Abstract
We determined the prevalence of IgG antibodies to hepatitis E virus (anti-HEV IgG) among travelers attending Boston-area travel health clinics from 2009 to 2010. Pre-travel samples were available for 1,356 travelers, with paired pre- and post-travel samples for 450 (33%). Eighty of 1,356 (6%) pre-travel samples were positive for anti-HEV IgG. Compared with participants who had never lived in nor traveled to a highly endemic country, the pre-travel prevalence odds ratio (POR) of anti-HEV IgG among participants born in or with a history of previous travel to a highly endemic country was increased (POR = 4.8, 95% CI = 2.3–10.3 and POR = 2.6, 95% CI = 1.4–5.0, respectively). Among participants with previous travel to a highly endemic country, anti-HEV IgG was associated with age > 40 years (POR = 3.7, 95% CI = 1.3–10.2) and travel history to ≥ 3 highly endemic countries (POR = 2.7, 95% CI = 1.2–5.9). Two participants may have contracted HEV infection during their 2009–2010 trip.
Introduction
Hepatitis E virus (HEV) is a single-strand RNA virus (family Hepeviridae, genus Orthohepevirus). HEV infection causes acute hepatitis that is generally self-limited. However, HEV infection can also lead to liver failure and death in pregnant women, as well as become chronic in immunocompromised individuals.1,2 There is no commercially available vaccine for HEV.
Globally, HEV causes an estimated 20 million infections, 3.3 million acute cases, and 56,600 deaths each year.3 A portion of infected individuals are asymptomatic.4 HEV occurs throughout much of the world, but it is highly endemic in many areas with inadequate sanitation and limited access to safe drinking water, where it is spread primarily via the fecal-oral route. The prevailing HEV genotypes in these areas are genotypes 1 and 2.5 In highly endemic areas, HEV causes waterborne outbreaks involving hundreds to thousands of cases and accounts for a substantial proportion of sporadic hepatitis infections.6
There have been reports of autochthonous HEV infections, unrelated to travel, in industrialized countries, including the United States. Symptomatic HEV infections in low-incidence countries generally occur among older adults.7 The prevailing HEV genotype in low-incidence countries is genotype 3, which is frequently isolated from mammals, including swine; zoonotic transmission is suspected.5 The National Health and Nutrition Evaluation Survey (NHANES) reported that the prevalence of IgG antibodies against HEV (anti-HEV IgG) among the general U.S. population aged ≥ 6 years declined from 10.2% during 1988–1994 to 6.0% during 2009–2010; the prevalence for those born in the United States declined from 9.6% to 5.2% over the same period.8
A proportion of individuals with anti-HEV IgG in low-incidence countries may have been infected with HEV while living in or traveling to high-incidence countries. Increased seroprevalence was reported among NHANES participants born outside of the United States.8,9 Studies from Spain and Italy have also found that immigrants from highly endemic countries have a higher prevalence of anti-HEV IgG compared with the general population.10,11 In a case–control study of blood donors from California, donors with anti-HEV IgG were more likely than those without antibodies to report history of travel to highly endemic areas.12
As HEV infection can have severe consequences for travelers who are pregnant or immunocompromised, it is important to identify factors associated with HEV infection. Although a few studies have examined seroconversion to antibodies against HEV during travel, the frequency of seroconversion and the demographic and travel history factors associated with pre- and post-travel HEV antibody status are not well understood.13–15 The Boston Area Travel Medicine Network (BATMN) was a research collaboration of five travel clinics in the greater Boston area that saw approximately 7,500 travelers each year in a variety of settings. From this network, we sought to 1) identify demographic and travel history predictors of pre-travel anti-HEV IgG and 2) determine frequency of seroconversion to anti-HEV IgG during travel.
Methods
Participants and procedures.
Participants were international travelers seeking pre-travel health consultations at BATMN clinics and enrolled in one of two dengue research studies, for whom serum was available for testing for anti-HEV IgG. Both research studies had convenience samples of participants ≥ 2 years old who came to the clinics for preparation for planned travel to dengue-endemic countries for at least 2 weeks. The first study was a dengue seroprevalence study with an enrollment period of August 2008 to June 2009.16 Participants enrolled in the seroprevalance study in November 2008 or later were eligible for testing for anti-HEV IgG. The second was a dengue seroconversion study with an enrollment period of January 2009 to September 2010.17 To be eligible for enrollment in the seroconversion study, participants must have indicated that they would be available to return for follow-up after travel.
Each BATMN clinic obtained institutional review board (IRB) approval for the samples to be tested for dengue and other viruses. The studies were also reviewed by the Centers for Disease Control and Prevention (CDC) Human Subjects Advisors, who approved reliance on Boston University Medical Center's IRB. We obtained written consent from participants ≥ 18 years of age, written assent and parental consent for participants aged 7–17 years, and parental consent for those < 7 years of age.
At the pre-travel consultation, participants were asked about demographic information, travel history, travel plans, medical history, and vaccination history. Blood samples were collected from each participant. At 2–3 months post-travel, participants enrolled in the seroconversion study returned to the clinic to provide repeat blood samples and answer questions regarding their travel. Serum samples were stored at −70°C for future testing.
Hepatitis E antibody testing.
All pre- and post-travel specimens were de-identified and shipped to the Laboratory Branch, Division of Viral Hepatitis, CDC, for anti-HEV IgG testing, which was performed using a commercially available enzyme immunoassay (Diagnostic System Ltd., DSI S.r.l., Saronno, Italy). Samples that were initially reactive were retested in duplicate for confirmation.
Data analysis.
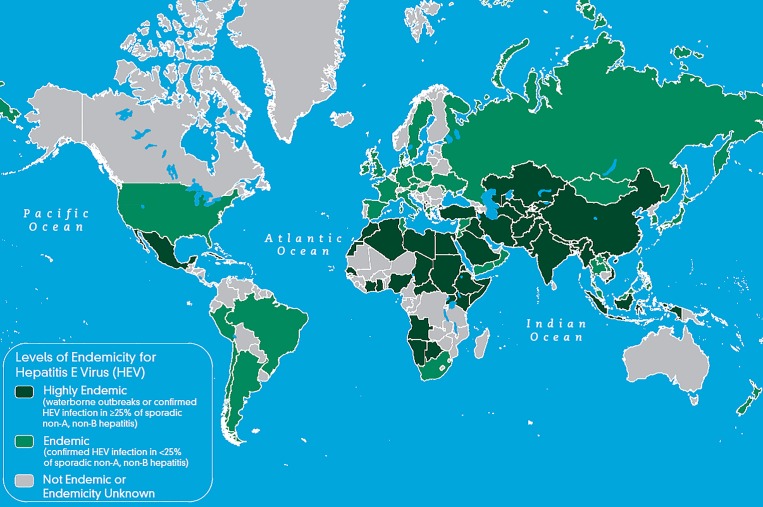
We used published maps that categorize highly endemic countries as those with either reported waterborne outbreaks or confirmed HEV infection in at least 25% of sporadic non-A, non-B hepatitis cases.18 To match the period of data collection, countries were categorized as highly endemic for HEV using 2010 data (Figure 1 ). For analysis, participants were stratified into four groups based on their level of previous time spent in highly endemic countries (Table 1). Group 1 included those born in highly endemic countries. Group 2 included participants who were not born in highly endemic countries, but who had lived in a highly endemic country for at least 1 year. Group 3 included participants who had never lived in a highly endemic country but had a history of travel to at least one highly endemic country prior to the study. Group 4 included participants who had never lived in nor traveled to a highly endemic country.
Figure 1.
Global distribution of hepatitis E virus infection, 2010.18
Table 1.
Demographics of 1,356 pre-travel participants tested for IgG antibodies against hepatitis E virus by the Boston Area Travel Medicine Network from November 2008 to September 2010, stratified by level of previous exposure to countries highly endemic for Hepatitis E
| Total | Group 1: born in a highly endemic country | Group 2: not born in a highly endemic country but lived in one for ≥ 1 year | Group 3: never lived in a highly endemic country but traveled to at least one | Group 4: never lived in or traveled to a highly endemic country | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 152 | 51 | 710> | 443 | 1,356> | ||||||
| Attribute | N | % | N | % | N | % | N | % | N | % |
| Country of birth | ||||||||||
| United States | N/A | N/A | 42 | 82 | 576 | 81 | 340 | 77 | 958 | 71 |
| Other | 152 | 100 | 9 | 18 | 117 | 16 | 98 | 22 | 376 | 28 |
| Missing | 0 | 0 | 0 | 0 | 17 | 2 | 5 | 1 | 22 | 2 |
| Sex | ||||||||||
| Female | 71 | 47 | 23 | 45 | 381 | 54 | 261 | 59 | 736 | 54 |
| Male | 80 | 53 | 28 | 55 | 327 | 46 | 180 | 41 | 615 | 45 |
| Missing | 1 | < 1 | 0 | 0 | 2 | < 1 | 2 | < 1 | 5 | < 1 |
| Race | ||||||||||
| White | 6 | 4 | 42 | 82 | 566 | 80 | 299 | 67 | 913 | 67 |
| Non-white | 140 | 92 | 7 | 14 | 116 | 16 | 128 | 29 | 391 | 29 |
| Missing | 6 | 4 | 2 | 4 | 28 | 4 | 16 | 4 | 52 | 4 |
| Age | ||||||||||
| < 20 | 3 | 2 | 1 | 2 | 17 | 2 | 14 | 3 | 35 | 3 |
| 20–40 | 48 | 32 | 15 | 29 | 209 | 29 | 232 | 52 | 504 | 37 |
| > 40 | 38 | 25 | 15 | 29 | 227 | 32 | 103 | 23 | 383 | 28 |
| Missing | 63 | 41 | 20 | 39 | 257 | 36 | 94 | 21 | 434 | 32 |
N/A = not applicable
Data were entered into a password-protected database (CS Pro, U.S. Census Bureau, Washington, DC). Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). Bivariate and multivariate comparisons between proportions were performed using logistic regression to estimateo prevalence odds ratios (POR) and Wald 95% confidenceintervals (CIs). Hosmer and Lemeshow goodness-of-fit tests were performed on multivariate models. Comparisons between medians were performed using Kruskal–Wallace χ2 tests. A P < 0.05 was considered to be statistically significant.
Results
HEV antibody test results.
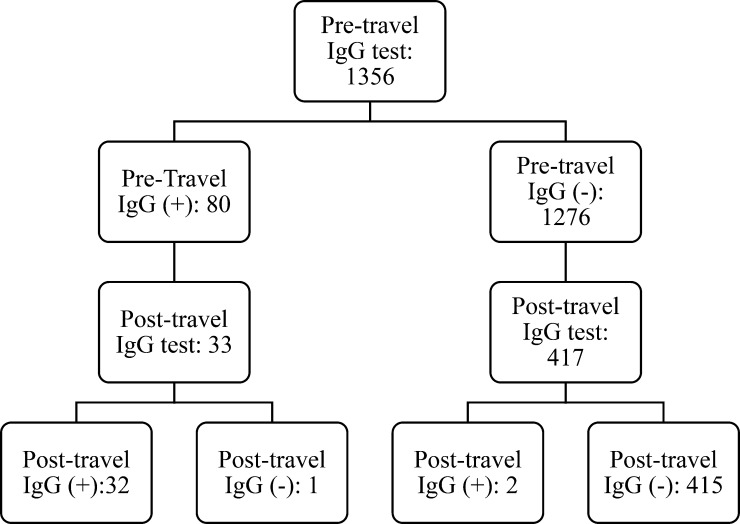
Of the 1,356 participants included in the analysis, 80 (6%) were positive for anti-HEV IgG before travel (Figure 2 ). After returning from travel, 450 (33%) of the 1,356 participants were retested. Of 417 initially negative participants who were retested, two (0.5%) seroconverted to anti-HEV IgG. Of 33 initially positive participants who were retested, anti-HEV IgG became undetectable in one (3%).
Figure 2.
Pre- and post-travel hepatitis E IgG antibody results among 1,356 participants who received pre-travel care at Boston Area Travel Medicine Network clinics during January 2009 to September 2010.
Participant characteristics.
Of 1,356 participants before travel, 958 (71%) were born in the United States, 736 (54%) were female, 913 (67%) were white, and ages ranged from 4 to 83 years (median = 35) (Table 1). There were 152 (11%) participants in Group 1, 51 (4%) in Group 2, 710 (52%) in Group 3, and 443 (33%) in Group 4. Participants in Group 4 had a median age of 30 years and were significantly younger than participants in all other groups: Group 1 (median age = 37, P = 0.001), Group 2 (median age = 39, P = 0.002), and Group 3 (median age = 41, P < 0.0001).
Pre-travel anti-HEV IgG status.
Anti-HEV IgG positivity status varied by group (Table 2). Compared with participants in Group 4, of whom 2% had anti-HEV IgG, the prevalence odds of anti-HEV IgG was 2.6 (95% CI = 1.4–5.0) for participants in Group 3, and 4.8 (95% CI = 2.3–10.3) for participants in Group 1.
Table 2.
Comparison of pre-travel prevalence odds of IgG antibodies against hepatitis E virus (anti-HEV IgG) to exposure history-level grouping among pre-travel participants evaluated by the Boston Area Travel Medicine Network before travel from January 2009 to September 2010
| Hepatitis E exposure level | Anti-HEV IgG (+) pre-travel | Anti-HEV IgG (−) pre-travel | Prevalence odds ratio (95% CI) | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Overall (N = 1,356) | 80 | 6 | 1,276 | 94 | Not applicable |
| Group 1: born in a highly endemic country (N = 152) | 18 | 12 | 134 | 88 | 4.8 (2.3–10.3) |
| Group 2: not born in a highly endemic country but lived in a highly endemic country for at least one year (N = 51) | 2 | 4 | 49 | 96 | 1.5 (0.3–6.7) |
| Group 3: not born in and never lived in a highly endemic country but traveled to a highly endemic country (N = 710) | 48 | 7 | 662 | 93 | 2.6 (1.4–5.0) |
| Group 4: never lived in or traveled to a highly endemic country (N = 443) | 12 | 2 | 431 | 97 | Referent |
CI = confidence interval.
Among participants in Group 1, participants born in Africa had 2.9 times (95% CI = 1.0–8.1) the prevalence odds of anti-HEV IgG pre-travel in comparison with participants born in Asia. Among participants in Group 4, participants older than age 40 had 7.1 times (95% CI = 1.4–35.9) the prevalence odds of anti-HEV IgG compared with participants aged 20–40. Sex and race were not significant predictors of anti-HEV IgG prevalence odds among participants in Group 1, Group 2, or Group 4.
Among participants in Group 3, history of travel to three or more highly endemic countries, in comparison with history of travel to 1–2 highly endemic countries, was associated with a POR of 2.7 (95% CI = 1.2–5.9) of anti-HEV IgG, controlling for region of birth, sex, race, and age (Table 3). Age older than 40 years, compared with age of 20–40 years, was also significantly associated with anti-HEV IgG (adjusted POR = 3.7, 95% CI = 1.3–10.2), after controlling for region of birth, sex, history of travel, and race. On bivariate analysis, white race, compared with non-white race, was associated a POR of 5.0 (95% CI = 1.2–21.1), but this association was not significant in the multivariate analysis.
Table 3.
Characteristics of 710 Boston Area Travel Medicine Network pre-travel participants in Group 3* and seropositivity status for IgG antibodies against hepatitis E (anti-HEV IgG)
| Characteristics | Anti-HEV IgG (+) (N = 48), N (%) | Anti-HEV IgG (−) (N = 662), N (%) | Bivariate prevalence odds ratio (95% CI) | Multivariate prevalence odds ratio (95% CI)† |
|---|---|---|---|---|
| Country of birth | ||||
| United States | 38 (79) | 538 (81) | Referent | Referent |
| Other | 10 (21) | 124 (19) | 1.1 (0.6–2.3) | 0.7 (0.2–2.6) |
| Sex | ||||
| Male | 21 (44) | 306 (46) | Referent | Referent |
| Female | 26 (54) | 355 (54) | 1.1 (0.6–1.9) | 1.2 (0.5–2.5) |
| Number of highly endemic countries traveled to | ||||
| 1–2 countries | 23 (48) | 460 (69) | Referent | Referent |
| Three or more countries | 25 (52) | 202 (31) | 2.5 (1.4–4.5) | 2.7 (1.2–5.9) |
| Race | ||||
| Non-white | 2 (4) | 114 (18) | Referent | Referent |
| White | 45 (94) | 521 (82) | 4.9 (1.2–20.6) | 3.8 (0.5–30.2) |
| Age | ||||
| < 20 years | 0 (0) | 17 (3) | Excluded | Excluded |
| 20–40 years | 5 (10) | 204 (31) | Referent | Referent |
| > 40 years | 24 (50) | 203 (31) | 4.8 (1.8–12.) | 3.7 (1.3–10.2) |
| Missing | 19 (40) | 238 (36) | Excluded | Excluded |
CI = confidence interval.
Group 3 travelers were not born in, and never lived in, a country highly endemic for hepatitis E but traveled to a highly endemic country.
Multivariate analysis included 419 participants; participants with missing values for any variable and/or who were younger than 20 years of age at the time of enrollment were excluded. Hosmer and Lemeshow goodness-of-fit test showed that the model fit well (P = 0.87).
Among participants in Group 3, anti-HEV IgG was also significantly associated with median days spent in highly endemic countries (P = 0.02). These participants who were positive for anti-HEV IgG had a history of travel in highly endemic countries for a median of 38 days (range = 7–385), whereas participants who were negative for anti-HEV IgG had a history of travel in highly endemic countries for a median of 24 days (range = 1–1,710). Among participants in Group 1 and Group 2, there was no association between anti-HEV IgG and time spent in a highly endemic country.
Among participants with history of birth in, travel to, or living in highly endemic countries, prevalence of anti-HEV IgG varied by specific country of exposure (Table 4). Of all highly endemic countries to which at least 20 participants reported prior exposure, prevalence was highest for participants who had been born in, lived in, or traveled to Ethiopia (29%). Prevalence was lowest for participants who had been born in, lived in, or traveled to Nepal (5%) or Indonesia (4%).
Table 4.
Prevalence of hepatitis E IgG antibody (anti-HEV IgG) among pre-travel participants who were born in, lived in, or traveled to individual highly endemic countries*
| Country | Total | No. of anti-HEV IgG (+) | % Anti-HEV IgG (+) |
|---|---|---|---|
| Ethiopia | 28 | 8 | 29 |
| Namibia | 24 | 4 | 17 |
| Turkey | 63 | 10 | 16 |
| Cuba | 21 | 3 | 14 |
| Egypt | 84 | 11 | 13 |
| Burma | 25 | 3 | 12 |
| Uganda | 26 | 3 | 12 |
| Botswana | 35 | 4 | 11 |
| Morocco | 54 | 6 | 11 |
| Ghana | 51 | 5 | 10 |
| Nigeria | 31 | 3 | 10 |
| India | 223 | 19 | 9 |
| China | 208 | 14 | 7 |
| Kenya | 104 | 7 | 7 |
| Vietnam | 100 | 6 | 6 |
| Mexico | 370 | 21 | 6 |
| Nepal | 37 | 2 | 5 |
| Indonesia | 50 | 2 | 4 |
Results limited to countries to which ≥ 20 participants reported history of travel. Participants were evaluated by the Boston Area Travel Medicine Network from January 2009 to September 2010.
Among participants in Group 4, history of travel to specific non-highly endemic countries was associated with higher prevalence of anti-HEV IgG. Of all countries to which at least 20 participants in Group 4 reported prior travel, prevalence was highest for participants who had been to Peru (10%) and Costa Rica (6%).
Seroconversion to anti-HEV IgG among travelers.
Of the 417 participants with pre-travel negative anti-HEV IgG results who were retested after travel, 221 (53%) reported recent travel to at least one highly endemic country. Two participants, both of whom reported recent travel to at least one highly endemic country, were positive for anti-HEV IgG after travel. This represents 1% of all initially negative participants retested after returning from a highly endemic country. No participants reporting travel to countries that were not highly endemic seroconverted to anti-HEV IgG.
The first participant who likely seroconverted while traveling was an Indian-born 41-year-old female who reported travel to India and Nepal, where she stayed in hotels and private homes. The second participant who likely seroconverted while traveling was a white U.S.-born 60-year-old female who reported travel to China, where she visited rural areas, had contact with animals, and stayed at hotels and hostels. Neither of these participants reported symptoms during or after travel.
Discussion
The prevalence of anti-HEV IgG among Boston area individuals seeking pre-travel consultations at travel clinics was 6%, which is identical to the seroprevalence in the general U.S. population found by the 2009–2010 NHANES survey.8,9 Among our study population, only 2.5% of participants who had never lived in or traveled to a highly endemic country had anti-HEV IgG. Therefore, it is likely that some participants were exposed to HEV while living or traveling outside of the United States.
Factors associated with anti-HEV IgG positivity among participants with history of travel to but not residence in highly endemic countries correspond to factors associated with anti-HEV IgG positivity in the 2009–2010 NHANES.8,9 Among NHANES participants, seroprevalence of anti-HEV IgG was associated with older age. Similarly, our study found that age > 40 was associated with increased seroprevalence. This could reflect a cohort effect, implying that incidence of HEV infection was higher in the past.9 It could also reflect a cumulative effect of exposures over time. White race was also associated with increased seroprevalence of anti-HEV IgG in both our bivariate analysis and NHANES. The reasons for this are less clear. Other variables collected by NHANES suggest that affluence may be associated with increased anti-HEV IgG seroprevalence among the U.S. population.8 However, we did not assess affluence in our study population.
Our study found a low incidence of seroconversion to anti-HEV IgG and no symptomatic HEV infection, which is consistent with the literature. Of three existing prospective seroconversion studies of travelers, two reported no seroconversion among more than 1,100 Dutch travelers and 84 young Israeli travelers, respectively.13,14 The third reported four asymptomatic HEV infections among 211 U.S. travelers.15 Discrepancies between study results may be explained by both laboratory methods used and epidemiology of HEV infection in the study populations. Although symptomatic HEV infection in travelers has not been found in any existing prospective studies, sporadic cases of symptomatic HEV infections following international travel have been described in four U.S. travelers and two travelers from the United Kingdom.19,20 A study of 154 U.S. patients with clinical samples negative for hepatitis A and B IgM found that 26, including 11 with a history of recent travel, tested positive for anti-HEV IgM.21
The results of this study are subject to a few limitations. The map provided in Figure 1 categorizes a number of countries as “not endemic or endemicity unknown.” Because HEV is present in much of the world, it is likely that most of these countries have varying levels of HEV endemicity but data about HEV incidence or outbreaks are not yet available.3 We did not include countries in this category as highly endemic countries. This may have led to an underestimate of the effect of previous travel to, or birth in, a highly endemic country. Also, a substantial proportion of participants were missing values for age. This limited our ability to draw conclusions about the effect of age and led to a limited number of participants included in the multivariate analysis. Further, the investigation was limited to travelers to dengue-endemic countries, which restricted our understanding about travelers to other areas. Because the participants in our study were individuals seeking pre-travel care at travel clinics in the Boston area, they do not represent the general traveling population of the United States.
In conclusion, previous time spent in highly endemic countries, as well as other demographic factors, affects seroprevalence of hepatitis E among travelers. Our results indicate a need for better data about HEV transmission and epidemiology in common travel destinations for which HEV endemicity is unknown. In our study, anti-HEV IgG seroconversion during travel was rare but present. Although we did not have any symptomatic infections in our study population, HEV infection can cause serious illness and is especially dangerous for high-risk groups such as pregnant women and immunocompromised travelers. It is important to address the risk of acquiring HEV infection during travel and emphasize preventative measures, such as hand hygiene and safe food and water practices, during pre-travel consultations, particularly with travelers who are pregnant or immunocompromised. In addition, HEV infection should be considered in the differential diagnosis for any returning traveler with signs and symptoms of viral hepatitis.
ACKNOWLEDGMENTS
We would like to acknowledge the following individuals and groups: Boston Area Travel Medicine Network clinics, study participants, Mary Wilson, Lin Chen, Adolf Karchmer, Winnie Ooi, Laura Kogelman, Christine Benoit, Manveen Bhussar, Rebecca Durfur, Deborah Gannon, Meghan Geary, Erika Gelva, Allison Kay, Natasha Soodoo, Millie Sosa, Hari Iyer, Racquel Wells, Maria del Milgaro Sosa, and Manuela Beltran.
Footnotes
Authors' addresses: Kira A. Barbre and Emily S. Jentes, Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA, E-mails: kirabarbre@gmail.com and ejentes@cdc.gov. Jan Drobeniuc, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), Atlanta, GA, E-mail: jqd6@cdc.gov. Saleem Kamili, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: sek6@cdc.gov. Davidson H. Hamer, Center for International Health and Development, Boston University School of Public Health, Boston, MA, E-mail: dhamer@bu.edu. Elizabeth D. Barnett, Department of Pediatrics, Boston Medical Center, Boston, MA, E-mail: elizabeth.barnett@bmc.org.
References
- 1.Navaneethan U, Mohajer MA, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 2008;28:1190–1199. doi: 10.1111/j.1478-3231.2008.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamar N, Selves J, Mansuy JM, Ouezzani L, Peron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Hepatitis E. 2015. http://www.who.int/mediacentre/factsheets/fs280/en/ Available at. Accessed August 31, 2016.
- 4.Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 5.Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16:5–36. doi: 10.1002/rmv.482. [DOI] [PubMed] [Google Scholar]
- 6.Khuroo MS, Khuroo MS. Hepatitis E: an emerging global disease—from discovery towards control and cure. J Viral Hepat. 2016;23:68–79. doi: 10.1111/jvh.12445. [DOI] [PubMed] [Google Scholar]
- 7.Kmush BL, Nelson KE, Labrique AB. Risk factors for hepatitis E virus infection and disease. Expert Rev Anti-infe. 2015;13:41–53. doi: 10.1586/14787210.2015.981158. [DOI] [PubMed] [Google Scholar]
- 8.Teshale EH, Denniston MM, Drobeniuc J, Kamili S, Teo CG, Holmberg SD. Decline in hepatitis E virus antibody prevalence in the United States from 1988–1994 to 2009–2010. J Infect Dis. 2015;211:366–373. doi: 10.1093/infdis/jiu466. [DOI] [PubMed] [Google Scholar]
- 9.Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988–1994. J Infect Dis. 2009;200:48–56. doi: 10.1086/599319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarrago D, Lopez-Velez R, Turrientes C, Baquero F, Mateos ML. Prevalence of hepatitis E antibodies in immigrants from developing countries. Eur J Clin Microbiol Infect Dis. 2000;19:309–311. doi: 10.1007/s100960050482. [DOI] [PubMed] [Google Scholar]
- 11.Scotto G, Martinelli D, Giammario A, Prato R, Fazio V. Prevalence of antibodies to hepatitis E virus in immigrants: a seroepidemiological survey in the district of Foggia (Apulia-southern Italy) J Med Virol. 2013;85:261–265. doi: 10.1002/jmv.23400. [DOI] [PubMed] [Google Scholar]
- 12.Mast EE, Kuramoto IK, Favorov MO, Schoening VR, Burkholder BT, Shapiro CN, Holland PV. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in northern California. J Infect Dis. 1997;176:34–40. doi: 10.1086/514037. [DOI] [PubMed] [Google Scholar]
- 13.Elfrink F, van Rijekevorsal GGC, van Gool T, van den Hoek A, Sonder GJB. Low risk of hepatitis E among Dutch short-term travelers. J Travel Med. 2012;19:202–204. doi: 10.1111/j.1708-8305.2012.00597.x. [DOI] [PubMed] [Google Scholar]
- 14.Potasman I, Koren L, Peterman M, Srugo I. Lack of hepatitis E infection among backpackers to tropical countries. J Travel Med. 2000;7:208–210. doi: 10.2310/7060.2000.00062. [DOI] [PubMed] [Google Scholar]
- 15.Ooi WW, Gawoski JM, Yarbough PO, Pankey GA. Hepatitis E seroconversion in United States travelers abroad. Am J Trop Med Hyg. 1999;61:822–824. doi: 10.4269/ajtmh.1999.61.822. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Vegas C, Hamer DH, Chen LH, Wilson ME, Benoit C, Hunsperger E, MacLeod WB, Jentes ES, Ooi WW, Karchmer AW, Kogelman L, Yanni E, Marano N, Barnett ED. Prevalence of dengue virus infection in United States travelers who have lived in or traveled to dengue-endemic countries. J Travel Med. 2013;20:352–360. doi: 10.1111/jtm.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivero RM, Hamer DH, MacLeod WB, Benoit CM, Sanchez-Vegas C, Jentes ES, Chen LH, Wilson ME, Marano N, Yanni EA, Ooi WW, Karchmer AW, Kogelman L, Barnett ED. Dengue virus seroconversion in travelers to dengue-endemic areas. Am J Trop Med Hyg. 2016;95:1130–1136. doi: 10.4269/ajtmh.16-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teo CG. Hepatitis E. In: Brunette GW, Kozarsky PM, Magill AJ, Shlim DR, Whatley A, editors. CDC Health Information for International Travel. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 19.Centers for Disease Control and Prevention Hepatitis E among U.S. travelers, 1989–1992. Morb Mortal Wkly Rep. 1993;42:1–4. [PubMed] [Google Scholar]
- 20.Skidmore SJ, Yarbough PO, Gabor KA, Tam AW, Reyes GR, Flower AJE. Imported hepatitis E in UK. Lancet. 1991;8756:1541. doi: 10.1016/0140-6736(91)93227-z. [DOI] [PubMed] [Google Scholar]
- 21.Drobeniuc J, Greene-Montfort T, Lee NT, Mixson-Hayden TR, Ganova-Raeva L, Dong C, Novak RT, Sharapov UM, Tohme RA, Teshale E, Kamili S, Teo CG. Laboratory-based surveillance for hepatitis E infection, United States, 2005–2012. Emerg Infect Dis. 2013;19:218–222. doi: 10.3201/eid1902.120961. [DOI] [PMC free article] [PubMed] [Google Scholar]