLoS Negl Trop Dis. 2011;5:e1292. doi: 10.1371/journal.pntd.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 17.Pilotte N, Papaiakovou M, Grant JR, Bierwert LA, Llewellyn S, McCarthy JS, Williams S. Improved PCR-based detection of soil transmitted helminth infections using a next-generation sequencing approach to assay design. PLoS Negl Trop Dis. 2016;10:e0004578. doi: 10.1371/journal.pntd.0004578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llewellyn S, Inpankaew T, Nery SV, Gray DJ, Verweij JJ, Clements ACA, Gomes SJ, Traub R, McCarthy JS. Application of a multiplex quantitative PCR to assess prevalence and intensity of intestinal parasite infections in a controlled clinical trial. PLoS Negl Trop Dis. 2016;10:e0004380. doi: 10.1371/journal.pntd.0004380. [DOI] [PMC free article] [PubMed] [Google Scholar]