Abstract
Purpose
Ephedra alata (E. alata) is perennial tough shrub plant that grows in Palestine and other regions. It is used often in folk's medicine for the treatment of various diseases. In this project, E. alata extract was tested for its ability to improve wound and burn healing.
Methods
An aqueous extract of E. alata was prepared and underwent several phytochemical analyses for the presence of the major classes of phytochemical compounds. After that, a polyethylene glycol-based ointment containing the extract of E. alata was prepared and its wound and burn healing activities were tested in-vivo using an animal model for deep wound and full thickness skin burn. The effect was compared against a placebo ointment. Skin biopsies were evaluated by a blinded clinical histopathologist, in addition to digital analysis.
Results
Phytochemical analysis demonstrated the presence of the major classes of phytochemical compounds in the prepared extract including flavonoids, alkaloids, phytosteroids, phenolic compounds, volatile oils and tannins. As compared to placebo ointment, E. alata ointment significantly improved the healing of the wound ulcers, whereas it showed no advantage on the quality of the healing of burn ulcers.
Conclusion
E. alata extract is rich in phytochemical compounds and can improve wound healing when applied topically.
Keywords: Ephedra alata, Ulcer, Wound healing, Burns, Phytochemicals
Introduction
Millions of patients worldwide suffer chronic or acute difficult wounds that normally take a prolonged time for adequate healing, rendering the site of ulceration prone to serious complicated infections, besides the sensation of pain and disability. According to a publication in the year 2010 around 6.5 million patients in the United States suffer chronic wounds, the majority of whom simultaneously suffer diabetes mellitus and obesity that hinder the healing process. In addition, the article reported that several other millions of patients require post-surgical wound care, and the numbers are increasing annually.1 Therefore, many research groups and pharmaceutical companies throughout the world are trying to develop treatments that can accelerate the healing process.
Throughout the history, plants have been the mainstay source for remedies, as they contain thousands of chemical compounds, known as phytochemicals, which serve different therapeutic effects. Nowadays, despite the huge development in the pharmaceutical field, many of the currently used drugs are either directly obtained from plants or are derivatives of phytochemical compounds, such as aspirin, cardiac glycosides, atropine, opioids, curare derivatives and pilocarpine.2 Ephedra alata (E. alata) is a perennial tough shrubs, with yellow-green and 50–100 cm tall twigs.3 It belongs to the ephedraceae family of gymnosperms, which contains several well-known pharmacologically active compounds such as ephedrine and pseudoephedrine alkaloids.4 E. alata contains a number of phytochemical classes such as cardiac glycoside, alkaloids, phenols and flavonoids.5 Despite the lack of clinical evidence, E. alata is being used quite often in folk medicine for treating various diseases like cancer. In addition, E. alata was suggested by some research groups for the treatment of diabetes mellitus.6, 7
This novel work aims to demonstrate whether the extract of E. alata can improve the healing process of ulcers caused by deep wounds and full thickness burns.
Materials and methods
FeCl3 was obtained from Riedeldehan Germany, n-hexane was obtained from Frutarom Haifa, NaOH was obtained from Gadot, and H2SO4 was obtained from Alfa Agar England. Chloroform and folin-ciocalteu's reagents were obtained from Sigma–Aldrich Germany. PEG4000 and PEG6000 were obtained from Sunpharma Palestine.
Preparation of E. alata plant extract
A large quantity of whole plant was dried for 2 weeks in a well-ventilated dark room before it was grinded. The extraction was performed in two steps. First the plant powder was incubated with n-hexane and 50% ethanol for 72 h at 30 °C in a shaking incubator. Next the mixture was filtrated and the plant sediment was kept for use in the second extraction step, and the aqueous phase was separated and freeze dried. In the next step, 800 ml of 50% ethanol was added to a bottle containing the sediment of the plant remnant obtained from the first step of extraction. The bottle was incubated at 30 °C for 72 h in a shaking incubator. After that, the mixture was filtered and the filtrate was transferred to a rotary evaporator at 40 °C for 2 h to get rid of ethanol. Next the solution was freeze dried and the obtained yields from the two steps were pooled together and stored at −20 °C until use.
Verification of chemical constituents of E. alata extract
The presence of the major classes of phytochemical compounds was verified using the method described by Jaradat and colleagues.5 Briefly, 1 ml of plant extract was mixed with 2 ml of 2% FeCl3 solution. A black coloration indicated the presence of tannins. The alkaline reagent test was used to check the presence of flavonoids, for which 2 ml of 2% NaOH solution was mixed with 1 ml of the plant extract turning the solution into yellow color. When a drop of diluted acetic acid was added the yellow color turned colorless, indicating the presence of flavonoids. In order to test the presence of phenols, 1 ml of the plant extract was added to 2 ml distilled water followed by 0.5 ml NaHCO3 solution and 0.5 ml folin-ciocalteu's reagent; the appearance of a blue color indicates the presence of phenol. Keller–Kilani test was used for testing the presence of glycosides. 10 mg of extract was mixed with 2 ml of glacial acetic acid containing a few drops of 2% FeCl3 solutions. Then the mixture was poured into another test tube containing 2 ml of concentrated H2SO4. The appearance of a brown ring confirmed the presence of cardiac glycosides. The presence of steroids was investigated by adding 2 ml of chloroform and concentrated H2SO4 to 1 ml of the plant extract. The appearance of a red color in the lower chloroform layer suggested the presence of steroids. Wagner's reagent test was used to test the presence of alkaloids, for which 1 ml of the plant extract was added to few drops of saturated Iodine solution, and the reddish-brown color indicated the presence of alkaloids. Finally, the presence of volatile oil was tested by adding 1 ml of plant extract to 2 ml of 50% KOH solution, and the formation of sharp needle crystals confirmed the presence of volatile oils.
In vivo testing for the effect of E. alata extract on skin ulcer healing
Preparation of E. alata ointment: Two types of ointments were prepared, placebo ointment consisting only of the base, and the test ointment containing the plant extract as shown in Table 1.
Table 1.
Formulation of placebo and test ointments.
| Placebo ointment | Test ointment | |
|---|---|---|
| PEG4000 (g) | 76.5 | 76.5 |
| PEG6000 (g) | 9 | 9 |
| Double distilled water (ml) | 5 | 5 |
| Powder of aqueous extract (g) | – | 1.5 |
Preparation of E. alata ointment: The powder of the E. alata extract was dissolved in water, and then the solution was mixed with PEG4000 and was warmed under stirring until the temperature was between 60 and 65 °C. After that PEG6000 was added with stirring until PEG6000 was fused. After that the mixture was left to cool down to room temperature under continuous stirring.
Preparation of the placebo ointment: A similar method to that used for the EA ointment was used except that no plant extract was added.
Experimental animals
Six male healthy adult syrian hamsters (Mesocricetusauratus), average body weight of 117 g, were used. The hamsters were left for 1 week in the cages for accommodation before running the experiment. They had free access to water and food all the time. The animal handling and the procedures were carried out according to the guidelines of the Ethics Committee of the International Association for the study of pain. The hamsters were distributed into groups A (3 animals) and B (3 animals). A commercial hair removing cream was used to remove fur from the dorsal surface of the animals.
Induction of skin wound, treatment application and sample collection: The hamsters were anesthetized by the inhalation of ether, and a full thickness piece of fur-free skin was excised with a diameter of around 1.7 cm. One wound ulcer was induced on each animal of group A, while two wound ulcers were induced on group B animals (right and left). On the next day, the ulcers on the right side of group B were treated with the placebo ointment, while the left ulcers were treated with the EA ointment. The ulcers on group A animals were left untreated (negative control). The treatment was applied daily for 15 days when the ulcers had mostly healed. At this time point, macroscopic images for the site of ulcers were documented and full thickness skin biopsies of entire ulcer site from each animal were collected under anesthesia. The samples were fixed in 10% neutral buffered formalin for 24 h at room temperature, and after that they were sent to An-Najah National University Hospital for histopathology studies. Induction of skin burn, treatment application and sample collection: Using similar experimental settings used for the induction of wound, a circular metal plate (1.7 cm in diameter) was warmed in boiling water bath for 5 min and was applied to the fur-free dorsal body surface of the hamster under general anesthesia. One burn ulcer was induced on each animal of group A, while two burn ulcers were induced on group B animals (right and left). The resulting burn sites were treated for another 15 days following the same protocol explained above. At the end of the experiment macroscopic images for the site of ulcers were documented, and full thickness skin biopsies were collected as explained before.
Histopathological investigation for skin samples: Skin samples were sent to the medical labs at An-Najah National University Hospital. The samples were processed and sectioned according to the standard professional procedure followed routinely in the lab for regular pathological samples. The samples were stained by masson-trichrome stain and were examined by a clinical pathologist who was blinded for the samples. In addition, the degree of fibrosis was further digitally evaluated by analyzing the image using ImageJ software, where “color deconvolution plug-in” was used to separate the blue color. After that at least 150 points were randomly selected on the deconvoluted blue image, and the mean gray value was measured for the blue color using the “measure function” in ImageJ software. Paired-sample t-test was used to compare the means, and p ≤ 0.05 was considered significant.
Results
Phytochemical constituents of E. alata extract
As previously published by Jaradat and colleagues,5 the phytochemical analysis of E. alata aqueous extract revealed that it contains a variety of organic compound classes such as alkaloids, glycosides, flavonoids and phytosterols (Table 2). Interestingly, many of the clinically used drugs nowadays belong to these chemical classes, and are known to exhibit a wide range of pharmacological and therapeutic activities. Therefore, it was rational to think of E. alata as a potential drug bank for the treatment of a variety of diseases.
Table 2.
Results of phytochemical analysis of E. alata extract.
| Result | Test | Phytochemical compound |
|---|---|---|
| Positive | Wagner's test | Alkaloids |
| Positive | Keller–Kilani test | Glycoside |
| Positive | Alkaline reagent test | Flavonoid |
| Positive | Liebermann test | Phytosteroid |
| Positive | Folinciocalteu test | Phenolic compound |
| Positive | KOH test | Volatile oil |
| Positive | Ferric chloride test | Tannin |
Investigation of wound healing activity of E. alata extract ointment
After the induction of wound, the negative control animals (group A) received no treatment; while for group B with each animal having two ulcers, one ulcer received placebo ointment and the other one received E. alata ointment. The treatment was applied once daily for 15 days. At the end of the experiment macroscopic images for the site of ulcers were documented, and biopsies from these sites were collected and examined histopathologically by a blinded clinical histopathologist.
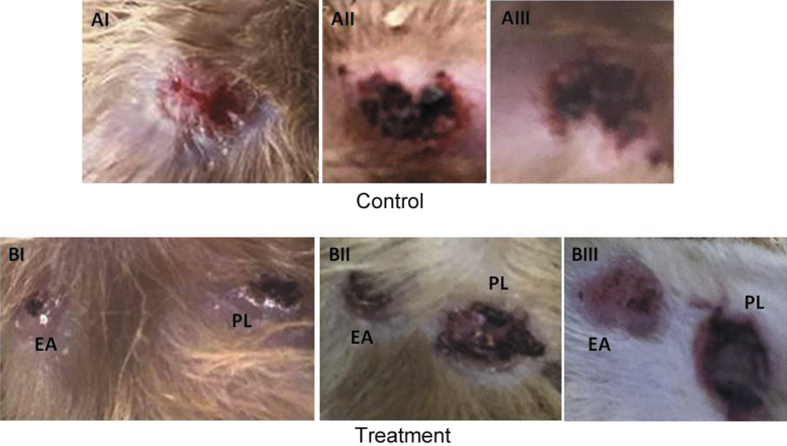
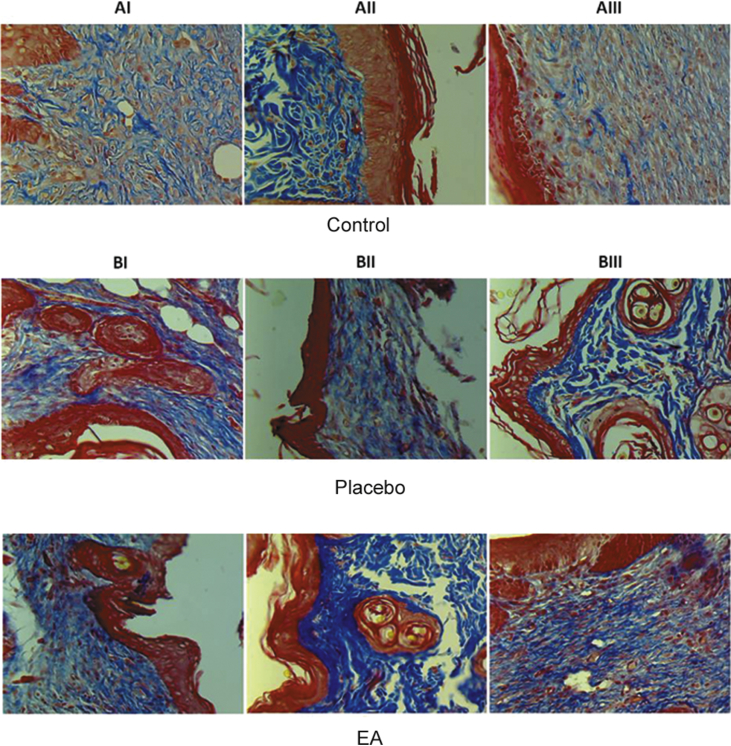
As shown by the macroscopic images for the site of ulceration, it was observed that the ulcers treated by the EA ointment healed faster than those of the control or with PL-treatment (Fig. 1). In order to investigate the quality of the healed tissue, microscopic examination was performed using masson-trichrome stain (Fig. 2). Two samples out of three of group A (AI and AIII) showed minimal fibrosis, while sample (AII) showed severe fibrosis. For group B, all ulcers treated with the EA ointment showed higher degree of fibrosis as compared to the corresponding placebo-treated ulcer of the same animal. In addition, the degree of fibrosis was further digitally evaluated by analyzing the image using ImageJ software. As shown in Fig. 3, the mean gray value of the blue channel showed significantly lower value for EA-treated samples as compared with the corresponding placebo-treated ones, which reflects darker blue values for EA-treated samples and therefore higher degree of fibrosis, and this indicates a better repair process.
Fig. 1.
Macroscopic examination for the wound ulcers at the 15th day of the experiment. Control group: AI–AIII, received no treatment. Treatment group: BI–BIII. EA: treated with E. alata ointment. PL: treated with a placebo ointment.
Fig. 2.
Histology for the skin samples obtained from the site of wound ulcers on day 15th of treatment. The samples were stained with masson-trichrome stain. Group A consists of control samples. In group B, all paired images belong to the same animal. The images in the upper panel are for ulcers treated with placebo ointment, while the images in the lower panel are for ulcers treated with E. alata ointment. AI: moderate degree of collagen fibers deposition. AII: severe degree of collagen fibers deposition. AIII: mild degree of collagen fibers deposition. BI placebo: moderate degree of collagen fibers deposition. BI EA: severe degree of collagen fibers deposition. BII placebo: mild degree of collagen fibers deposition. BII EA: moderate degree of collagen fibers deposition. BIII placebo: mild degree of collagen fibers deposition. BIII EA: moderate degree of collagen fibers deposition. Magnification 400×.
Fig. 3.
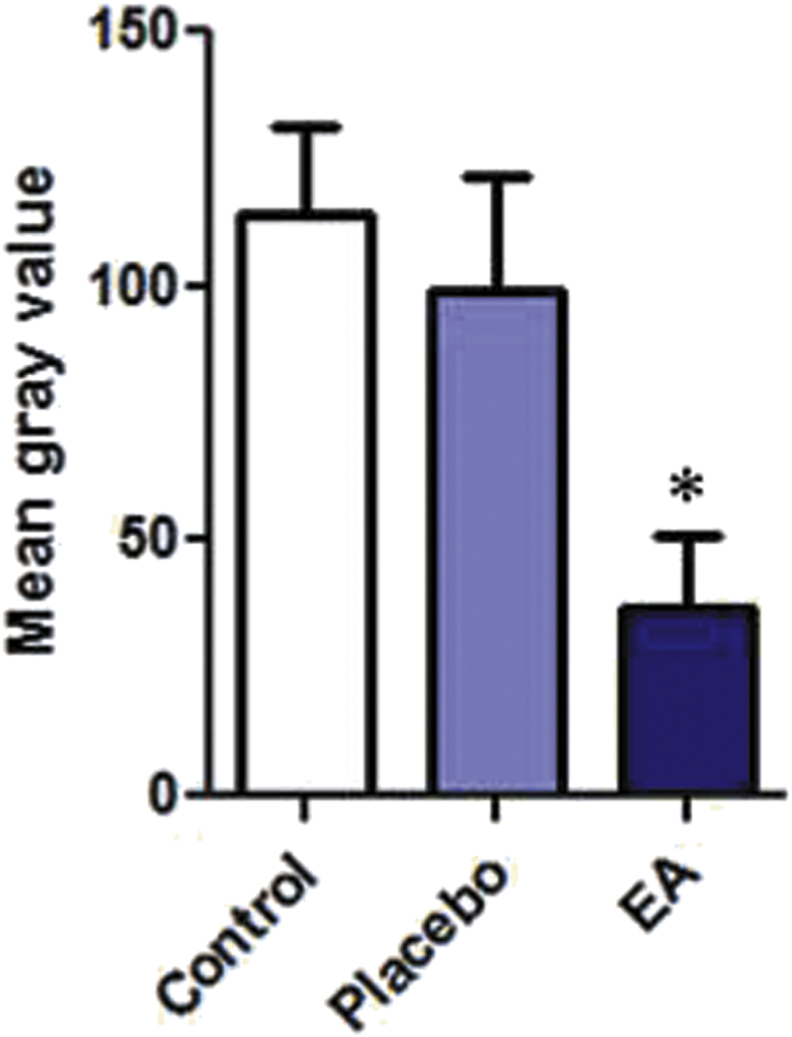
Image analysis for the degree of fibrosis or wound ulcers. The analysis was performed using ImageJ software. Color deconvolution plug-in was used to separate the blue color. After that at least 150 points were randomly selected on the deconvoluted blue image, and the mean gray value was measured for the blue color using the measure function in ImageJ software. The paired-sample t-test showed a statistically significant lower mean gray values for EA-treated samples as compared to the placebo, which reflects darker blue values for EA-treated samples and therefore higher degree of fibrosis. n = 3, p ≤ 0.05.
Investigation of burn healing activity of E. alata extract ointment
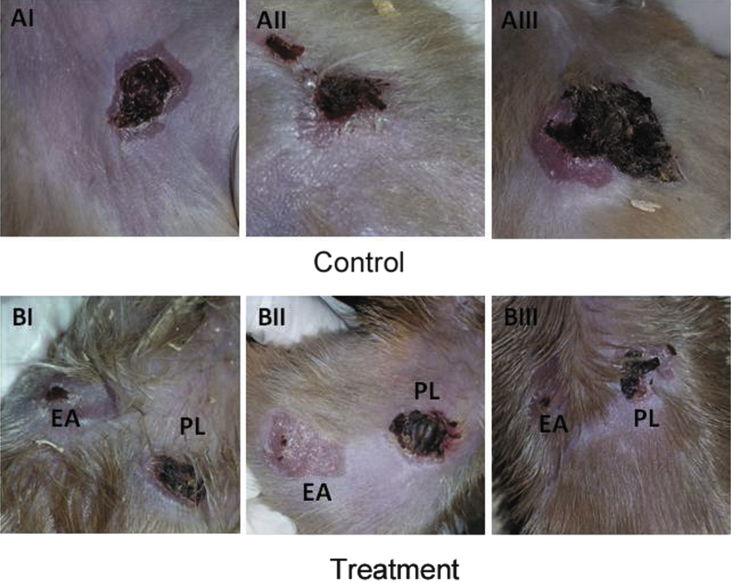
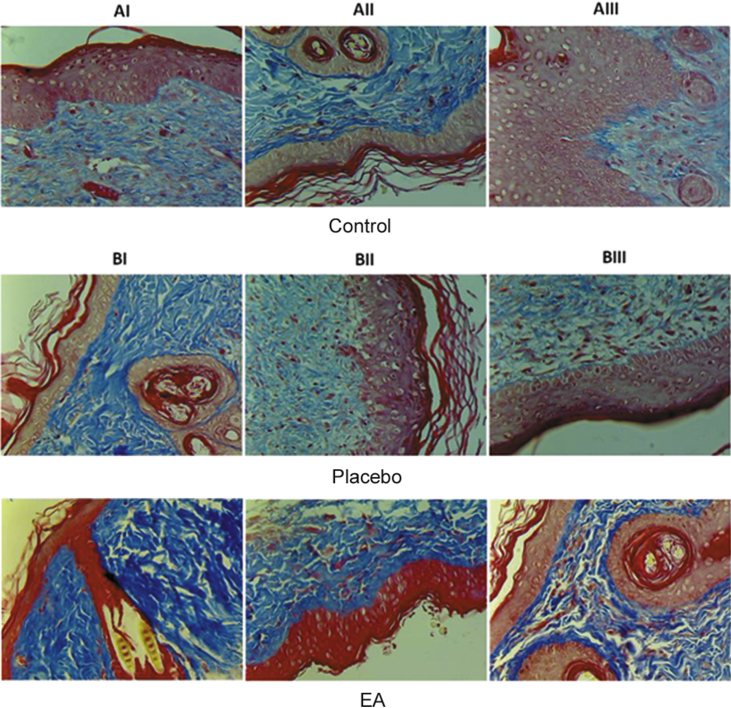
The same protocol used for testing the healing activity of the wound ulcers was used to evaluate the effect of EA ointment on the healing of burn ulcers. As shown by the macroscopic images for the site of ulceration, it was observed that the ulcers treated by the EA ointment healed faster than those of the control or with PL-treatment (Fig. 4). In order to check the quality of the healed tissue, microscopic examination for tissue biopsies was performed using the masson-trichrome stain as shown in Fig. 5. Two samples from group A (negative control) AI and AIII showed mild degree of collagen fibers deposition, whereas sample AII demonstrated severe fibrosis. Coming to group B, the ulcer treated with EA ointment was always compared with its internal control ulcer for the same animal treated with the placebo ointment. As compared to the corresponding placebo-treated sample, much better (severe) fibrosis and collagen fibers deposition was identified in sample IIB, which indicates a better repair process. As detailed in the previous experiment, the images were analyzed by ImageJ software in order to digitally compare the degree of fibrosis between the placebo and the EA-treated burn ulcers (Fig. 6).
Fig. 4.
Macroscopic examination for the wound ulcers at the 15th day of the experiment. Control group: AI–AIII, received no treatment. Treatment group: BI–BIII. EA: treated with E. alata ointment. PL: treated with a placebo ointment.
Fig. 5.
Histology for the skin samples obtained from the site of burn ulcers on day 15th of treatment. The samples were stained with masson-trichrome stain. Group A consists of control samples. In group B, all paired images belong to the same animal. The images in the upper panel are for ulcers treated with placebo ointment, while the images in the lower panel are for ulcers treated with E. alata ointment. AI: mild degree of collagen fibers deposition. AII: severe fibrosis, indicating the collagen fibers deposition. AIII: minimal fibrosis. BI placebo: mild degree of collagen fibers deposition. BI EA: moderate fibrosis. BII placebo: mild degree of collagen fibers deposition. BII EA: severe fibrosis. BIII placebo: moderate degree of collagen fibers deposition. BIII EA: moderate fibrosis. Magnification 400×.
Fig. 6.
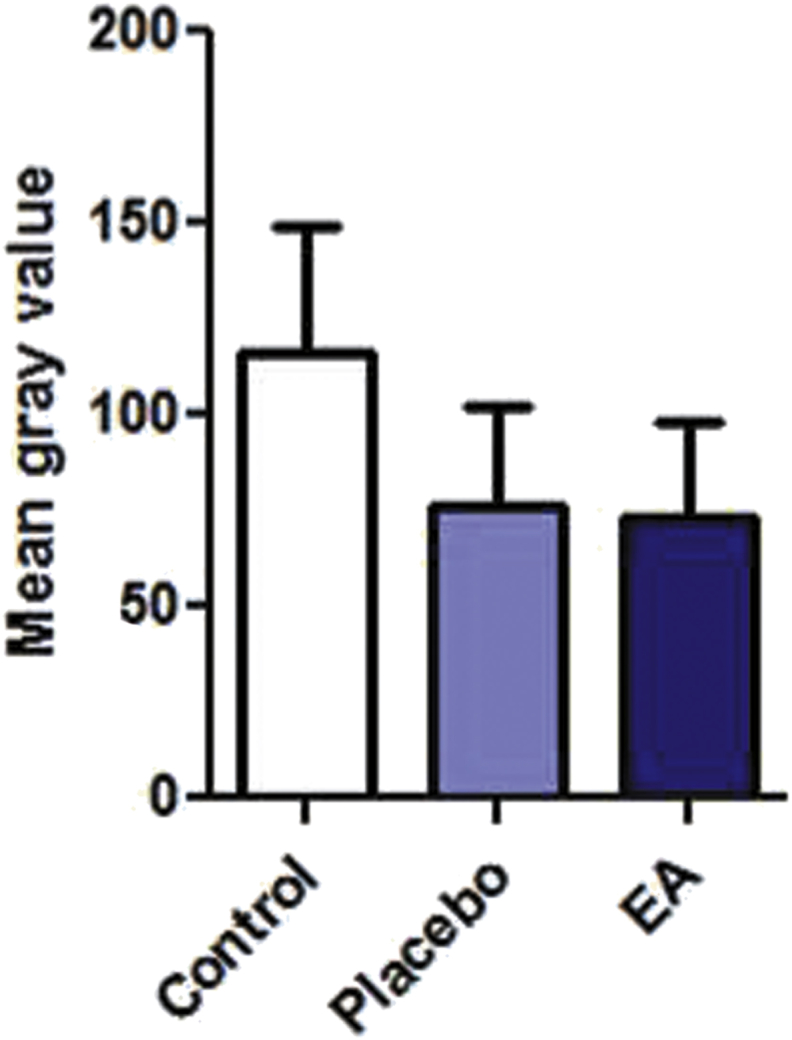
Image analysis for the degree of fibrosis of burn ulcers. The analysis was performed using ImageJ software. Color deconvolution plug-in was used to separate the blue color. After that at least 150 points were randomly selected on the deconvoluted blue image, and the mean gray value was measured for the blue color using the measure function in ImageJ software. The paired-sample t-test showed no statistically significant difference between the mean gray values for EA-treated samples and the placebo.
Discussion
The management of different kinds of skin ulcers, such as those associated with deep wounds, diabetic foot, bed sores and severe burns is a real clinical challenge. Normally, such ulcers require a prolonged healing time rendering the ulcers prone to difficult infections and disfiguring scaring. Therefore there is a demand towards developing new treatments that can accelerate the process of healing with a suitable quality of the regenerated skin.8, 9, 10 Besides keratinocyte proliferation and migration, another important event during the healing process is myofibroblasts differentiation and collagen fibers deposition, which is often driven by factors such as transforming growth factor- β (TGF-β) that are upregulated upon tissue injury. Another important event is the reformation of new blood capillary network at the site of tissue repair, which is induced by the vascular endothelial growth factor (VEGF).11 Several research groups have shown that alkaloids such as taspine and flavonoids such as baicalin and quercetin could improve the process of wound healing via the upregulation of TGF-β and VEGF.12, 13, 14, 15, 16 In our project, the phytochemical analysis of E. alata extracts revealed that the plant is rich of a variety of organic compounds including flavonoids and alkaloids, which was also shown previously by Jaradat and colleagues.5 Therefore, in this project it was hypothesized that E. alata extract could have a wound healing properties. Full thickness wound or full thickness burn ulcers were induced on the dorsal side of the animals.
Treatment of wound ulcers with EA ointment accelerated the healing process, enhanced the deposition of collagen and improved the process of fibrosis as compared to the placebo ointment, indicating a better repair process. We speculate the observed therapeutic effects of EA ointment to be a result of enhanced production of pro-fibrotic factors such as TGF-β and VEGF at the site of drug application. We recommend future detailed studies to test this hypothesis. On the other hand, although the macroscopic examination demonstrated that EA ointment could also accelerate the healing process of the burn ulcers, the analysis of the microscopic images of the histological sections showed that the degree of fibrosis did not actually change significantly with EA treatment as compared to placebo. Unlike the excision wound, high temperature during the induction of burn causes the death of keratinocytes at the edges of the formed ulcer. These cells are known to interact with dermal fibroblasts, which are important for fibroblasts activity and consequently critical for the quality of the healing process.17, 18 Therefore we hypothesize that the mechanism of action for the active ingredients of E. alata might require viable healthy marginal tissue that is capable to produce profibrotic mediators. However, further follow-up studies are required in the future in order to specifically determine the phytochemical compounds responsible for the observed therapeutic effects, and to elucidate the underlying mechanism.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Sen C.K., Gordillo G.M., Roy S. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veeresham C. Natural products derived from plants as a source of drugs. J Adv Pharm Technol Res. 2012;3:200–201. doi: 10.4103/2231-4040.104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natho G., Mandaville J.P. Flora of Eastern Saudi Arabia. Feddes Repertor. 1991;102:444. [Google Scholar]
- 4.Kim H.K., Choi Y.H., Chang W.T. Quantitative analysis of ephedrine analogues from Ephedra species using 1H-NMR. Chem Pharm Bull (Tokyo) 2003;51:1382–1385. doi: 10.1248/cpb.51.1382. [DOI] [PubMed] [Google Scholar]
- 5.Jaradat N., Hussen F., Al-Ali A. Preliminary phytochemical screening, quantitative estimation of total flavonoids, total phenols and antioxidant activity of Ephedra alata Decne. J Mater Environ Sci. 2015;6:1771–1778. [Google Scholar]
- 6.Jaradat N.A., Al-Ramahi R., Zaid A.N. Ethnopharmacological survey of herbal remedies used for treatment of various types of cancer and their methods of preparations in the West Bank-Palestine. BMC Complement Altern Med. 2016;16:93. doi: 10.1186/s12906-016-1070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shabana M.M., Mirhom Y.W., Genenah A.A. Study into wild Egyptian plants of potential medicinal activity. Ninth communication: hypoglycaemic activity of some selected plants in normal fasting and alloxanised rats. Arch Exp Veterinarmed. 1990;44:389–394. [PubMed] [Google Scholar]
- 8.Mulder G., Tenenhaus M., D'Souza G.F. Reduction of diabetic foot ulcer healing times through use of advanced treatment modalities. Int J Low Extrem Wounds. 2014;13:335–346. doi: 10.1177/1534734614557925. [DOI] [PubMed] [Google Scholar]
- 9.Andrews K.L., Houdek M.T., Kiemele L.J. Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prosthet Orthot Int. 2015;39:29–39. doi: 10.1177/0309364614534296. [DOI] [PubMed] [Google Scholar]
- 10.Mudge E.J. Recent accomplishments in wound healing. Int Wound J. 2015;12:4–9. doi: 10.1111/iwj.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastar I., Stojadinovic O., Yin N.C. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3:445–464. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y., He L., Chen F. Enhancement of wound healing by taspine and its effect on fibroblast. Zhong Yao CAi. 2005;28:579–582. [PubMed] [Google Scholar]
- 13.Zhang K., Lu J., Mori T. Baicalin increases VEGF expression and angiogenesis by activating the ERR{alpha}/PGC-1{alpha} pathway. Cardiovasc Res. 2011;89:426–435. doi: 10.1093/cvr/cvq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopalakrishnan A., Ram M., Kumawat S. Quercetin accelerated cutaneous wound healing in rats by increasing levels of VEGF and TGF-beta1. Indian J Exp Biol. 2016;54:187–195. [PubMed] [Google Scholar]
- 15.Ambiga S., Narayanan R., Gowri D. Evaluation of wound healing activity of flavonoids from Ipomoea carnea Jacq. Anc Sci Life. 2007;26:45–51. [PMC free article] [PubMed] [Google Scholar]
- 16.Hasanoglu A., Ara C., Ozen S. Efficacy of micronized flavonoid fraction in healing of clean and infected wounds. Int J Angiol. 2001;10:41–44. doi: 10.1007/BF01616343. [DOI] [PubMed] [Google Scholar]
- 17.Sawicki G., Marcoux Y., Sarkhosh K. Interaction of keratinocytes and fibroblasts modulates the expression of matrix metalloproteinases-2 and -9 and their inhibitors. Mol Cell Biochem. 2005;269:209–216. doi: 10.1007/s11010-005-3178-x. [DOI] [PubMed] [Google Scholar]
- 18.Tandara A.A., Mustoe T.A. MMP- and TIMP-secretion by human cutaneous keratinocytes and fibroblasts—impact of coculture and hydration. J Plast Reconstr Aesthet Surg. 2011;64:108–116. doi: 10.1016/j.bjps.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]