Abstract
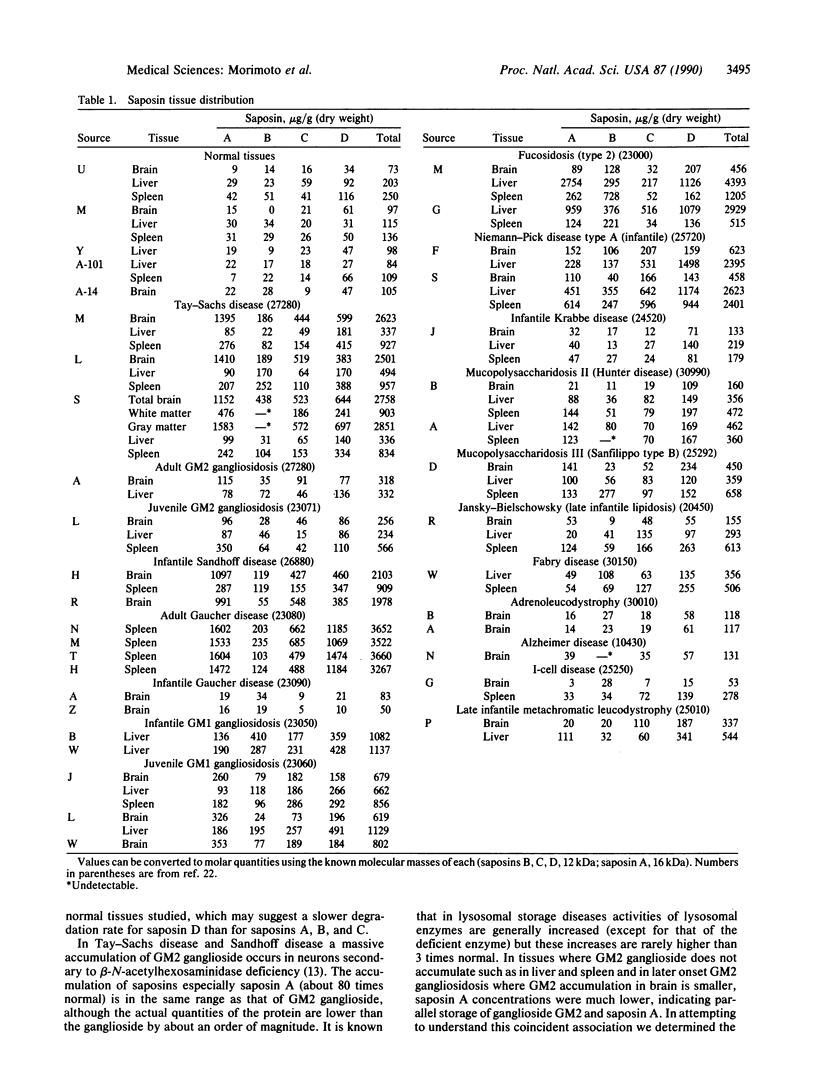
Saposins (A, B, C, and D) are small glycoproteins required for the hydrolysis of sphingolipids by specific lysosomal hydrolases. Concentrations of these saposins in brain, liver, and spleen from normal humans as well as patients with lysosomal storage disease were determined. A quantitative HPLC method was used for saposin A, C, and D and a stimulation assay was used for saposin B. In normal tissues, saposin D was the most abundant of the four saposins. Massive accumulations of saposins, especially saposin A (about 80-fold increase over normal), were found in brain of patients with Tay-Sachs disease or infantile Sandhoff disease. In spleen of adult patients with Gaucher disease, saposin A and D accumulations (60- and 17-fold, respectively, over normal) were higher than that of saposin C (about 16-fold over normal). Similar massive accumulations of saposins A and D were found in liver of patients with fucosidosis (about 70- and 20-fold, respectively, over normal). Saposin D was the primary saposin stored in the liver of a patient with Niemann-Pick disease (about 30-fold over normal). Moderate increases of saposins B and D were found in a patient with GM1 gangliosidosis. Normal or near normal levels of all saposins were found in patients with Krabbe disease, metachromatic leukodystrophy, Fabry disease, adrenoleukodystrophy, I-cell disease, mucopolysaccharidosis types 2 and 3B, or Jansky-Bielschowsky disease. The implications of the storage of saposins in these diseases are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berent B. L., Radin N. S. beta-Glucosidase activator protein from bovine spleen ("coglucosidase"). Arch Biochem Biophys. 1981 Apr 15;208(1):248–260. doi: 10.1016/0003-9861(81)90147-8. [DOI] [PubMed] [Google Scholar]
- Collard M. W., Sylvester S. R., Tsuruta J. K., Griswold M. D. Biosynthesis and molecular cloning of sulfated glycoprotein 1 secreted by rat Sertoli cells: sequence similarity with the 70-kilodalton precursor to sulfatide/GM1 activator. Biochemistry. 1988 Jun 14;27(12):4557–4564. doi: 10.1021/bi00412a050. [DOI] [PubMed] [Google Scholar]
- Conzelmann E., Sandhoff K. AB variant of infantile GM2 gangliosidosis: deficiency of a factor necessary for stimulation of hexosaminidase A-catalyzed degradation of ganglioside GM2 and glycolipid GA2. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3979–3983. doi: 10.1073/pnas.75.8.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. C., Radin N. S. Glucosylceramide and the level of the glucosidase-stimulating proteins. Lipids. 1986 Nov;21(11):702–709. doi: 10.1007/BF02537244. [DOI] [PubMed] [Google Scholar]
- Datta S. C., Snider R. M., Radin N. S. Uptake by neuroblastoma cells of glucosylceramide, glucosylceramide glucosidase, its stimulator protein, and phosphatidylserine. Biochim Biophys Acta. 1986 Jul 18;877(3):387–398. doi: 10.1016/0005-2760(86)90204-3. [DOI] [PubMed] [Google Scholar]
- Fischer G., Jatzkewitz H. The activator of cerebroside-sulphatase. A model of the activation. Biochim Biophys Acta. 1978 Jan 27;528(1):69–76. doi: 10.1016/0005-2760(78)90053-x. [DOI] [PubMed] [Google Scholar]
- Fujibayashi S., Wenger D. A. Biosynthesis of the sulfatide/GM1 activator protein (SAP-1) in control and mutant cultured skin fibroblasts. Biochim Biophys Acta. 1986 Feb 28;875(3):554–562. doi: 10.1016/0005-2760(86)90077-9. [DOI] [PubMed] [Google Scholar]
- Fujibayashi S., Wenger D. A. Synthesis and processing of sphingolipid activator protein-2 (SAP-2) in cultured human fibroblasts. J Biol Chem. 1986 Nov 15;261(32):15339–15343. [PubMed] [Google Scholar]
- Fürst W., Machleidt W., Sandhoff K. The precursor of sulfatide activator protein is processed to three different proteins. Biol Chem Hoppe Seyler. 1988 May;369(5):317–328. doi: 10.1515/bchm3.1988.369.1.317. [DOI] [PubMed] [Google Scholar]
- Ho M. W., O'Brien J. S. Gaucher's disease: deficiency of 'acid' -glucosidase and reconstitution of enzyme activity in vitro. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2810–2813. doi: 10.1073/pnas.68.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui K., Wenger D. A. Concentrations of an activator protein for sphingolipid hydrolysis in liver and brain samples from patients with lysosomal storage diseases. J Clin Invest. 1983 Nov;72(5):1622–1628. doi: 10.1172/JCI111121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. C., Kihara H., Serizawa S., Li Y. T., Fluharty A. L., Mayes J. S., Shapiro L. J. Activator protein required for the enzymatic hydrolysis of cerebroside sulfate. Deficiency in urine of patients affected with cerebroside sulfatase activator deficiency and identity with activators for the enzymatic hydrolysis of GM1 ganglioside and globotriaosylceramide. J Biol Chem. 1985 Feb 10;260(3):1867–1871. [PubMed] [Google Scholar]
- Li S. C., Sonnino S., Tettamanti G., Li Y. T. Characterization of a nonspecific activator protein for the enzymatic hydrolysis of glycolipids. J Biol Chem. 1988 May 15;263(14):6588–6591. [PubMed] [Google Scholar]
- Li S. C., Wan C. C., Mazzotta M. Y., Li Y. T. Requirement of an activator for the hydrolysis of sphingoglycolipids by glycosidases of human liver. Carbohydr Res. 1974 May;34(1):189–193. doi: 10.1016/s0008-6215(00)80383-3. [DOI] [PubMed] [Google Scholar]
- Mehl E., Jatzkewitz H. Eine Cerebrosidsulfatase aus Schweineniere. Hoppe Seylers Z Physiol Chem. 1964;339(1):260–276. [PubMed] [Google Scholar]
- Morimoto S., Kishimoto Y., Tomich J., Weiler S., Ohashi T., Barranger J. A., Kretz K. A., O'Brien J. S. Interaction of saposins, acidic lipids, and glucosylceramidase. J Biol Chem. 1990 Feb 5;265(4):1933–1937. [PubMed] [Google Scholar]
- Morimoto S., Martin B. M., Kishimoto Y., O'Brien J. S. Saposin D: a sphingomyelinase activator. Biochem Biophys Res Commun. 1988 Oct 14;156(1):403–410. doi: 10.1016/s0006-291x(88)80855-6. [DOI] [PubMed] [Google Scholar]
- Morimoto S., Martin B. M., Yamamoto Y., Kretz K. A., O'Brien J. S., Kishimoto Y. Saposin A: second cerebrosidase activator protein. Proc Natl Acad Sci U S A. 1989 May;86(9):3389–3393. doi: 10.1073/pnas.86.9.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. S., Kretz K. A., Dewji N., Wenger D. A., Esch F., Fluharty A. L. Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science. 1988 Aug 26;241(4869):1098–1101. doi: 10.1126/science.2842863. [DOI] [PubMed] [Google Scholar]
- Purpura K. P., Baker H. J. Neurite induction in mature cortical neurones in feline GM1-ganglioside storage disease. Nature. 1977 Apr 7;266(5602):553–554. doi: 10.1038/266553a0. [DOI] [PubMed] [Google Scholar]
- SAMUELS S., GONATAS N. K., WEISS M. FORMATION OF THE MEMBRANOUS CYTOPLASMIC BODIES IN TAY-SACHS DISEASE: AN IN VITRO STUDY. J Neuropathol Exp Neurol. 1965 Apr;24:256–264. doi: 10.1097/00005072-196504000-00007. [DOI] [PubMed] [Google Scholar]
- TERRY R. D., WEISS M. Studies in Tay-Sachs disease. II. Ultrastructure of the cerebrum. J Neuropathol Exp Neurol. 1963 Jan;22:18–55. doi: 10.1097/00005072-196301000-00003. [DOI] [PubMed] [Google Scholar]
- Walkley S. U., Blakemore W. F., Purpura D. P. Alterations in neuron morphology in feline mannosidosis. A Golgi study. Acta Neuropathol. 1981;53(1):75–79. doi: 10.1007/BF00697187. [DOI] [PubMed] [Google Scholar]
- Wenger D. A., Sattler M., Roth S. A protein activator of galactosylceramide beta-galactosidase. Biochim Biophys Acta. 1982 Sep 14;712(3):639–649. doi: 10.1016/0005-2760(82)90293-4. [DOI] [PubMed] [Google Scholar]


