Abstract
Snake bite is an important health hazard in tropics. Snake envenomation in pregnancy may cause fetal death and maternal mortality or morbidity. However, little is known about the toxic effects and optimal management during pregnancy after snake envenomation because of the rarity of cases. Herein, we report a case of a pregnant woman who was successfully treated for snake bite–induced acute kidney injury during the third trimester. She was treated with equine-derived polyvalent anti-snake venom without development of any adverse effects, hemodialysis, and supportive therapy. She fully recovered and subsequently gave birth to a healthy child.
Introduction
Snake bite is a significant public health problem causing considerable morbidity and mortality worldwide, particularly in tropics. Much information is available regarding the effect of snake venom on nonpregnant woman and treatment protocols. However, little is known about snake bite during pregnancy. Acute kidney injury (AKI) is an important complication of snake bite and a major cause of mortality. Snake bite in pregnancy leading to AKI is not reported in literature. Herein, we report a case of a pregnant woman who was successfully treated for snake bite–induced AKI. Informed consent was obtained from the patient and institutional ethics committee for publication.
Case Report
A 30-year-old pregnant woman was admitted with a history of snake bite on the left foot 4 days earlier. She had severe local pain, followed by swelling and bruising of the foot. She was initially treated at a local hospital where she had progressive swelling of the left leg and ecchymosis around the fang marks. She developed hematuria and decreased urine output, and was referred to our hospital on fifth day. There was history of generalized abdominal pain but there was no history of bleeding from gums, epistaxis, hemoptysis, hematemesis, melena, vaginal bleeding, breathing difficulty, focal neurological deficit, or unconsciousness. She was a multigravida in 33rd week of gestation. On examination she was conscious and oriented. She was hemodynamically stable with a pulse rate of 100/minute, blood pressure of 120/70 mmHg, and a respiratory rate of 20/minute. There was pallor but no cyanosis, clubbing, lymphadenopathy, edema, and the jugular venous pressure was not elevated. Her left leg was swollen up to the knee and had ecchymosed around the fang marks. On abdominal examination, the height of uterus was 28–30 weeks and fetal heart sounds were present. The other systemic examination was unremarkable. Obstetric consultation was performed; ultrasonographic examination showed a 32-week-old live fetus demonstrating normal biometry and anatomy and a normal placenta. Fetal movements were normal at ultrasonography, and there were no uterine contractions at fetal cardiotocography.
The investigations were as follows: hemoglobin 5.5 g/dL, white blood cell count 16.2 × 103/mm3 with differential count of 86% neutrophils, 10% lymphocytes, 2% eosinophils, monocytes 2%, platelet count 80 × 103/mm3, erythrocyte sedimentation rate 20 mm first hour, 20-minute whole blood clotting test (20WBCT) > 20 minutes, fibrin degradation products > 15 μg/mL, d-dimer 2.9 mg/L, serum urea 213 mg/dL, creatinine 8.9 mg/dL, sodium 134 mEq/L, potassium 5.9 mEq/L, chloride 108 mEq/L, calcium 6.1 mg/dL, phosphorous 5.8 mg/dL, uric acid 7.2 mg/dL, protein 5.5 g/dL, albumin 2.9 g/dL, bilirubin 1.6 mg/dL, aspartate aminotransferase 724 IU/L, alanine aminotransferase 534 IU/L, alkaline phosphatase 432 U/L, cholesterol 130 mg/dL, random blood sugar 89 mg/dL, serum creatine phosphokinase 18,000 U/L, and lactate dehydrogenase 2,412 IU/L. Arterial blood gas revealed pH 7.35, paO2 83.3 mmHg, paCO2 23.6, HCO3 12.7 mEq/L, and SaO2 92.2%. Urine examination showed albumin 2+, sugar nil, pus cells 6–8 per high power field, the culture was sterile, and 24-hour urine protein was 0.4 g. Viral serology for hepatitis B, hepatitis C, and human immunodeficiency virus were negative. Electrocardiogram was normal. Ultrasound examination showed intrauterine pregnancy with a single living fetus.
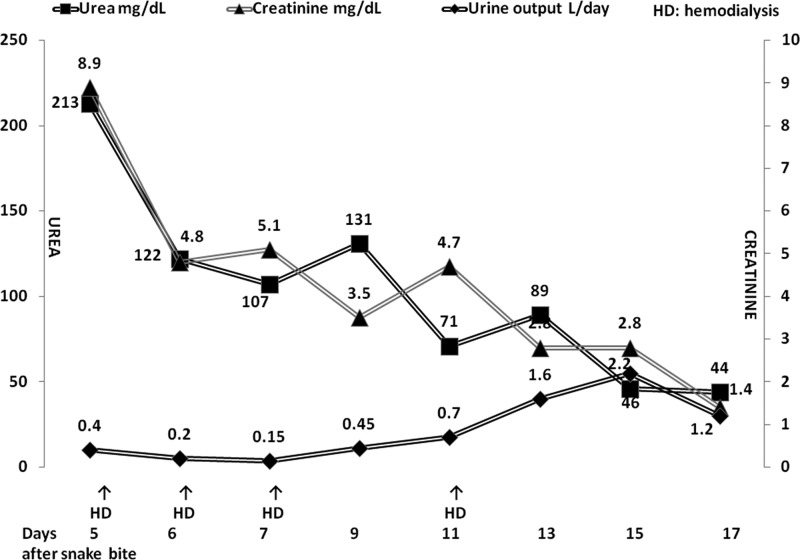
Patient was diagnosed as snake envenomation and AKI due to viper bite. She was treated with polyvalent anti-snake venom (ASV) serum injection manufactured by the Haffkine Institute (Mumbai, India), which neutralizes four most important venomous species in India (Indian cobra, Naja naja; common krait, Bungarus caeruleus; Russell's viper, Daboia russelli; saw-scaled viper, Echis carinatus). It was administered as an intravenous infusion over 1 hour after reconstituting 10 vials of lyophilized ASV in 250 mL of isotonic saline. No adverse reaction to its administration was observed. The coagulation abnormality was corrected as observed by a repeat 20WBCT done at 6 hours. She was transfused four units of blood and provided other supportive treatment. She received four sessions of hemodialysis. The course of renal function tests, hemodialysis treatment, and urine output are shown in Figure 1 . The fetal well-being was confirmed by ultrasound examination at discharge. The renal functions had normalized on follow-up at 2 weeks. She delivered a live healthy male baby at 40 weeks of gestation.
Figure 1.
Course of renal function tests, urine output, and the hemodialysis treatment.
Discussion
Worldwide occurrence of snake bite is common but relatively few cases among pregnant women are reported in the literature. In a review published in the year 2010, only 213 cases of snakebite in pregnant females were identified.1 Snake bites during pregnancy may have a significant adverse effect on the fetus as well as the mother, which are related to the severity of envenomation.2 Early gestational age and delay in the treatment are associated with an unfavorable outcome. Overall rate of fetal loss is around 20% and maternal case-fatality rate is about 4–5%.1
Twelve to thirty percent of patients bitten by venomous snakes, primarily vipers, develop AKI. Hemorrhage, hypotension, disseminated intravascular coagulation, intravascular hemolysis, and rhabdomyolysis contribute to the development of AKI. Enzymatic activities of snake venoms account for direct nephrotoxicity. Immunologic mechanism plays a minor role. Mortality in snake bite–induced AKI is 1–20%.3
The basic therapeutic approach for AKI in patients bitten by snakes is the same as that for AKI due to any other cause. Early administration of ASV is a vital therapeutic measure.3 ASV is recommended when a patient with proven or suspected snake bite develops one or more of signs of systemic envenomation—hemostatic abnormalities: spontaneous systemic bleeding, coagulopathy (20WBCT), or thrombocytopenia (< 100 × 103/mL); neurotoxic signs: ptosis, external ophthalmoplegia, paralysis, etc.; cardiovascular abnormalities: hypotension, shock, cardiac arrhythmia, or abnormal electrocardiogram; AKI: oliguria/anuria, rising blood urea, and creatinine (> 2 mg/dL); hemoglobinuria/myoglobinuria: dark brown urine, urine dipsticks, other evidence of intravascular hemolysis, or generalized rhabdomyolysis; or local envenomation—local swelling involving more than half of the bitten limb (in the absence of a tourniquet) swelling after bites on the digits (toes and fingers); rapid extension of swelling (beyond the wrist or ankle within a few hours of bites on the hands or feet); and development of an enlarged tender lymph mode draining the bitten limb.4,5 Pregnant women are treated in exactly the same way as other victims and the same dosage of ASV is given.4–6 The victim should be assessed by an obstetrician for any impact on the fetus.5 In patients with intravascular hemolysis and/or rhabdomyolysis, maintenance of a high urine output by increasing fluid intake and giving a loop diuretic, as well as rendering the urine alkaline, helps in the prevention of AKI, provided it is performed early.3,7 Treatment of established AKI is largely supportive in nature, renal replacement therapy being the cornerstone.8
Snake bite in pregnancy leading to AKI is not reported in the literature. Our patient is the first reported case of snake bite due to a viper leading to AKI during the pregnancy. She was treated with equine-derived polyvalent ASV without development of any adverse effects, hemodialysis, and supportive therapy. She fully recovered and subsequently gave birth to a healthy child.
Footnotes
Authors' addresses: Sanjay Vikrant, Department of Nephrology, Indira Gandhi Medical College, Shimla, India, E-mail: sanjayvikrant@rediffmail.com. Anupam Parashar, Department of Community Medicine, Indira Gandhi Medical College, Shimla, India, E-mail: anupamvikrant@yahoo.co.in.
References
- 1.Langley RL. Snakebite during pregnancy: a literature review. Wilderness Environ Med. 2010;21:54–60. doi: 10.1016/j.wem.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar S, Bhattacharya P, Paswan A. Snakebite in pregnancy: preliminary study. Br J Anaesth. 2008;101:128–129. doi: 10.1093/bja/aen157. [DOI] [PubMed] [Google Scholar]
- 3.Kanjanabuch T, Sitprija V. Snakebite nephrotoxicity in Asia. Semin Nephrol. 2008;28:363–372. doi: 10.1016/j.semnephrol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Warrel DA. Guidelines for the Management of Snake-Bites. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 5.Ghosh S, Mukhopadhyay P, Chatterjee T. Management of snake bite in India. J Assoc Physicians India. 2016;64:11–14. [PubMed] [Google Scholar]
- 6.Habib A, Abubakar S, Abubakar IS, Larnyang S, Durfa N, Nasidi A, Yusuf PO, Garnvwa J, Theakston RD, Salako L, Warrell DA. EchiTab Study Group Envenoming after carpet viper (Echis ocellatus) bite during pregnancy: timely use of effective antivenom improves maternal and foetal outcomes. Trop Med Int Health. 2008;13:1–4. doi: 10.1111/j.1365-3156.2008.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553–1561. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 8.Fieghen H, Wald R, Jaber BL. Renal replacement therapy for acute kidney injury. Nephron Clin Pract. 2009;112:c222–c229. doi: 10.1159/000224788. [DOI] [PubMed] [Google Scholar]