Abstract
Bradycardia is the most common form of dysrhythmia developing after disruption of the sympathetic pathway by a spinal cord injury (SCI), and it can have fatal consequences, including cardiac arrest. Here, we report a case of cardiac arrest developing after cervical SCI attributable to sympathetic hypoactivity. A 26-year-old male pedestrian was admitted after a traffic accident. Radiologically, fractures were apparent at the C6–7 bilateral articular facets, and cord contusion with hemorrhage was evident at C4–7. During his stay in ICU, intermittent bradycardia was noted, but the symptoms were not specific. On the 22nd postoperative day, the patient was taken to the computed tomography suite for further evaluation and experienced cardiac arrest during a positional change. After immediate cardiac massage, the patient was resuscitated. We scheduled Holter monitoring, which detected 26 pauses, the longest of which was 17.9 s. The patient underwent cardiac pacemaker insertion. No further cardiac events were noted.
Keywords: Heart arrest, Parasympathetic nervous system, Spinal cord injuries, Sympathetic nervous system
Introduction
Spinal cord injury (SCI) is the most devastating form of neurological injury, disturbing the normal sensory, motor, and/or autonomic functions.1 The annual incidence of SCI in the USA is about 40 cases per million of population.2 SCI is caused principally by trauma (e.g. traffic injuries, falls, and violence). The risk of death is highest in the first year after SCI and varies by level and severity of injury.3 Cardiovascular disease is one of the principal causes of death.3, 4 In the USA, diseases of the circulatory system are the most common causes of death.4 In Norway, the main causes of death following the experience of a SCI are pneumonia/influenza, ischemic heart disease, and urogenital disease.3
In patients with acute cervical SCIs (rather than thoracolumbar SCIs), cardiovascular deficits, including severe bradycardia, asystole, and loss of peripheral vascular tone, are known complications.5, 6 Acute autonomic imbalances developing after disruption of the sympathetic pathway (located in the cervical cord) are thought to trigger such cardiovascular events.5, 6, 7 Although autonomic dysfunction developing after SCI can have fatal consequences, including cardiac arrest, many clinicians do not recognize such risks, and the conditions are often misdiagnosed. Here, we report a case of cardiac arrest developing after cervical SCI. The immediate cause was autonomic nervous system dysfunction. The patient improved after pacemaker insertion.
Case report
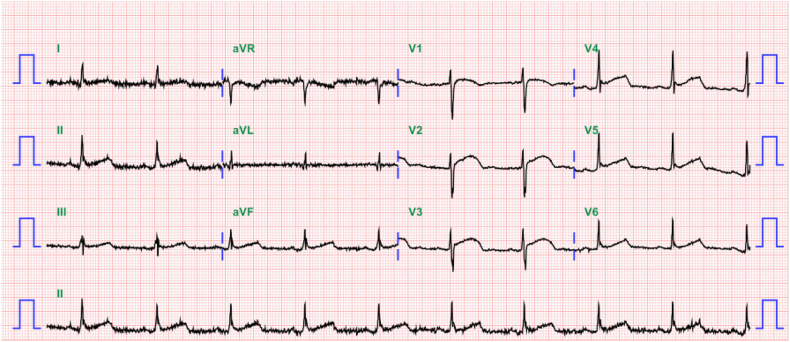
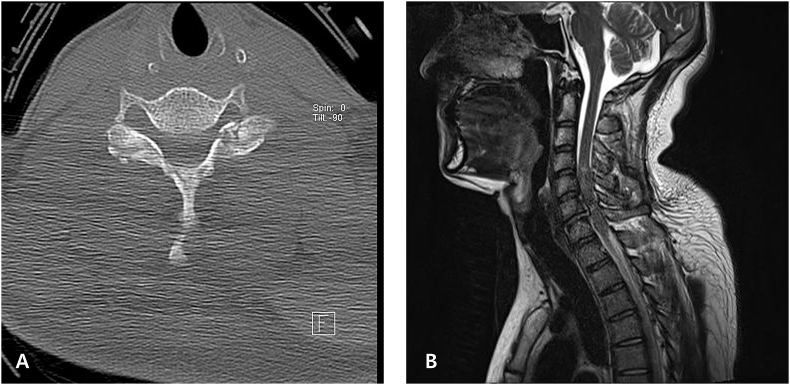
A 26-year-old male pedestrian was admitted to hospital via the emergency room after a traffic accident. He was mentally alert and complained of weakness and numbness of both the upper and lower extremities. On physical and neurological examination, no external injuries were evident except for an abrasion on the left arm. However, paraplegia was apparent. The baseline electrocardiogram (ECG) revealed sinus bradycardia (57 beats/min, Fig. 1). Initial chest and abdominal computed tomography (CT) did not reveal any abnormality or trauma-related injury. A skull fracture, with a small subdural hemorrhage at the right frontal convexity was evident on brain and facial CT. Spinal CT revealed acute fractures at the C6–7 bilateral articular facets and the right-side lamina of C6 (Fig. 2A). Magnetic resonance imaging revealed cord contusion with hemorrhage at C4–7 (Fig. 2B).
Fig. 1.
The baseline electrocardiogram (ECG) revealed sinus bradycardia (57 beats/min).
Fig. 2.
A: A spinal computed tomography revealed acute fractures at the C6–7 bilateral articular facets and the right-side lamina of C6. B: The magnetic resonance image showed contusion with hemorrhage at C4–7.
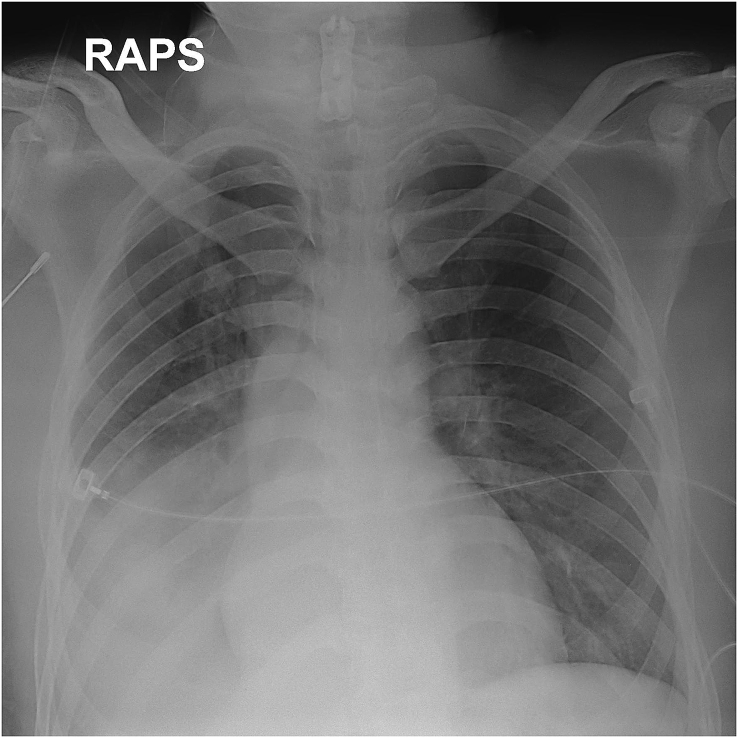
On the first day of admission, the patient underwent microscopy-assisted corpectomy and discectomy with anterior interbody fusion. Postoperatively, the patient remained in the ICU for monitoring. On the fifth postoperative day (POD), a pneumonic infiltration with pleural effusion was evident on a follow-up chest X-ray (Fig. 3). Fever developed, and self-expectoration was impossible because respiration was shallow. Bronchoscopy was performed, and a large volume of purulent secretion was evident in the dependent portion. The patient was transferred to the Division of Pulmonology for further respiratory treatment in an ICU. Antibiotics were commenced, ventilator support put in place, and a tracheostomy performed.
Fig. 3.
On the 5th postoperative day, a pneumonic infiltration with pleural effusion was evident on a follow-up chest X-ray.
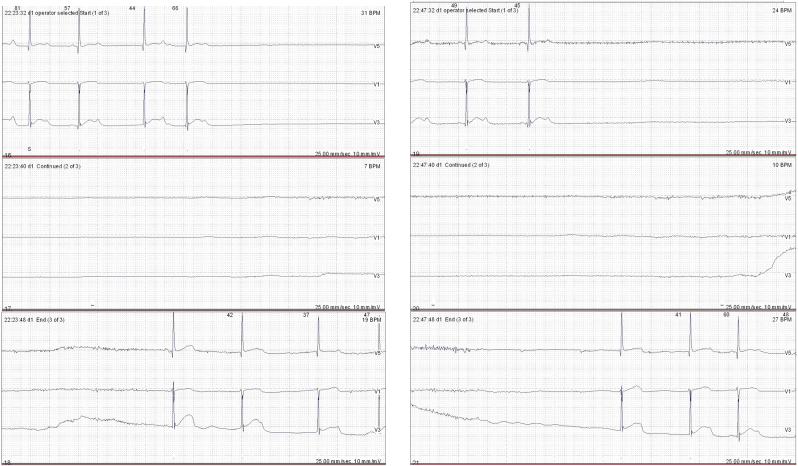
During the ICU stay, intermittent bradycardia (30–55 beats/min) was evident, without specific symptoms; his vital signs were stable. Intravenous atropine (0.25 mg) was given twice to treat severe bradycardia; the heart rate was recovered in a few seconds. Echocardiography revealed no specific abnormality, and the left ventricle exhibited normal systolic function (ejection fraction 60%). On the 22nd POD, chest CT was scheduled for differential diagnosis of a pulmonary thromboembolism. During a positional change prior to imaging in the CT suite, the heart rate fell to 23 beats/min, and blood pressure was not palpable at the carotid artery. After immediate cardiac massage over 1 min, the patient was resuscitated and exhibited stable vital signs. Holter monitoring was performed on the day after cardiac arrest (Fig. 4). The average heart rate was 67 beats/min. However, 26 pauses were evident, the longest of which was 17.9 s. A temporary cardiac pacemaker was initially placed because of the patient's poor general condition and the risk of infection. On the 31st POD, a permanent cardiac pacemaker was inserted in consultation with the Division of Cardiology. No further cardiac event was noted. The patient was transferred to the Department of Rehabilitation after ventilator weaning on the 90th POD and was discharged 2 months later.
Fig. 4.
Holter monitoring showed that the average heart rate was 67 beats/min. However, 26 pauses were evident, the longest of which was 17.9 s.
Discussion
Cardiovascular complications, such as bradycardia, arterial hypotension, and loss of peripheral vascular tone, are common complications of acute SCI.3, 4 Such cardiovascular phenomena are usually observed in patients with upper thoracic or cervical SCIs.8 Bradycardia is the most common form of dysrhythmia developing after SCI, especially in the acute phase of injury.9 Several studies have shown that almost all patients with complete cervical SCIs will develop bradycardia, and 16% will suffer cardiac arrest.5, 9 Cardiac dysfunction usually commences within 3–5 days after injury, and it typically resolves in 6–8 weeks.8, 10 Although bradycardia developing after acute SCI is usually temporary, there is a real risk of cardiac arrest, and adequate management is required. Treatment of bradycardia in SCI patients generally involves administration of oxygen, atropine, inotropes, and aminophylline.11 Temporary and permanent pacemakers have been placed in patients who are refractory to general treatment and experience life-threatening events such as cardiac arrest.10, 11
We recommend that patients with acute SCIs should be admitted to ICUs or similar high-care settings in order to facilitate acute cardiopulmonary management; this is especially important if a patient has a high-level SCI or exhibits hemodynamic instability.12 Several studies have shown that ICU monitoring and aggressive medical management improve the outcomes of patients with acute SCIs.12, 13 Additionally, such patients can develop multiple (cardiovascular, respiratory, and urinary) complications, trophic skin changes, and heterotopic ossification.14 Hence, a multidisciplinary ICU approach is needed to minimize various problems.13 However, most clinicians do not recognize the risk of severe bradycardia that can trigger cardiac arrest and often misdiagnose the conditions that then would develop.
In our case, the patient was transferred from the Department of Orthopedics to the Division of Pulmonology because he developed respiratory complications and thus required further ICU care after surgery. Ventilator support was put in place, and a tracheostomy was performed. The ICU medical team did not pay much attention to the intermittent bradycardia. The patient usually self-recovered and was stable, and showed no specific symptoms. On the 22nd POD, a CT to explore the possibility of pulmonary thromboembolism was planned because the hypoxia was persistent and the D-dimer level was elevated. However, the patient went into cardiac arrest during a positional change in the CT suite located outside the ICU. Consequently, a cardiac pacemaker was inserted after asystole was confirmed by 24-h Holter monitoring.
Disruption of the supraspinal sympathetic pathways, inducing parasympathetic dominance, by a cervical SCI, is the major cause of cardiovascular instability.15, 16 The preganglionic sympathetic nerve fibers exit the spinal cord at the first-to-fourth thoracic vertebrae (T1–T4) and are controlled by higher centers via messages transmitted through the cervical spinal cord.7 However, the parasympathetic system is controlled by the vagus nerve, which originates in the medulla.6 Thus, the extent of sympathetic dysfunction is related to the location and severity of the SCI.15 Any increase in vagal reflexes, as might be induced by tracheal suctioning, a hypoxic episode, or a change in posture, can precipitate a bradycardia event.17 Other studies have shown that the sympathetic hypoactivity gradually improves within 4–6 weeks after injury.5, 16
The proportion of SCI patients requiring a pacemaker ranges from 9% to 17%.5, 10, 18 Percutaneous cardiac pacing is only a temporary tool employed in emergency situations. Such a pacemaker is associated with risks of injury, skeletal muscle contraction, and pacing failure, and it suffices only until permanent transvenous cardiac pacing can be established.18 Cardiac pacemaker implantation is indicated for patients with high-level SCIs and continuing symptomatic bradycardia and they do not respond to medical management or suffer recurrent cardiac arrests.19 Sanghvi et al described a patient with a traumatic C7–D1 dislocation who experienced continuous severe bradycardia and cardiac arrest for 2 months after injury.7 Ultimately, a permanent cardiac pacemaker was inserted. Gilgoff et al reported a patient with a C2 SCI causing complete quadriplegia; the patient experienced symptomatic bradycardia for 21 months after injury despite intensive medical management.19 Clinical improvement was evident after insertion of a cardiac pacemaker.
In conclusion, patients who suffer SCIs are at risk of severe bradycardia and cardiac arrest attributable to dysfunction of the autonomic nervous system. The risks are highest during the acute phase of injury and in patients with upper thoracic or cervical SCIs. Monitoring in an ICU, careful assessment of autonomic dysfunction, and aggressive medical management, are required. In addition, procedures that may increase vagal reflexes should be very carefully performed after prior preparation for a possible emergency situation that may develop very rapidly.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Singh A., Tetreault L., Kalsi-Ryan S. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–331. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devivo M.J. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 3.Lidal I.B., Snekkevik H., Aamodt G. Mortality after spinal cord injury in Norway. J Rehabil Med. 2007;39:145–151. doi: 10.2340/16501977-0017. [DOI] [PubMed] [Google Scholar]
- 4.Garshick E., Kelley A., Cohen S.A. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43:408–416. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehmann K.G., Lane J.G., Piepmeier J.M. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: incidence, time course and severity. J Am Coll Cardiol. 1987;10:46–52. doi: 10.1016/s0735-1097(87)80158-4. [DOI] [PubMed] [Google Scholar]
- 6.Bilello J.F., Davis J.W., Cunningham M.A. Cervical spinal cord injury and the need for cardiovascular intervention. Arch Surg. 2003;138:1127–1129. doi: 10.1001/archsurg.138.10.1127. [DOI] [PubMed] [Google Scholar]
- 7.Sanghvi A.V., Chhabra H.S., Nigam V. Permanent cardiac pacemaker for cardiac arrest following cervico-dorsal spinal injury. Eur Spine J. 2009;18(suppl 2):254–257. doi: 10.1007/s00586-009-0944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rollstin A., Carey M.C., Doherty G. Oral albuterol to treat symptomatic bradycardia in acute spinal cord injury. Intern Emerg Med. 2015;11:101–105. doi: 10.1007/s11739-015-1324-3. [DOI] [PubMed] [Google Scholar]
- 9.Grigorean V.T., Sandu A.M., Popescu M. Cardiac dysfunctions following spinal cord injury. J Med Life. 2009;2:133–145. [PMC free article] [PubMed] [Google Scholar]
- 10.Pasnoori V.R., Leesar M.A. Use of aminophylline in the treatment of severe symptomatic bradycardia resistant to atropine. Cardiol Rev. 2004;12:65–68. doi: 10.1097/01.crd.0000096418.72821.fa. [DOI] [PubMed] [Google Scholar]
- 11.Weant K.A., Kilpatrick M., Jaikumar S. Aminophylline for the treatment of symptomatic bradycardia and asystole secondary to cervical spine injury. Neurocrit Care. 2007;7:250–252. doi: 10.1007/s12028-007-0067-z. [DOI] [PubMed] [Google Scholar]
- 12.Ryken T.C., Hurlbert R.J., Hadley M.N. The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery. 2013;72(suppl 2):84–92. doi: 10.1227/NEU.0b013e318276ee16. [DOI] [PubMed] [Google Scholar]
- 13.Reines H.D., Harris R.C. Pulmonary complications of acute spinal cord injuries. Neurosurgery. 1987;21:193–196. doi: 10.1227/00006123-198708000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Aito S. Complications during the acute phase of traumatic spinal cord lesions. Spinal Cord. 2003;41:629–635. doi: 10.1038/sj.sc.3101513. [DOI] [PubMed] [Google Scholar]
- 15.Furlan J.C., Fehlings M.G. Cardiovascular complications after acute spinal cord injury: pathophysiology, diagnosis, and management. Neurosurg Focus. 2008;25:E13. doi: 10.3171/FOC.2008.25.11.E13. [DOI] [PubMed] [Google Scholar]
- 16.Teasell R.W., Arnold J.M., Krassioukov A. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506–516. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Arango A.F., Robinson V.J., Sharma G.K. Characteristics of patients with cervical spinal injury requiring permanent pacemaker implantation. Cardiol Rev. 2006;14:e8–e11. doi: 10.1097/01.crd.0000184453.29079.0f. [DOI] [PubMed] [Google Scholar]
- 18.Rangappa P., Jeyadoss J., Flabouris A. Cardiac pacing in patients with a cervical spinal cord injury. Spinal Cord. 2010;48:867–871. doi: 10.1038/sc.2010.48. [DOI] [PubMed] [Google Scholar]
- 19.Gilgoff I.S., Ward S.L., Hohn A.R. Cardiac pacemaker in high spinal cord injury. Arch Phys Med Rehabil. 1991;72:601–603. [PubMed] [Google Scholar]