Abstract
The overwhelming majority of evidence about the health of children in low- and middle-income countries is based on reports by parents. There is limited evidence on whether these reports suffer from systematic bias, particularly related to the gender of the child. We investigate differences in symptom reporting by child gender in a sample of countries in sub-Saharan Africa. Data from 35 Demographic and Health Surveys and 10 Malaria Indicator Surveys conducted since 2005 were analyzed. Parental reports of child symptoms were compared for girls and boys. In a subsample of data from Nigeria, we also compared the accuracy of parental reports of fever between girls and boys. Then, potential explanations for observed reporting differences were explored. Finally, country-level relationships between gender differences in symptom reporting and differences in child health outcomes were estimated. Parents reported fewer episodes of fever and diarrhea for girls as compared with boys. Less frequent symptom reporting for girls does not appear to be due to reduced exposure to illness-causing agents nor increased treatment seeking. Lower fever reporting for girls relative to boys is associated with higher relative infant mortality for girls at the country level, consistent with a potential link between underreporting and health outcomes. From a measurement perspective, estimates of gender imbalances in child morbidity and treatment based on parental reports may be inaccurate. From a public health perspective, parental underreporting of symptoms in girls may indicate untreated illness that goes unnoticed.
Introduction
Questions about the accuracy of self-reported health and morbidity information have increased in recent years. Sen,1 echoing an established anthropology literature,2,3 has argued that individuals' perceptions of their own health are heavily mediated by their social environment. As a result, relying on self-reports to inform population-level health policies is problematic. Furthermore, parental reports of child health and morbidity may be even more fraught with inaccuracy than adults own self-reports. Not only must parents observe illness in children to report accurately, the complexities of parent–child relationships and the related social context have the potential to distort the interpretation of observations. Although Subramanian and others4 find that socioeconomic gradients in adult self-reported health in India have face validity and are therefore useful (if not entirely accurate), Manesh and others5 find that socioeconomic gradients in parental reports of child morbidity in Iran strongly contradict expected patterns.
The current understanding of gender gradients in child morbidity in developing countries relies heavily on parental reports collected as part of national surveys like the Demographic and Health Surveys (DHS). For example, a recent United Nations Children's Emergency Fund report on the state of diarrheal disease and pneumonia worldwide presents prevalences for boys and girls based on these data.6 However, the relationship between child gender and parental reporting of illness remains unclear, raising questions about the validity of using reporting data to estimate gender gradients in child illness. In one of the few papers to investigate this relationship, Hill and Upchurch analyze a large cross-national dataset collected as part of the DHS, and find that parents reported symptoms of diarrhea and acute respiratory infection less in girls than they did in boys.7 The authors suggest the possibility that, “the lower morbidity rates among girls result from reporting differentials by sex of the child: that mothers are less likely to report girls as sick, even given equivalent symptom.”
We update and expand on the analysis presented in Hill and Upchurch, with a focus on further analysis of gender differences in reporting of child illness symptoms. If parents are indeed less likely to report girls sick even when they have the same symptoms as boys, we should expect this to have important implications not only for our understanding and measurement of child health, but for the health of children themselves. Specifically, it is unlikely that children receive timely treatment of illnesses that their parents do not recognize and report. The majority of child deaths are avoidable with timely access to treatment,8,9 and delays in treatment can have severe detrimental effects on child physical and cognitive development.10 Hill and Upchurch observe a substantial amount of excess under-five mortality among girls as compared with boys, mitigating the well-documented biological survival advantage for girls.11,12
We first document gender differences in symptom reporting in a sample of sub-Saharan African countries, using data from recent DHS. We then attempt to understand whether these differences can be explained by differences in exposure status or treatment-seeking behavior. Finally, we look at the relationship between gender differences in symptom reporting and differences in key child health outcomes, including physical development and mortality.
Materials and Methods
Data.
The main source of data for this analysis is the DHS. The DHS asks parents to report on symptoms of child illness, as well as treatment sought for reported symptoms.13 We restrict our analysis to countries in sub-Saharan Africa, as data on fever are regularly collected as part of DHS interviews in these countries, but are not regularly collected in other regions. We also use data from several Malaria Indicator Surveys (MIS) with a particular focus on fever reporting and its relationship to malaria infection.14 In a subanalysis, we restrict our sample to MIS data collected in Nigeria in 2010, as this survey was unique in measuring children's body temperatures at the time of the interview, and therefore allows us to analyze whether parents' perceptions of child fever are objectively accurate.15 Finally, we use estimates of infant and under-five mortality derived from the same DHS datasets used to measure child symptom reporting. We restrict all analyses to data collected since 2005, around the time of the first MIS.
Variables.
Parental reports of child illness symptoms are key variables in this analysis. For each child under the age of five residing in a DHS household at the time of the interview, parents are asked whether the child had each of the three symptoms in the preceding 2 weeks: fever, diarrhea, and cough. The same data on child fever (but not diarrhea or cough) are collected as part of MIS interviews. Parents that report symptoms are then asked if they sought treatment of the child. As part of the MIS, a random subsample of children under the age of 5 years is tested for malaria parasitemia using a rapid diagnostic test. As part of the 2010 Nigeria MIS, study interviewers measured axillary body temperature for children 6–59 months of age during the household interview; this was the only MIS where temperature measurements were taken. Finally, gender-specific infant mortality rate (IMR) and under-five mortality rate (U5MR) were collected from the World Health Organization Global Health Observatory, and were estimated based on parental reports of child death.
Analysis.
We present a series of graphs showing gender differences in child illness symptom reporting. For each symptom, we plot the country-level probability of reporting for boys on the x-axis and the probability of reporting for girls on the y-axis. For each figure, the solid 45-degree line describes the points at which reporting for boys and girls are equal. All observations below the line indicate lower reporting for girls as compared with boys. The same relationship between gender and symptom reporting that is presented in the figures is then formally estimated in a series of logistic regression models. Observations from the full sample of DHS and MIS surveys were included in these models. Additional models were fit to investigate the relationship between gender and cough reporting within child age strata. Adjusted models included child's age and cluster fixed-effects as control variables. Standard errors in all models were adjusted to account for clustering at the level of the country and survey.
Next, we investigate potential explanations for observed gender differences in symptom reporting. We first examine whether differences in treatment seeking can explain our results. In particular, we might expect parents of girls to report fewer symptoms if girls are treated more quickly for the symptoms they have. In the full DHS sample, we estimate gender differences in the probability of a child receiving treatment of fever/cough (the DHS asks about treatment of these two symptoms together) and diarrhea by fitting unadjusted and adjusted logistic regression models for each, clustering standard errors at the level of the country and survey. Adjusted models include controls for child's age and cluster fixed-effects. Then, we look at whether differential exposure to infections can explain gender differences in symptom reporting. We might expect girls to have a lower probability of fever at the interview if they have lower exposure to fever-causing agents such as malaria parasites. We investigate the relationship between gender and malaria parasitemia, and how this relationship influences fever reporting, using MIS data. Finally, we investigate the relationship between actual child body temperature and fever reporting using data from the 2010 Nigeria MIS.
We conclude by estimating the relationships between gender differences in symptom reporting and differences in three child health and development outcomes: height-for-age z-score (HAZ), IMR, and U5MR.
Results
Study population.
Data from 223,758 children under 5 years of age, collected as part of 35 DHS in 27 sub-Saharan African countries, were included in the main analysis. In addition, data from 52,202 children collected as part of 10 MIS in eight sub-Saharan African countries were included in the analysis of malaria parasitemia (see Supplemental Table 1 for a summary of all included datasets). In Table 1, we describe study children and households from each cross-national dataset.
Table 1.
Characteristics of the study population
| DHS dataset (N = 223,758) | MIS dataset (N = 52,202) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Child characteristics | ||||
| Female | 0.495 | (0.500) | 0.497 | (0.500) |
| Age (months) | 27.834 | (17.250) | 29.982 | (16.786) |
| Child health | ||||
| Probability of reported fever | 0.200 | (0.200) | 0.337 | (0.473) |
| Probability of reported diarrhea | 0.139 | (0.346) | – | – |
| Probability of reported cough | 0.194 | (0.396) | – | – |
| Probability of parasitemia | – | – | 0.260 | (0.438) |
| Parent characteristics | ||||
| Age (years) | 29.092 | (6.897) | 28.626 | (7.056) |
| Education (years) | 4.299 | (3.715) | 4.612 | (7.413) |
| Household characteristics | ||||
| Rural | 0.704 | (0.456) | 0.735 | (0.441) |
| Residents | 7.145 | (3.827) | 8.589 | (6.170) |
DHS = Demographic and Health Survey; MIS = Malaria Indicator Survey; SD = standard deviation. Diarrhea and cough not reported as part of MIS.
Children were on average around 28 (DHS) and 30 months (MIS) of age, with a relatively uniform distribution across the under-five age range. In the full DHS dataset, 20% of children were reported by their parent to have had a fever in the previous 2 weeks; the probability of reported fever was higher in the MIS dataset (34%), likely due to the fact that countries where the MIS is administered usually have higher rates of malaria endemicity. In the MIS dataset, malaria parasitemia was found in 26% of children. In the DHS dataset, 40% of children reported to have fever or cough and 41% reported to have diarrhea in the previous 2 weeks were taken for treatment. In both datasets, parents were on average around 29 years of age, and study households were predominantly located in rural settings.
Gender differences in symptom reporting.
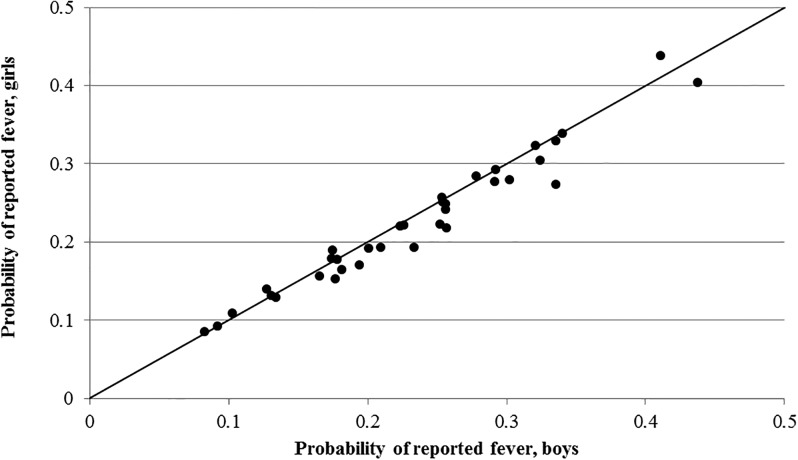
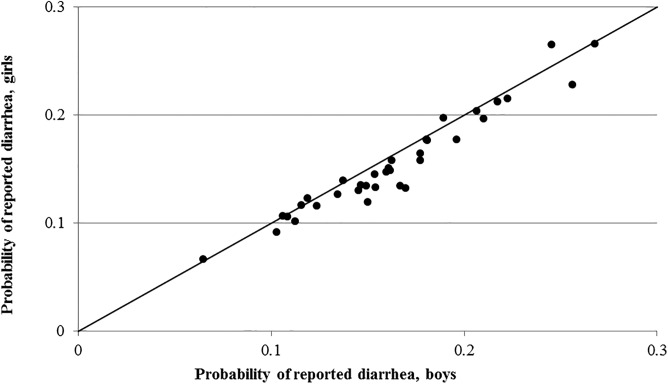
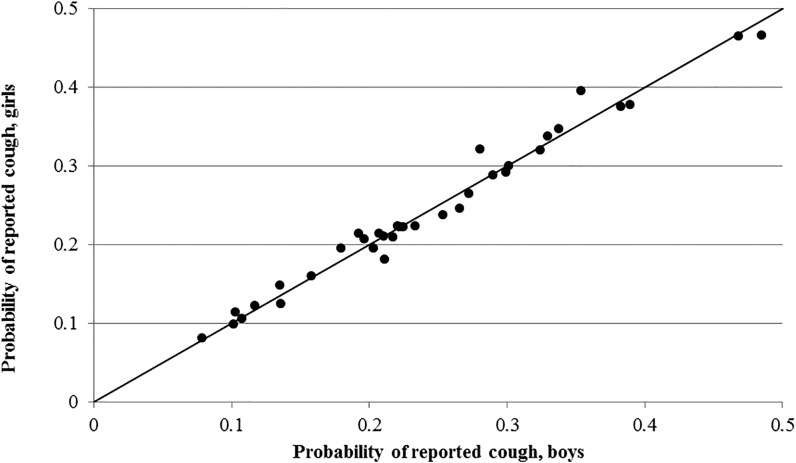
Overall, both fever (Figure 1 ) and diarrhea (Figure 2 ) are reported less often for girls consistently across the DHS datasets. Indeed, fever and diarrhea reporting was lower for girls in 66% and 80% of surveys, respectively. For cough (Figure 3 ), there does not appear to be a relationship between gender and reporting.
Figure 1.
Country-level probability of reported fever for boys and girls.
Figure 2.
Country-level probability of reported diarrhea for boys and girls.
Figure 3.
Country-level probability of reported cough for boys and girls.
In adjusted models, we find that fever (odds ratio [OR] = 0.949, 95% confidence interval [CI] = 0.931–0.967; P < 0.001) and diarrhea (OR = 0.929, 95% CI = 0.907–0.950; P < 0.001) reporting was significantly lower for girls than for boys (Table 2). There was not a significant gender difference in reporting of cough when pooling children of all ages together (OR = 1.002, 95% CI = 0.983–1.020; P = 0.870). Among infants 0–5 months of age (OR = 0.914, 95% CI = 0.858–0.975; P = 0.006) and 6–11 months of age (OR = 0.962, 95% CI = 0.911–1.016; P = 0.161), the odds of cough reporting for girls was lower than for boys. This relationship was not found postinfancy (OR = 1.018, 95% CI = 0.996–1.040; P = 0.105).
Table 2.
Relationship between child gender and symptom reporting
| Fever | Diarrhea | Cough | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All ages | 0–5 months | 6–11 months | 12+ months | ||||||
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Female | 0.951 (0.929–0.973) | 0.949 (0.931–0.967) | 0.933 (0.909–0.958) | 0.929 (0.907–0.950) | 1.007 (0.984–1.030) | 1.002 (0.983–1.020) | 0.914 (0.858–0.975) | 0.962 (0.911–1.016) | 1.018 (0.996–1.040) |
| Constant | 0.257 (0.213–0.309) | 0.337 (0.329–0.345) | 0.166 (0.144–0.192) | 0.281 (0.274–0.288) | 0.241 (0.189–0.307) | 0.348 (0.339–0.356) | 0.105 (0.097–0.114) | 0.318 (0.276–0.367) | 0.433 (0.420–0.448) |
| Observations | 223,758 | 223,758 | 223,707 | 223,707 | 223,793 | 223,793 | 25,610 | 25,693 | 172,490 |
| Clusters | 15,874 | 15,874 | 15,874 | 15,874 | 15,874 | 15,874 | 11,380 | 11,398 | 15,811 |
| Surveys | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
CI = confidence interval; OR = odds ratio. Standard errors clustered within survey. Given in parentheses are 95% CIs. Adjusted models include as controls: child's age (months) and cluster fixed-effects.
Child gender and treatment seeking.
One potential explanation for the observed gender differences in fever and diarrhea reporting could be that girls are actually less likely to have those symptoms at a given point in time as a result of quicker treatment. If girls are indeed treated more quickly than boys, we should see a higher probability of treatment of girls conditional on reported symptoms, as the most recent symptoms for girls should be more likely to have received treatment. We find the opposite relationship (Table 3), with parents of girls rather less likely to seek care for reported fever/cough (OR = 0.947, 95% CI = 0.919–0.976; P < 0.001) and diarrhea (OR = 0.964, 95% CI = 0.923–1.007; P = 0.101), suggesting that treatment differences do not explain the reporting differences observed in Table 2.
Table 3.
Relationship between child gender and treatment of reported symptoms
| Fever/cough treatment | Diarrhea treatment | |||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Female | 0.947 (0.914–0.982) | 0.947 (0.919–0.976) | 0.967 (0.928–1.009) | 0.964 (0.923–1.007) |
| Constant | 0.690 (0.571–0.833) | 0.867 (0.838–0.898) | 0.634 (0.512–0.784) | 0.736 (0.702–0.772) |
| Observations | 63,309 | 63,309 | 31,003 | 31,003 |
| Clusters | 13,923 | 13,923 | 11,191 | 11,191 |
| Surveys | 35 | 35 | 35 | 35 |
CI = confidence interval; OR = odds ratio. Standard errors clustered within survey. Given in parentheses are 95% CIs. Adjusted models include as controls: child's age (months) and cluster fixed-effects.
Malaria exposure and fever reporting.
A second potential explanation for gender differences in symptom reporting could be that girls have lower exposure to infection-causing agents. In this section, we look at the relationship between child gender and exposure to one important cause of fever: the malaria parasite. We use data on malaria parasitemia included in nine of the 35 DHS datasets, as well as 10 additional MIS datasets.
In Table 4, we present estimates from three logistic regression models. In Model 1, we find that girls are nearly as likely as boys to be infected with the malaria parasite at the time of the interview (OR = 0.988, 95% CI = 0.965–1.012; P = 0.328). Furthermore, controlling for malaria parasitemia has minimal effect on the relationship between gender and fever reporting.
Table 4.
Relationship between gender, fever reporting, and malaria parasitemia
| Malaria parasitemia | Fever reporting | ||
|---|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Female | 0.988 (0.965–1.012) | 0.963 (0.934–0.992) | 0.964 (0.935–0.994) |
| Malaria parasitemia | 1.859 (1.782–1.939) | ||
| Constant | 0.238 (0.228–0.250) | 0.493 (0.476–0.511) | 0.437 (0.422–0.454) |
| Observations | 88,294 | 88,174 | 88,174 |
| Clusters | 7,939 | 7,939 | 7,939 |
| Surveys | 23 | 23 | 23 |
CI = confidence interval; OR = odds ratio. Standard errors clustered within survey. Given in parentheses are 95% CIs. All models include as controls: child's age (months) and cluster fixed-effects.
Child body temperature and fever reporting in Nigeria.
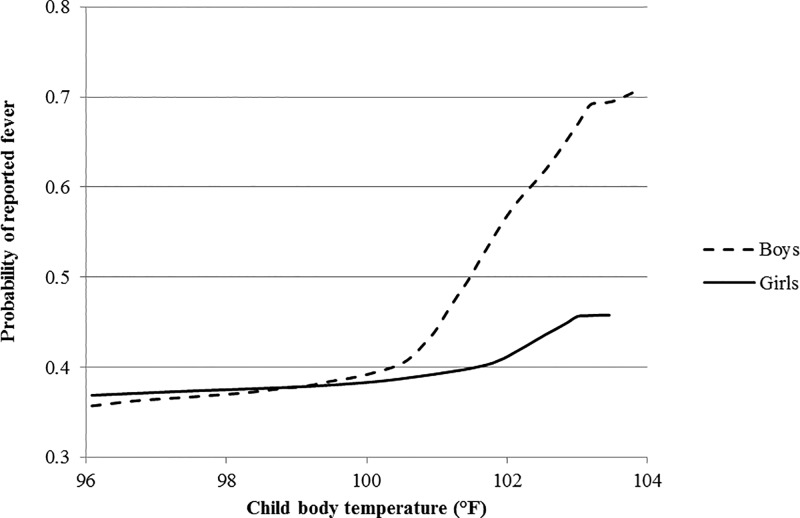
The 2010 Nigeria MIS was unique in that in addition to asking parents to report on child fevers, body temperatures of children were also measured at the time of the interview. This allows us to compare parental reports to objective measures of fever. In Figure 4 , we present data on the relationship between child body temperature and the probability of reported fever for girls and boys, using a local polynomial smoothing function. Reports were in reference to the previous 2 weeks, explaining the relatively high probability of reported fever (37%) for children with body temperatures below 98.5°F at the time of measurement (these children may have recovered from earlier fevers by the time of the interview). More relevant to this study is the fact that for children with fevers at the time of measurement (usually defined as temperatures above 99.5°F), the probability of parents reporting fever was consistently higher for boys than for girls. However, in the Nigeria sample fever reporting did not differ by gender for children without fevers at the time of measurement.
Figure 4.
Child gender, body temperature, and fever reporting in Nigeria.
Symptoms reporting and health outcomes.
The evidence that we have presented thus far suggests that lower reporting of symptoms for girls as compared with boys does not reflect underlying differences in the prevalence of actual symptoms. Underreporting of symptoms for girls may have important health consequences if parents do not seek treatment of symptoms that they do not report. We examined the relationship between gender differences in symptom reporting and differences in child health outcomes (Table 5). For this analysis, we use the full DHS dataset. We focus on three outcomes: mean HAZ, IMR, and U5MR.
Table 5.
Relationship between gender difference in symptom reporting and difference in health
| Gender difference in mean HAZ | Gender difference in IMR | Gender difference in U5MR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | |
| Gender difference in fever reporting | −0.039 (−1.035 to 0.956) | −115.835 (−204.199 to −27.472) | −124.553 (−246.784 to −2.323) | ||||||
| Gender difference in diarrhea reporting | 0.327 (−1.557 to 2.212) | 11.058 (−219.090 to 241.206) | −81.922 (−343.830 to 179.987) | ||||||
| Gender difference in cough reporting | 0.721 (−0.865 to 2.307) | −123.594 (−325.439 to 78.252) | −136.560 (−349.507 to 76.387) | ||||||
| Constant | 0.183 (0.160 to 0.205) | 0.186 (0.156 to 0.217) | 0.183 (0.164 to 0.202) | −14.392 (−17.318 to −11.465) | −13.148 (−16.531 to −9.766) | −13.233 (−15.558 to −10.907) | −16.877 (−20.639 to −13.115) | −16.463 (−20.330 to −12.596) | −15.630 (−18.542 to −12.719) |
| Observations | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
HAZ = height-for-age z-score; IMR = infant mortality rate; U5MR = under-five mortality rate. All gender difference variables are constructed: girls minus boys. Standard errors clustered within survey. Given in parentheses are 95% confidence intervals.
We find a strong and significant relationship between gender differences in fever reporting and differences in IMR (β = −115.8, 95% CI = −204.2 to −27.5; P = 0.012), that is, the stronger the gender difference in reporting, the weaker the survival advantage of infant girls in a country. We also observe a relationship between differences in fever reporting and differences in U5MR (β = −124.6, 95% CI = −246.8 to −2.3; P = 0.046), but the point estimate is similar to that found for IMR, suggesting that the relationship of interest occurs primarily in the 1st year of life. A plot of the country-level relationship between gender differences in fever reporting and differences in IMR is presented in Supplemental Figure 1.
Discussion
We investigated the relationship between child gender and parental reporting of child illness symptoms in several recent national surveys conducted in sub-Saharan Africa. Our analysis produced three main findings. First, consistent with Hill and Upchurch, we found lower reporting of fever and diarrhea for girls as compared with boys.7 We also find lower reporting of cough for infant girls, though the gender difference in cough reporting is not present in the postinfancy period. Although our data cannot explain why gender differences in reporting do not persist for cough symptoms for older children, future surveys and qualitative interviews might help to elucidate differences in reporting patterns across different illness symptoms. Second, our analysis provides suggestive evidence that parents report symptoms differently for boy and girl children, even when there are no differences in underlying illness. We found evidence against explanations that reporting differences were due to differences in treatment or exposure to illness-causing agents. Furthermore, we present evidence from Nigeria showing that for children with measured fever, parents were substantially less likely to report fever for girls as compared with boys. Third, we show that gender differences in fever reporting are significantly correlated with gender differences in IMR at the country level. It is well documented that boys have higher infant mortality than girls in many settings, including sub-Saharan Africa.16 Although the exact mechanisms for this phenomenon are not fully known, most research points to a biological explanation.17 We find that the survival advantage for girls is smaller in countries where girls are reported to have fewer fevers than boys, the opposite of what would be expected if girls were indeed healthier than boys.
One potential explanation for differential symptom reporting by gender is that parents often have a difficult time recognizing illness in children, and gender-related factors may exacerbate the challenge of recognizing symptoms.18 Parents may not closely monitor physical symptoms, which may be the only means of detecting illness in young preverbal children. If parents have closer or more frequent physical interactions with boys than with girls, for example, as a result of greater breastfeeding,19,20 this might lead to better recognition of illness in boys. In some cases symptoms may very subtle, especially in infants21; symptoms may be more obvious or salient in boys as compared with girls, though there is no clear evidence of this in the biomedical literature. Finally, parents and verbal children may not effectively communicate about illness. If parents communicate more effectively with boys than with girls, we might expect greater awareness of illness in boys. Even when parents are able to gather good information on illness in their children, they may interpret this information differently for girls and boys through pathways strongly mediated by local culture. In Nigeria, we find that lower fever reporting for girls is limited to children with current fevers, which may suggest that parents are slower to recognize fevers in girls, an explanation that is consistent with the rest of the data that we present. We also cannot rule out the possibility that there is a biological mechanism that would lead to girls recovering more quickly from symptoms than boys.
Alternatively, gender differences in symptom reporting could reflect a more general form of gender inequality in society. There is increasing recognition of gender as a social determinant of health, and our results contribute to the evidence base on this important issue.22 Previous literature has shown that in many low-resource settings parents invest less in girls.23 There is evidence of boys being breastfed for longer durations,19,20 being more likely to receive vaccinations and needed healthcare,24 receiving better nutrition,25 and attending school earlier and for longer periods.26 These differential investments early in life can in some cases lead to excess mortality among girls relative to boys and contribute to the well-documented “missing women” phenomenon.27 Flatø and Kotsadam28 find that infant mortality in sub-Saharan Africa increases more for girls than for boys during a drought; they further explain that at least part of this difference is due to gender discrimination, since the effect is larger in communities more likely to discriminate. In the same vein, Friedman and Schady29 find that in Africa, girls are more exposed than boys to mortality risk in case of aggregate economic shocks. Furthermore, underinvestment in the health and nutrition of girls leads to deficits in physical and cognitive development, thereby limiting opportunities for women into adulthood and sustaining the social roots of gender discrimination.30 Although the evidence we present is consistent with this explanation, more qualitative and behavioral research is needed to document links between gender discrimination and recognition of child illness symptoms.
Although we find that gender differences in fever reporting are correlated with differences in infant mortality, the same is not true of differences in diarrhea reporting. Recognition of fever by parents is arguably more subjective than recognition of diarrhea,31,32 and as a result, the mechanisms through which the observed gender differences manifest for these two symptoms may be quite different. We were unable to explore gender differences in exposure to diarrhea-causing agents, and it may very well be that girls have lower rates of actual diarrhea. One of the few studies to estimate population prevalence of childhood diarrhea found that girls in Matlab, Bangladesh, had slightly fewer annual episodes than boys, though these differences were not statistically significant.33
There were several important limitations to this analysis. First, our data do not provide direct evidence for why parents report symptoms differentially for girls and boys, so we must rely on stylized facts and corroborative evidence to build our argument as to the possible underlying causes. Future work should distinguish the degree to which gender differences in reporting are due to challenges in recognizing illness versus deliberate underinvestment in girls relative to boys. Furthermore, there is not enough evidence from sub-Saharan Africa on the underlying phenomena that might explain our findings, and future work could focus on understanding the potential mechanisms that could lead to the observed gender differences in symptom reporting. This research would allow for greater certainty in interpreting the implications of differences in reporting based on child gender. It might also be illuminating to explore in future research village- and country-level factors that contribute to gender differences in symptom reporting using multilevel modeling methods. In addition, the country-level analysis presented in Table 5 cannot be interpreted as evidence of a causal relationship between gender differences in fever reporting and differences in health outcomes, as there may be unmeasured confounding and ecological fallacy. In particular, a general atmosphere of discrimination against girls could produce the observed relationship without a direct causal link between fever reporting and infant mortality.
This analysis has important implications for understanding gender disparities and child health more generally. From a measurement perspective, estimates of gender imbalances in child illness and treatment based on parental reports may be inaccurate. Previous studies have already documented that parents do a poor job at distinguishing diseases in their children,34 and also that recall periods have a biasing effect on illness reporting.35 Parental reports collected as part of the DHS are a primary means for estimating illness prevalence among children in developing countries, but alternative or supplementary methods should perhaps be considered. More important, from a public health perspective, parental underreporting of symptoms in girls may indicate a tremendous amount of untreated illness that goes unnoticed. Using symptom reporting for boys as a benchmark for what can reasonably be expected from parents, our findings suggest that every 2 weeks, around 1.8% of girls under 5 years of age have fever or diarrhea that goes unnoticed. If this relationship holds true throughout sub-Saharan Africa, where there are roughly 80 million girls under 5 years of age, this amounts to nearly 1.5 million illness episodes that go unnoticed in any 2-week period. Although more research is needed to understand the practical impact of the observed gender difference in symptom reporting, the scope of the problem has the potential to be quite large. On this point, our article contributes to a large and growing research agenda on reducing gender disparities in health and increasing gender equality more generally.36
Supplementary Material
Footnotes
Authors' addresses: Peter C. Rockers, Department of Global Health, Boston University School of Public Health, Boston, MA, E-mail: prockers@bu.edu. Margaret McConnell, Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Boston, MA, E-mail: mmcconne@hsph.harvard.edu.
References
- 1.Sen A. Health: perception versus observation. BMJ. 2002;324:860–861. doi: 10.1136/bmj.324.7342.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88:251–258. doi: 10.7326/0003-4819-88-2-251. [DOI] [PubMed] [Google Scholar]
- 3.Young A. The anthropologies of illness and sickness. Annu Rev Anthropol. 1982;11:257–285. [Google Scholar]
- 4.Subramanian SV, Subramanyam MA, Selvaraj S, Kawachi I. Are self-reports of health and morbidities in developing countries misleading? Evidence from India. Soc Sci Med. 2009;68:260–265. doi: 10.1016/j.socscimed.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manesh AO, Sheldon TA, Pickett KE, Carr-Hill R. Accuracy of child morbidity data in demographic and health surveys. Int J Epidemiol. 2008;37:194–200. doi: 10.1093/ije/dym202. [DOI] [PubMed] [Google Scholar]
- 6.United Nations Children's Emergency Fund (UNICEF) Pneumonia and Diarrhoea: Tackling the Deadliest Diseases for the World's Poorest Children. Geneva, Switzerland: UNICEF; 2012. [Google Scholar]
- 7.Hill K, Upchurch DM. Gender differences in child health: evidence from the demographic and health surveys. Popul Dev Rev. 1995;21:127–151. [Google Scholar]
- 8.Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS. Bellagio Child Survival Study Group How many child deaths can we prevent this year? Lancet. 2003;362:65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 9.United Nations Children's Emergency Fund (UNICEF) Levels and Trends in Child Mortality: 2012 Report. Estimates Developed by the UN Inter-Agency Group for Child Mortality Estimation. New York, NY: UNICEF, WHO, World Bank, United Nations Population Division; 2012. [Google Scholar]
- 10.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, Carter JA. International Child Development Steering Group Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 11.Nathanson CA. Sex differences in mortality. Annu Rev Sociol. 1984;10:191–213. doi: 10.1146/annurev.so.10.080184.001203. [DOI] [PubMed] [Google Scholar]
- 12.Waldron I. Sex differences in human mortality: the role of genetic factors. Soc Sci Med. 1983;17:321–333. doi: 10.1016/0277-9536(83)90234-4. [DOI] [PubMed] [Google Scholar]
- 13.Corsi DJ, Neuman M, Finlay JE, Subramanian SV. Demographic and health surveys: a profile. Int J Epidemiol. 2012;41:1602–1613. doi: 10.1093/ije/dys184. [DOI] [PubMed] [Google Scholar]
- 14.MEASURE DHS, MEASURE Evaluation, President's Malaria Initiative, Roll Back Malaria, United Nations Children's Fund . Malaria Indicator Survey: Basic Documentation for Survey Design and Implementation. Calverton, MD: MEASURE Evaluation; 2013. [Google Scholar]
- 15.National Population Commission of Nigeria, National Malaria Control Programme of Nigeria, ICF International . Nigeria Malaria Indicator Survey 2010. Abuja, Nigeria: NPC, NMCP, and ICF International; 2012. [Google Scholar]
- 16.Garenne M. Sex differences in health indicators among children in African DHS surveys. J Biosoc Sci. 2003;35:601–614. doi: 10.1017/s0021932003006047. [DOI] [PubMed] [Google Scholar]
- 17.Waldron I. Too Young to Die: Genes or Gender? New York, NY: United Nations; 1998. Sex differences in infant and early childhood mortality: major causes of death and possible biological causes; pp. 64–83. [Google Scholar]
- 18.Choi Y, El Arifeen S, Mannan I, Rahman S, Bari GL, Darmstadt R, Black RE, Baqui AH. Projahnmo Study Group Can mothers recognize neonatal illness correctly? Comparison of maternal report and assessment by community health workers in rural Bangladesh. Trop Med Int Health. 2010;15:743–753. doi: 10.1111/j.1365-3156.2010.02532.x. [DOI] [PubMed] [Google Scholar]
- 19.Jayachandran S, Kuziemko I. Why do mothers breastfeed girls less than boys? Evidence and implications for child health in India. Q J Econ. 2011;126:1485–1538. doi: 10.1093/qje/qjr029. [DOI] [PubMed] [Google Scholar]
- 20.Chakravarty A. Gender Bias in Breastfeeding and Missing Girls in Africa: The Role of Fertility Choice. London, United Kingdom: University College; 2012. http://www.homepages.ucl.ac.uk/∼uctpabc/Gender%20Bias%20in%20Breastfeeding%20in%20Africa.pdf Mimeo. Available at. [Google Scholar]
- 21.Young Infants Clinical Signs Study Group Clinical signs that predict severe illness in children under age 2 months: a multicentre study. Lancet. 2008;371:135–142. doi: 10.1016/S0140-6736(08)60106-3. [DOI] [PubMed] [Google Scholar]
- 22.Sen G, Östlin P. Gender inequity in health: why it exists and how we can change it. Glob Public Health. 2008;3:1–12. doi: 10.1080/17441690801900795. [DOI] [PubMed] [Google Scholar]
- 23.Kornrich S, Furstenberg F. Investing in children: changes in parental spending on children, 1972–2007. Demography. 2013;50:1–23. doi: 10.1007/s13524-012-0146-4. [DOI] [PubMed] [Google Scholar]
- 24.Pande RP, Yazbeck AS. What's in a country average? Wealth, gender, and regional inequalities in immunization in India. Soc Sci Med. 2003;57:2075–2088. doi: 10.1016/s0277-9536(03)00085-6. [DOI] [PubMed] [Google Scholar]
- 25.Chen LC, Huq E, D'Souza S. Sex bias in the family allocation of food and health care in rural Bangladesh. Popul Dev Rev. 1981;7:55–70. [Google Scholar]
- 26.Grant MJ, Behrman JR. Gender gaps in educational attainment in less developed countries. Popul Dev Rev. 2010;36:71–89. [Google Scholar]
- 27.Sen A. New York Review of Books. New York, NY: New York Review of Books; 1990. More Than 100 Million Women are Missing.http://www.nybooks.com/articles/1990/12/20/more-than-100-million-women-are-missing/ Available at. Accessed December 1, 2015. [Google Scholar]
- 28.Flatø M, Kotsadam A. Droughts and Gender Bias in Infant Mortality in Sub-Saharan Africa. Oslo, Norway: Department of Economics, Oslo University; 2014. Working Paper No. 02/2014. [Google Scholar]
- 29.Friedman J, Schady N. How Many More Infants Are Likely to Die in Africa as a Result of the Global Financial Crisis? Washington, DC: World Bank; 2012. Working Paper, World Bank Policy Research. [Google Scholar]
- 30.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. International Child Development Steering Group Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banco L, Veltri D. Ability of mothers to subjectively assess the presence of fever in their children. Am J Dis Child. 1984;138:976–978. doi: 10.1001/archpedi.1984.02140480078024. [DOI] [PubMed] [Google Scholar]
- 32.Einterz EM, Bates ME. Fever in Africa: do patients know when they are hot? Lancet. 1997;350:781. doi: 10.1016/S0140-6736(97)24037-7. [DOI] [PubMed] [Google Scholar]
- 33.Black RE, Brown KH, Becker S, Yunus MD. Longitudinal studies of infectious diseases and physical growth of children in rural Bangladesh I. Patterns of morbidity. Am J Epidemiol. 1982;115:305–314. doi: 10.1093/oxfordjournals.aje.a113307. [DOI] [PubMed] [Google Scholar]
- 34.Eisele TP, Silumbe K, Yukich J, Hamainza B, Keating J, Bennett A, Miller JM. Measuring coverage in MNCH: accuracy of measuring diagnosis and treatment of childhood malaria from household surveys in Zambia. PLoS Med. 2013;10:e1001417. doi: 10.1371/journal.pmed.1001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das J, Hammer J, Sanchez-Paramo C. The impact of recall periods on reported morbidity and health seeking behavior. J Dev Econ. 2012;98:76–88. [Google Scholar]
- 36.United Nations Children's Emergency Fund (UNICEF) Women and Children: The Double Dividend of Gender Equality. The State of the World's Children. Vol. 7. Geneva, Switzerland: UNICEF; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.