Abstract
We describe a case of human infection with Gongylonema pulchrum acquired in southeast Georgia. The patient presented with intermittent yet persistent nausea and vomiting for months. This case describes the need for extraction of worms on two occasions each followed by courses of albendazole treatment. Gongylonema pulchrum infections with high worm burden may relapse after extraction of the worm and a 3-day short course of albendazole therapy. Longer courses of albendazole may be indicated in selected circumstances.
Nematode parasites can migrate within superficial soft tissues after ingestion or after entering through the skin. Such activity is typically restricted to the cutaneous layers of the skin. Migration of parasites in the oral cavity is unusual and is primarily caused by Gongylonema pulchrum. This gullet nematode has been characterized as commonly occurring in various animals from captive primates to sheep, donkeys, squirrel monkeys, and other animals.1–3 Cases remain rare in humans.3–6 Approximately 60 human cases worldwide have been described.2 This is the second case reported from the state of Georgia.7
We present a case of G. pulchrum infection that was acquired in rural southeast Georgia. The patient presented with intermittent, but persistent nausea and vomiting occurring every few days for 3 months. Most human cases have been treated by worm extraction with or without 3 days of albendazole. This treatment of worm extraction followed by 3 days of albendazole failed in our patient. A longer duration of albendazole therapy was needed to prevent recurrent relapses, resulting in a cure.
Case Report
A healthy 37-year-old man who lived in rural Georgia presented to the Emergency Department with a 3-month history of persistent nausea and vomiting. There were no associated fevers, chills, night sweats, or malaise. He described recurrent buccal mucosa “zig zagging blister-” like sensations that lasted for several days at a time for the preceding 7 months. On the day of presentation, he had self-extracted a thin hair-like object with a needle from his buccal mucosa. He placed this object in a jar and brought it to the hospital. The Emergency Department discharged the patient on anti-emetics and antacids with a referral to see an outpatient infectious disease consultant. Delusional parasitosis was considered.
During the infectious diseases consultation, the patient continued to complain of intermittent nausea and vomiting. He recalled a heavy rainy season the prior year with many flying roaches in his yard and home. He stored grains for consumption in the back of a double wide trailer. Physical examination was normal except for a lesion in his upper right buccal mucosa (Figure 1 ). All laboratory studies were normal. He had no eosinophilia. He was advised to exterminate the trailer. The worm extracted from the patient was identified by microscopy as G. pulchrum. He received 400 mg of albendazole twice a day for 3 days. He experienced resolution of the nausea and vomiting for 1 week.
Figure 1.
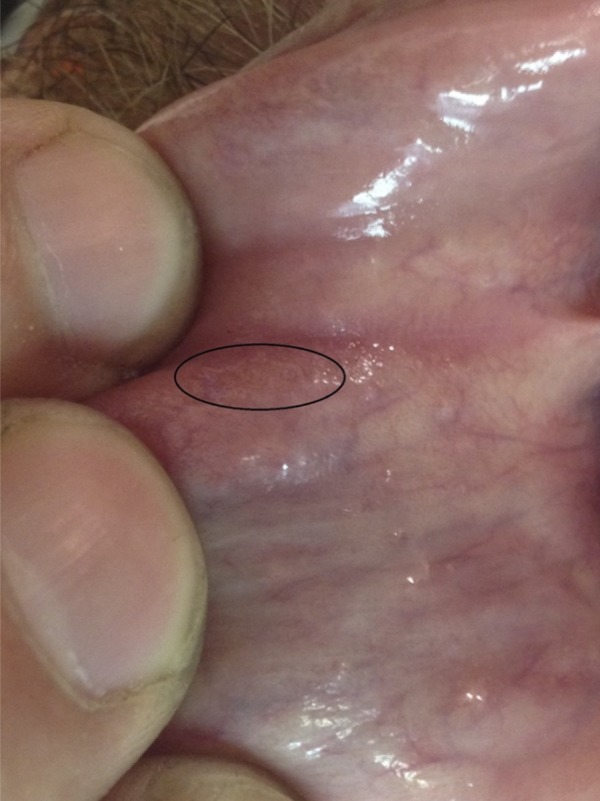
Oral lesion that migrated under mucosa in oral cavity.
Two weeks later, he presented with reoccurrence of symptoms associated with the “zig zagging” sensation in his mouth and nausea with vomiting. He had self-extracted another specimen from under his tongue. Gongylonema pulchrum was identified a second time. Given the persistent Gongylonema oral infection, he received a 30-day course of albendazole with complete resolution of his symptoms. No recurrence has been documented in more than 2 years.
Specimen.
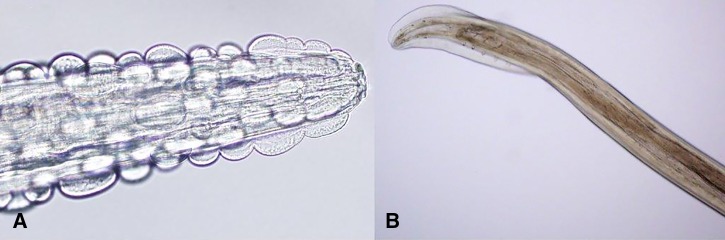
On two occasions, light colored, thread-like filariform worms were removed from the patient's buccal mucosa. The female specimen consisted of a long, thin nematode, approximately 25–30 mm long, with a diameter of 0.25–0.5 mm. The anterior end of the worm was notable for a short esophagus, and the presence of longitudinal rows of cuticular bosses or scutes characteristic for G. pulchrum (Figure 2A ). Transverse striations were noted posterior to the bosses, and continuing to the tail (Figure 2A). The posterior end of the male worm was slightly wider than the anterior and tapered; rows of caudal papillae were noted (Figure 2B). Embryonated eggs (50 × 25 μm) with smooth, thick shells were noted both in utero and external to the specimen (Figure 3 ).
Figure 2.
(A) Anterior end of the worm (male and female) demonstrating a short esophagus and presence of longitudinal rows of cuticular bosses or scutes characteristic for Gongylonema pulchrum. (B) Posterior end (male) was slightly wider than the anterior end and tapered; rows of caudal papillae are visible.
Figure 3.
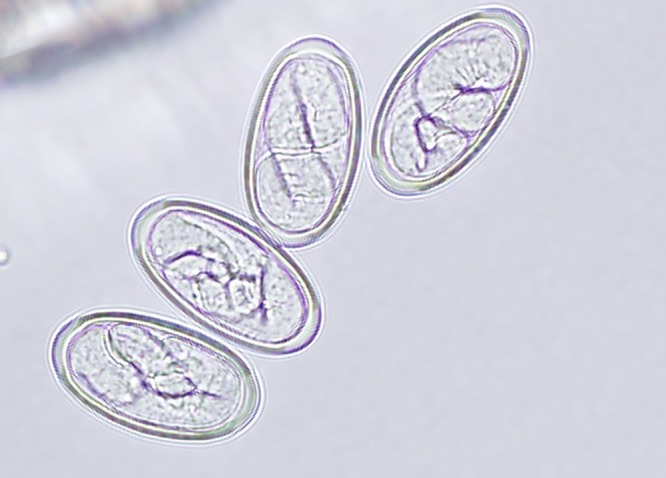
Embryonated eggs (50 × 25 μm) with smooth thick shells were noted both in utero and external to the specimen.
Discussion
Gongylonema infections are rare in humans, but commonly occur as parasitic infections of domestic cattle and other animals.8,9 Animals acquire the infection by feeding on insects (the intermediate host). Accidental ingestion by man occurs by consuming an obligate intermediate host (parts of beetles or cockroaches). Infective larvae burrow into the wall of the stomach or duodenum of the human host, and after 60–80 days of development migrate to the oral cavity or esophagus.5 Movement of the larvae through the oral tissues results in a serpiginous track.10 We hypothesize that the acquisition of Gongylonema in our patient was from consuming cockroach-contaminated grains. The history of the excessive presence of cockroaches during the rainy season in his trailer supports this, but the grains were discarded before analysis for Gongylonema could be done.
Published literature on Gongylonema human infections exist. Patients are described as having the sensation of an object moving in their mouth, and typically, as in this case, the patients remove the worms themselves. Removal of the parasite typically clears the infection.10 All reported cases describe oral lesions as the major component in the clinical presentation. Nausea and vomiting are often not noted,3–5 but some of the early case reports of Gongylonema infection did present with nausea.11 The oral symptoms lasted 7 months. The nausea with vomiting persisted for 3 months suggesting that Gongylonema migrated into the esophagus. Although, endoscopic studies were not performed, the presence of Gongylonema from the esophagus of cattle and a donkey in Iran has been well documented.8,9 Recently, Huang and others12 showed multiple infections of a human esophagus by Gongylonema using endoscopy.
The efficacy of anti-helminth agents is quite limited in animal studies,13 and none exist in humans for this parasite. The patient self-extracted the worm from the oral cavity, took 3 days of albendazole, and ate no further stored grains. Yet, the treatment failed. The poor efficacy of this treatment was likely related to the total nematode burden in the oral and upper digestive tract. Kudo and others13 conducted in vitro and in vivo studies to evaluate the effects of thiabendazole, mebendazole, levamisole, and ivermectin against G. pulchrum in mice. The in vivo study showed that levamisole, mebendazole, and ivermectin reduced worm burdens by 63%, 22%, and 25%, respectively, with worm burdens not reduced by thiabendazole. Surviving worms were found in the esophagus with the remainder distributed among the buccal or pharyngeal mucosa. The most effective agent was levamisole, and the least effective with no reduction in worm burden was thiabendazole. Mebendazole was withdrawn from the U.S. market in 2011. Albendazole was not tested. None of these anti-helminthic agents resulted in complete effectiveness at reducing worm burdens to zero. Albendazole was the agent used in prior case reports of gongylonemiasis. The observation of improvement of nausea and vomiting for 1 week after a 3-day course of albendazole only to be followed by a relapse of the gongylonemiasis (confirmed by a second extraction of the nematode) supports the finding in mice by Kudo and others13 that the efficacy of anti-helminthic agents varies in vivo. This observation in our patient is also consistent with the findings at necropsy of mice where surviving worms were found in the esophagus and pharyngeal mucosa after 3 days of treatment. Practitioners may want to assign longer treatment with albendazole to patients who report removing more than a single worm for the oral cavity or esophagus.
In conclusion, human gongylonemiasis may involve the oral cavity and migrate to the esophagus in humans as it does in animals. Patients may have nausea and vomiting as major symptoms at presentation. The burden of worms and duration of the illness suggest that longer durations of albendazole may be indicated.
Disclaimer: Mayo Clinic does not endorse specific products or services included in this article.
Footnotes
Authors' addresses: Claudia R. Libertin, Division of Infectious Diseases, Mayo Clinic, Jacksonville, FL, and Department of Microbiology, Mayo Clinic Health System, Waycross, GA, E-mail: libertin.claudia@mayo.edu. Mohammed Reza, Division of Hospital Internal Medicine, Mayo Clinic, Jacksonville, FL, E-mail: reza.mohammed@mayo.edu. Joy H. Peterson, Jason Lewis, and D. Jane Hata, Departments of Pathology and Laboratory Medicine, Mayo Clinic Health System, Waycross, GA, E-mails: peterson.joy1@mayo.edu, lewis.jason@mayo.edu, and hata.donna@mayo.edu.
References
- 1.Eslami AH, Nabavi L. Species of gastro-intestinal nematodes of sheep from Iran. Bull Soc Pathol Exot. 1976;69:92–95. [PubMed] [Google Scholar]
- 2.Movassaghi AR, Razmi GR. Oesophageal and gastric gongylonemiasis in a donkey. Iran J Vet Res. 2008;9:84–86. [Google Scholar]
- 3.Eberhard ML, Busillo C. Human Gongylonema infection in a resident of New York City. Am J Trop Med Hyg. 1999;61:51–52. doi: 10.4269/ajtmh.1999.61.51. [DOI] [PubMed] [Google Scholar]
- 4.Wilson ME, Lorente CA, Allen JE, Eberhard ML. Gongylonema infection of the mouth in a resident of Cambridge, Massachusetts. Clin Infect Dis. 2001;32:1378–1380. doi: 10.1086/319991. [DOI] [PubMed] [Google Scholar]
- 5.Jelinek T, Loscher T. Human infection with Gongylonema pulchrum: a case report. Trop Med Parasitol. 1994;45:329–330. [PubMed] [Google Scholar]
- 6.Allen JD, Esquela-Kerscher A. Gongylonema pulchrum infection in a resident of Williamsburg, Virginia, verified by genetic analysis. Am J Trop Med Hyg. 2013;89:755–757. doi: 10.4269/ajtmh.13-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimuke JC, Jr, Routh CF. Human infection with Gongylonema in Georgia. Am J Trop Med Hyg. 1963;12:73–74. doi: 10.4269/ajtmh.1963.12.73. [DOI] [PubMed] [Google Scholar]
- 8.Kheirandish R, Radfar MH, Sharifi H, Mohammadyari N, Alidadi S. Prevalence and pathology of Gongylonema pulchrum in cattle slaughtered in Rudsar, northern Iran. Scientia Parasitologica. 2013;14:37–42. [Google Scholar]
- 9.Halajian A, Eslami A, Salehi N, Ashrafi-Helan J, Sato H. Incidence and genetic characterization of Gongylonema pulchrum in cattle slaughtered in Mazandaran province, northern Iran. Iran J Parasitol. 2010;5:10–18. [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson ME. Worms that cause lumps in the mouth. Lancet. 2001;357:1888. doi: 10.1016/S0140-6736(00)04998-9. [DOI] [PubMed] [Google Scholar]
- 11.Ward HB. Gongylonema in the role of a human parasite. J Parasitol. 1916;2:119–125. [Google Scholar]
- 12.Huang Q, Wang J, Yang T, Liu Y. Multiple Gongylonema pulchrum worms in a human esophagus. Endoscopy. 2016;48((Suppl 1)):E24–E25. doi: 10.1055/s-0035-1569657. UCTN. [DOI] [PubMed] [Google Scholar]
- 13.Kudo N, Kubota H, Gotoh H, Ishida H, Ikadai H, Oyamada T. Efficacy of thiabendazole, mebendazole, levamisole and ivermectin against gullet worm, Gongylonema pulchrum: in vitro and in vivo studies. Vet Parasitol. 2008;151:46–52. doi: 10.1016/j.vetpar.2007.10.005. [DOI] [PubMed] [Google Scholar]