Abstract
The sphenopalatine ganglion (SPG) is a parasympathetic ganglion, located in the pterygopalatine fossa. The SPG block has been used for a long time for treating headaches of varying etiologies. For anesthesiologists, treating postdural puncture headaches (PDPH) has always been challenging. The epidural block patch (EBP) was the only option until researchers explored the role of the SPG block as a relatively simple and effective way to treat PDPH. Also, since the existing evidence proving the efficacy of the SPG block in PDPH is scarce, the block cannot be offered to all patients. EBP can be still considered if an SPG block is not able to alleviate pain due to PDPH.
Keywords: Epidural blood patch, Headache, Pain management, Postdural puncture headache, Pterygopalatine fossa, Sphenopalatine ganglion block
INTRODUCTION
Management of postdural puncture headache (PDPH) has always been challenging for anesthesiologists. PDPH not only increases the misery of the patient, but the length of stay and overall cost of treatment in the hospital also increases. Although the EBP is an effective way of treating the problem, the procedure itself could cause another inadvertent dural puncture (DP). Moreover, sometimes patients need to have a second EBP, if the first one is not completely effective. This can be difficult to explain to the patient who has already suffered a lot.
Recently, the sphenopalatine ganglion block has been successfully used in patients with PDPHs, although fewer patients were offered this block. If further prospective studies are able to establish its efficacy, patients would be benefited by early recovery and less suffering.
MAIN BODY
1. The problem of PDPH
PDPH is a distressing complication that occurs due to inadvertent DP while locating the epidural space with a Tuohy's needle. PDPH can also occur after an intentional dural puncture while performing a spinal anesthetic for lower abdominal surgeries, diagnostic or therapeutic lumbar punctures, or intrathecal anticancer drug or antibiotics injections.
The incidence and severity of PDPH is influenced by the types of needle (Whitacre spinal needle or Tuohy's epidural needle), multiple attempts, or in working with obstetric patients [1].
An autologous EBP is the gold standard for treating PDPH when the headache is persistent even after conservative management such as supine position, hydration, an abdominal binder, analgesics, caffeine, sumatriptan, and laxatives. Use of the prophylactic EBP in patients who have a DP is not recommended [2]. EBP has a success rate of around 75% in relieving PDPH [3].
In a few patients, a second EBP is required, if pain relief is not adequate after the first EBP. Problems with using the EBP include chances of another possible DP, infection, and neurological complications in some rare situations. It is also more difficult to convince the patient and family members to accept a second EBP.
Complications like meningitis, arachnoiditis, seizures, loss of hearing or vision, radicular pain, and neural deficits have also been reported after an EBP [4].
2. Relevant anatomy and the role of the sphenopalatine ganglion
The sphenopalatine ganglion (SPG) is a triangular-shaped parasympathetic ganglion, located superficially in the pterygopalatine fossa, posterior to the middle nasal turbinate, and anterior to the pterygoid canal (Fig. 1). It is also referred to as the pterygopalatine ganglion, Meckel's ganglion, or the nasal ganglion. It is about 5 mm in size [5]. There is a 1 to 1.5 mm-thick layer of connective tissue and mucous membrane surrounding the ganglion, so the drug enters well by a topical application or by injection. The lacrimal gland, glands of the nasal cavity, paranasal sinuses, palate, and upper pharynx receive fibers from the SPG. It is referred to as a parasympathetic ganglion because only the pre-ganglionic parasympathetic axons synapse within the ganglion. Post-ganglionic sympathetic neurons, along with somatic sensory afferent branches of the maxillary division of the trigeminal nerve, also pass through the ganglion. Postganglionic parasympathetic, sympathetic neurons, and the somatic sensory afferents can all be blocked by an SPG block.
Fig. 1. Sphenopalatine ganglion as seen in pterygopalatine fossa through pterygomaxillary fissure (Source: Khonsary SA, Ma Q, Villablanca P, Emerson J, Malkasian D. Clinical functional anatomy of the pterygopalatine ganglion, cephalgia and related dysautonomias: a review. Surg Neurol Int 2013; 4: S422-8).
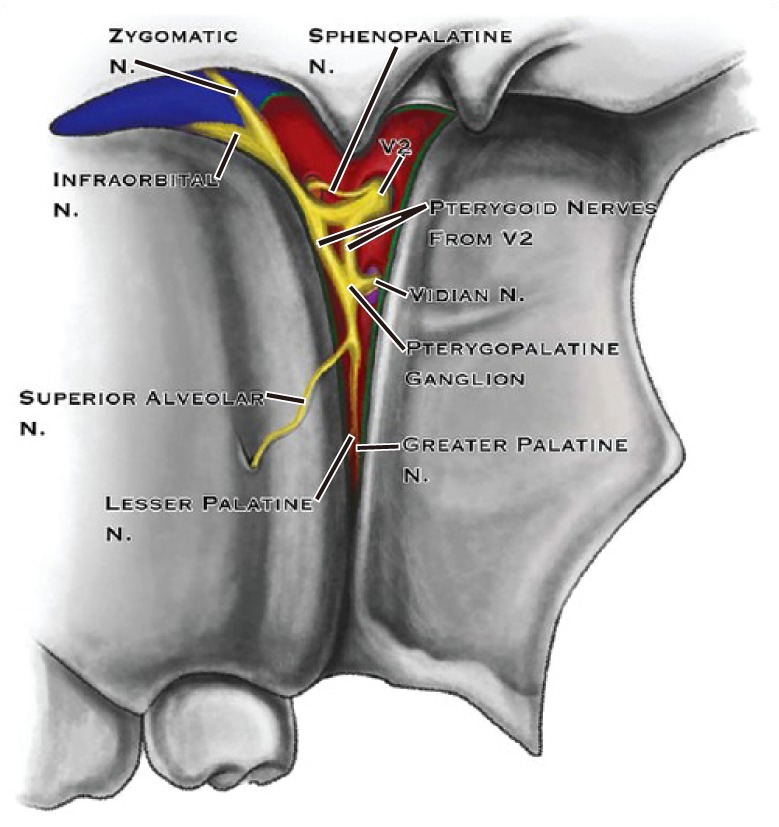
Thus, essentially, the SPG is a junction which has sympathetic, parasympathetic, and sensory innervation overlapping in a minute area [6]. This could be the reason behind the fact that the block diffuses the conduction of pain due to several etiologies.
The preganglionic parasympathetic neurons originate from the superior salivatory nucleus located in the pons through the nervous intermedius of the facial nerve. The preganglionic sympathetic neurons start from the intermediate horn of spinal gray matter of the spinal cord situated at the first thoracic vertebrae (T1), ascending the cervical sympathetic trunk to the superior cervical ganglion to synapse with the postganglionic neurons. The postganglionic fibers follow along the internal carotid artery and enter the skull as the deep petrosal nerve (DPN).
3. Indications for SPG block
An SPG block is indicated in acute and chronic facial/head pain like cluster headaches, trigeminal neuralgia, temporomandibular joint pain, postherpetic neuralgia, Sluder's neuralgia, paroxysmal hemicranias, atypical facial pain, pain due to head and neck cancer, complex regional pain syndrome (CRPS) I and II, and vasomotor rhinitis. The block is used for surgical anesthesia and post-operative analgesia in oral and maxillofacial surgery done without general anesthesia under the fluoroscope [7].
There are trans-nasal, trans-oral, sub-zygomatic, and lateral infratemporal approaches. The ganglion can be identified by injecting radiopaque contrast medium. Further treatments, such as an SPG block with local anesthetics, radiofrequency ablation, or stereotactic radiosurgery, should be decided after identification of the SPG [8].
4. Mechanism of action (Fig. 2)
Fig. 2. Components of sphenopalatine ganglion block.
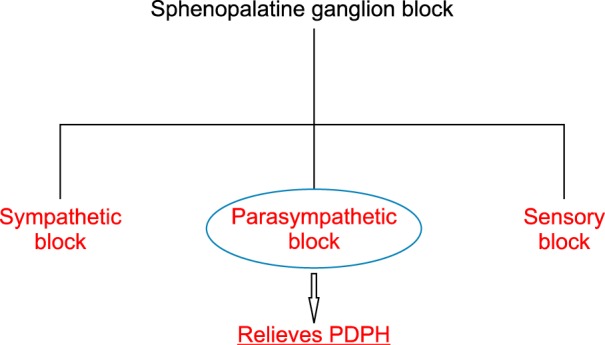
According to the Monro-Kellie doctrine or hypothesis, the sum of the volumes of the brain, cerebrospinal fluid (CSF), and blood in the intracranial compartment remains constant [9]. This means if the volume of one constituent reduces, the volume of another constituent increases to maintain an equilibrium and vice versa.
After a DP, the CSF is continuously lost. In such a situation, the intracranial volume is restored by compensatory vasodilatation, because it is not possible for the brain to swell to restore the normal volume. This vasodilatation is responsible for the excruciating headache after a DP. One of the contributors to this vasodilatation is mediated by parasympathetic activity by the neurons which have synapses in the SPG. This is how an SPG block helps in alleviating a headache which is mediated by the neurons in the SPG [10].
The parasympathetic activity is blocked which inhibits the cerebral vasodilatation. The vasodilatation occurs to restore intracranial volume. However, the blood flow does not stop after optimally filling the cranial cavity. The excruciating headache is the result of uncontrolled vasodilatation. By attenuating this with a sphenopalatine ganglion block, the vasodilatation is addressed, and the patient also receives symptomatic relief.
5. Technique (Fig. 3)
Fig. 3. Technique of sphenopalatine ganglion block.
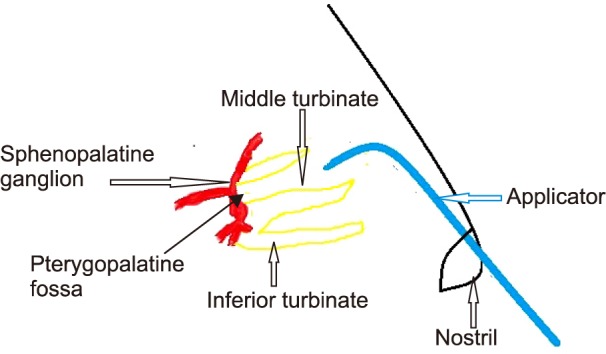
The approaches practiced by pain physicians are not only invasive, but also necessary training for use of fluoroscopy, the operation room, and sedation in some patients to facilitate the procedure. The SPG block practiced for PDPH is simple, and therefore, it can be done bedside in wards, or in the out-patient department, and does not need fluoroscopy or an operating room.
The patient needs to be in a supine position with the neck extended. The extension can be facilitated with a pillow or a folded sheet under both shoulders. A long applicator with a cotton swab at the tip is soaked with 2%-4% lidocaine or viscous lidocaine. It is then inserted parallel to the floor of the nose until resistance is encountered. The swab will be at the posterior pharyngeal wall superior to the middle turbinate. The applicator should be retained in the nostril for 5-10 minutes and then removed. The procedure is similarly repeated in the other nostril. The swab does not come into direct contact with the ganglion, however the local anesthetic infiltrates around it in that position [11]. The connective tissue and mucous membrane covering facilitates the spread and penetration of the drug.
6. Present evidence
The evidence available which can highlight the efficacy of the SPG block for relieving PDPH is limited, and is available in the form of case reports and case series. The first published paper describing the use of the SPG block for management of PDPH was by Cohen et al. [12] in 2009. However, the suggestion was made by the same author in a correspondence published in 2001, were they used the block in 22 parturients who had tension headaches, migraines, low backaches, and neck pain, and it was found to be effective. After this experience, Cohen et al. [12] suggested use of the SPG block in PDPH in that correspondence.
Eight years later, in 2009, Cohen et al. [13] published their experience with 13 parturients with moderate to severe PDPH who were treated with SPG blocks. Out of 13, 11 patients had good pain relief and did not require an EBP, while the remaining 2 patients were relieved only after an EBP.
Patel et al. [14] presented the retrospective data of 72 patients collected over 17 years in the form of a poster. They divided the 72 patients who had PDPH into 2 groups. The 33 patients in one group received an SPG block and the 39 patients in second group received EBP. They followed up the patients in both groups after the intervention at 30 m, 1 h, 24 h, 48 h, and 1 week. At the end of 1 h, the SPB block patients had good pain relief compared to the EBP group. However, after 24 hours there was no significant difference observed in either group. More complications were observed in the EBP group.
Kent and Mehaffey [15] performed SPG blocks in 3 patients with confirmed PDPH in the emergency room using 2% viscous lidocaine. All 3 patients had good relief after the intervention. The authors suggested that the procedure can be safely and accurately performed in the emergency room which will reduce the visit time, provide good pain relief, and the EBP can be deferred. Recently, Kent and Mehaffey [16] published their experience with 3 parturients diagnosed with PDPH who were offered an SPG block trans-nasally. All 3 patients had good pain relief and none of them required an EBP.
CONCLUSIONS
The SPG block appears to be a simple, minimally invasive block which can be done bedside. If successful, the patient does not need an EBP which is an established modality with a high success rate for managing PDPH. However, the data available is scarce at the moment. Lot of prospective studies done earlier have established the efficacy of the SPG block by several approaches for headaches of varied etiology. But before declaring the SPG block to be superior to the time-tested EBP, we need to have more data on successfully treated patients.
However, after reviewing the available evidence, it appears that we can offer the SPG block to all patients diagnosed with moderate to severe PDPH. If the block is ineffective in alleviating pain, an EBP can be planned. Clinicians should continue ongoing supportive management after performing the SPG block.
References
- 1.Bano F, Haider S, Aftab S, Sultan ST. Comparison of 25-gauge, Quincke and Whitacre needles for postdural puncture headache in obstetric patients. J Coll Physicians Surg Pak. 2004;14:647–650. [PubMed] [Google Scholar]
- 2.Boonmak P, Boonmak S. Epidural blood patching for preventing and treating post-dural puncture headache. Cochrane Database Syst Rev. 2010:CD001791. doi: 10.1002/14651858.CD001791.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Ylönen P, Kokki H. Epidural blood patch for management of postdural puncture headache in adolescents. Acta Anaesthesiol Scand. 2002;46:794–798. doi: 10.1034/j.1399-6576.2002.460707.x. [DOI] [PubMed] [Google Scholar]
- 4.Desai MJ, Dave AP, Martin MB. Delayed radicular pain following two large volume epidural blood patches for post-lumbar puncture headache: a case report. Pain Physician. 2010;13:257–262. [PubMed] [Google Scholar]
- 5.Khonsary SA, Ma Q, Villablanca P, Emerson J, Malkasian D. Clinical functional anatomy of the pterygopalatine ganglion, cephalgia and related dysautonomias: a review. Surg Neurol Int. 2013;4:S422–S428. doi: 10.4103/2152-7806.121628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rusu MC, Pop F. The anatomy of the sympathetic pathway through the pterygopalatine fossa in humans. Ann Anat. 2010;192:17–22. doi: 10.1016/j.aanat.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Oluigbo CO, Makonnen G, Narouze S, Rezai AR. Sphenopalatine ganglion interventions: technical aspects and application. Prog Neurol Surg. 2011;24:171–179. doi: 10.1159/000323049. [DOI] [PubMed] [Google Scholar]
- 8.Candido KD, Massey ST, Sauer R, Darabad RR, Knezevic NN. A novel revision to the classical transnasal topical sphenopalatine ganglion block for the treatment of headache and facial pain. Pain Physician. 2013;16:E769–E778. [PubMed] [Google Scholar]
- 9.Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001;56:1746–1748. doi: 10.1212/wnl.56.12.1746. [DOI] [PubMed] [Google Scholar]
- 10.Piagkou M, Demesticha T, Troupis T, Vlasis K, Skandalakis P, Makri A, et al. The pterygopalatine ganglion and its role in various pain syndromes: from anatomy to clinical practice. Pain Pract. 2012;12:399–412. doi: 10.1111/j.1533-2500.2011.00507.x. [DOI] [PubMed] [Google Scholar]
- 11.Schaffer JT, Hunter BR, Ball KM, Weaver CS. Noninvasive sphenopalatine ganglion block for acute headache in the emergency department: a randomized placebo-controlled trial. Ann Emerg Med. 2015;65:503–510. doi: 10.1016/j.annemergmed.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, Trnovski S, Zada Y. A new interest in an old remedy for headache and backache for our obstetric patients: a sphenopalatine ganglion block. Anaesthesia. 2001;56:606–607. [PubMed] [Google Scholar]
- 13.Cohen S, Sakr A, Katyal S, Chopra D. Sphenopalatine ganglion block for postdural puncture headache. Anaesthesia. 2009;64:574–575. doi: 10.1111/j.1365-2044.2009.05925.x. [DOI] [PubMed] [Google Scholar]
- 14.Patel P, Zhao R, Cohen S, Mellender S, Shah S, Grubb W. Sphenopalatine ganglion block (SPGB) versus epidural blood patch (EBP) for accidental postdural puncture headache (PDPH) in obstetric patients: a retrospective observation; 32nd Annual Meeting of the American Academy of Pain Medicine; 2016 Feb 18-21; Palm Springs (CA). Poster #145. [Google Scholar]
- 15.Kent S, Mehaffey G. Transnasal sphenopalatine ganglion block for the treatment of postdural puncture headache in the ED. Am J Emerg Med. 2015;33:1714.e1–1714.e2. doi: 10.1016/j.ajem.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Kent S, Mehaffey G. Transnasal sphenopalatine ganglion block for the treatment of postdural puncture headache in obstetric patients. J Clin Anesth. 2016;34:194–196. doi: 10.1016/j.jclinane.2016.04.009. [DOI] [PubMed] [Google Scholar]


