To the Editor
Discuss this article on the JACI Journal Club blog: www.jacionline.blogspot.com.
Cutaneous granulomas are a well-recognized pathologic feature in patients with various primary immunodeficiency diseases (PIDs) and may be self-limited or can progress to a persisting granulomatous disorder.1,2 Rubella virus (RV) vaccine strain RA27/3 has been recently detected in disseminated cutaneous granulomas of 2 patients with ataxia telangiectasia (AT) and a patient with Simpson-Golabi-Behmel syndrome (who had combined immunodeficiency [CID]).3 However, a more detailed study of a larger series of granuloma cases in patients with different PIDs was required to confirm and extend this observation.
Patients with cutaneous granulomas and with diverse PIDs were selected from the United States Immune Deficiency Network registry. Additional cases (cases 1 and 11) were recruited from the Clinical Immunology Society immune deficiency Listserv. Presence of RV in granuloma-containing tissue samples (Fig 1, A–C) was examined by immunofluorescence staining with 2 different RV capsid-specific antibodies (see this article’s Methods section in the Online Repository at www.jacionline.org) by a reader blinded to the diagnosis. Seven out of 14 patients (50%) exhibited positive RV antigen staining (Table I), whereas the tissue samples of 5 non-PID granuloma patients were negative.
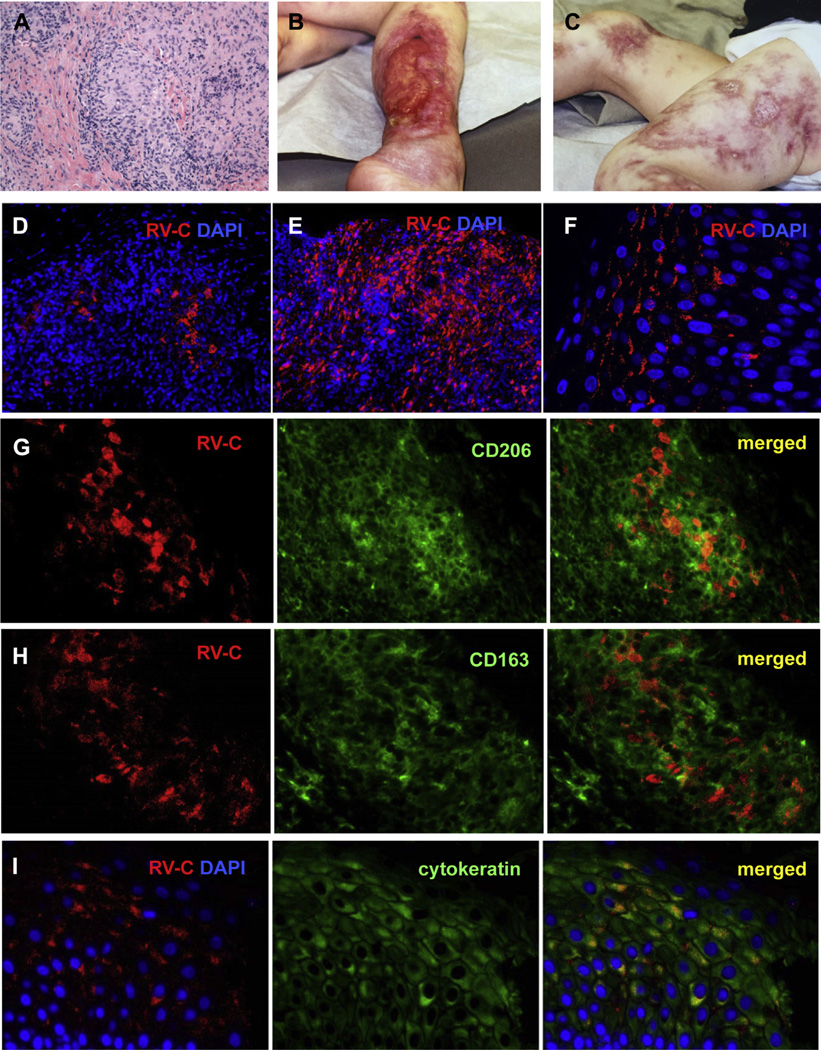
FIG 1.
Cutaneous granulomas in patients. A, Hematoxylin and eosin staining of a cutaneous granuloma from case 1. A well-formed granuloma is centrally located. B and C, Cutaneous skin lesions from case 3. Acute and chronic ulcers are observed. D–F, Distribution of RV-infected cells in skin samples of patients with PID. Histological immunofluorescent staining showing focal (Fig 1, D, case 1) or widespread (Fig 1, E, case 2) distribution of RV capsid in granulomas and focal capsid localization in the epidermis (Fig 1, F, case 5). Activation status of macrophages in granulomas (G–I, case 6). Double immunofluorescent staining of granulomas with RV capsid antibody (red) and M2 macrophage-specific antibodies, CD206 (Fig 1, G, green) or CD163 (Fig 1, H, green). I, RV antigen expression in the suprabasal cell layer of skin epidermis (case 1). Double immunofluorescent staining with RV capsid antibody (red) and keratinocyte-specific antibody (cytokeratin, green). Nuclei were counterstained with DAPI. DAPI, 4′-6-Diamidino-2-phenylindole, dihydrochloride; RV-C, rubella virus capsid.
TABLE I.
Information on patients with PID
| Case | Country | Immune deficiency |
Age at biopsy (y) |
Current status* | Biopsy description (all patients were negative for mycobacteria) |
Other sites |
|---|---|---|---|---|---|---|
| 1† | Finland | CID | 21, 24, & 28 | Alive (MMR, 5 and 9 y) |
Dermis with strong lymphocytic infiltrate, granulomatous inflammation. Some Langerhan giant cells. Over time, progressive necrosis |
Three skin biopsies were all positive |
| 2† | USA | CID | 11 | Alive (MMR) | Epidermal hyperplasia, dermal edema, suppurative granulomatous inflammation |
|
| 3† | USA | CHH | 8 | Deceased (MMR 15 mo) |
Skin and subcutaneous tissue incorporating predominantly dermal lymphohistiocytic infiltrates with occasional multinucleated giant cells, extending into the subcutaneous fat, and with inflammation focally extending into the epidermis |
|
| 4† | USA | AT | 9 | Deceased | Necrotizing granulomatous inflammation | |
| 5† | USA | AT | 1 | Alive (MMR 1 y) | The dermis contains chronic inflammatory infiltrate. The deep dermis/subcutaneous tissue is markedly abnormal. The tissue is replaced by large and small granulomas. The granulomas are necrotizing. Outlines of fat cells are seen within pink amorphous matrix, which includes necrotic nucleoli |
|
| 6† | USA | AT | 5 | Deceased | Necrotic vasculitis with neutrophilic infiltrates at some locations and necrotizing granulomatous inflammation of the dermis at other locations. Bone biopsy showed histiocytic infiltrate with early myelofibrosis |
Multiple skin sites, not all positive but bone biopsy positive |
| 7† | USA | AT | 7 | Alive | Noninfectious granulomatous process | |
| 8 | USA | MWS | 3 | Alive (MMR) | Marked hyperkeratosis, parakeratosus with focal scale, crust, and follicular plugging. Epidermal disruption, dermal lymphoplasmacytic infiltrate with prominent dermal granulomas with central necrosis |
Multiple skin sites |
| 9 | USA | CVID | 28 | Alive | Superficial and deep dermal diffuse granulomatous infiltrate composed of giant cells and epithelial cells |
Granulomas in 2 skin biopsies both negative |
| 10 | USA | CVID | 47 | Alive | Noncaseating dermal granulomatous inflammation with eosinophils | |
| 11 | Germany | XLA | 38 | Alive (MMR) | Perivascular T-cell infiltrates, with oligoclonality. PAS-positive (PAS reaction) particles in the subepidermal region |
|
| 12 | USA | NEMO | 10 | Alive | Subacute spongiotic dermatitis with focal parakeratosis, mild acanthosis, mild spongiosis with superficial dermal lymphohistiohistic infiltrate and absence of eccrine glands. CD3, CD5, and CD7 staining is seen on the lymphocytes. CD68 and CD163 decorate admixed histiocytes |
|
| 13 | USA | AT | 10 | Alive | Widespread collagen necrobiosis associated with granulomatous inflammation |
|
| 14 | USA | AT | 3 | Alive (MMR) | Sarcoidal granulomatous dermatitis with many associated CD8-positive lymphocytes, which focally obscure the dermoepidermal junction. The overlying epidermis is acanthotic with compact hyperkeratosis, parakeratosis, and plugged infundibula. Collections of epithelioid histiocytes, some of which are multinucleated, that palisade around central foci of fibrin and mucin |
CHH, Cartilage hair hypoplasia; CVID, common variable immunodeficiency; MWS, Marden-Walker syndrome; NEMO, nuclear factor kappa B essential modulator deficiency; PAS, periodic acid–Schiff; XLA, X-linked agammaglobulinemia.
Vaccination status and age of vaccination for rubella were indicated in parentheses, if known.
Indicates rubella antigen positive.
Staining intensity varied substantially between patients and did not correlate with the severity of granulomatous disease. RV immunostaining was typically observed in both epidermis and granulomas in dermis (Fig 1, D–F); however, staining only in granulomas (case 3) was seen (Table I). Multiple granulomas within a sample contained RV antigen with typically a few positive cells in the middle except cases 2 and 6, in which virtually all cells in the granulomas were positive. RV was found exclusively in patients with CIDs: CID cause unknown (n = 2), AT (n = 4), and cartilage hair hypoplasia (n = 1). The immune deficiencies in which granulomas were not found to be positive for rubella were common variable immune deficiency (n = 2), AT (n = 2), X-linked agammaglobulinemia (n = 1), Marden-Walker syndrome (n = 1), and nuclear factor kappa B essential modulator (n = 1). None of the nonimmune deficient samples was positive.
RV-positive cells in granulomas were positive for CD14 and CD68, markers of monocyte/macrophage cell lineage, and CD206 and CD163, activation markers for M2 macrophages, but negative for iNOS, an M1 macrophage marker (see this article’s Methods section; see Table E1 in this article’s Online Repository at www.jacionline.org; Fig 1, G–H). Endothelial cells (vWF+), T cells (CD3+), B cells (CD20+), dermal Langerhans (CD1a+), and dendritic (CD11c+) cells were negative for RV antigen (see this article’s Results section in the Online Repository at www.jacionline.org; Table E1). These results demonstrate that M2 macrophages were the cell type harboring RV antigen in granulomas. There was a high production of cytokeratin in many RV-positive keratinocytes, suggesting that RV replication in keratinocytes can lead to dysregulation of keratin synthesis (Fig 1, I). Overexpression of keratin is known to alter the architecture of the epidermis and impact healing of ulcers.4
The RV-immunostaining patterns were unchanged in the 3 skin samples obtained within a 7-year period (case 1), indicating long-term persistence of RV antigen. Antigen persistence is a hallmark of granulomas. Biopsy specimens collected from different body sites contained both RV-positive and RV-negative specimens (case 3), indicating focal distribution of RV antigen-positive cells in patient tissues. In addition to the skin samples from case 6, a bone periosteum tissue (collected 5 years later) contained RV-positive M2 macrophages in the granulomas.
PCR fragments covering the entire genome were amplified and sequenced from 1 patient. Phylogenetic analysis revealed that rubella virus of genotype 1a was present in the patient skin (RVs/Oulu.FIN/22.15/PID; see Fig E1 in this article’s Online Repository at www.jacionline.org). The sequence was similar (97.4% identity) to that of the RA27/3 vaccine virus. In RVs/Oulu.FIN/22.15/PID, 2 out of 7 RA27/3-specific amino acid residues had reverted to the wild type (see this article’s Results section; see Table E2 in this article’s Online Repository at www.jacionline.org). There were 69 amino acid substitutions in RVs/Oulu.FIN/22.15/PID compared with RA27/3; 52 of them were not found in wild-type RV genomes (see Table E3 in this article’s Online Repository at www.jacionline.org). Most neutralizing epitopes, which are located in the E1 protein, were conserved including the immunodominant epitope E1214–233, whereas each of 3 known CD8+ T-cell epitopes (largely predicted to be A2 binding), all located in the capsid, contained single mutations (see this article’s Results section; see Table E4 in this article’s Online Repository at www.jacionline.org), suggesting a role for CD8+ T-cell–selective pressure in viral evolution during chronic RA27/3 infection.
Similar to the previous report,3 AT was the most common single diagnosis among RV-positive patients. We have also identified additional PIDs (cartilage hair hypoplasia and CID) in which RV was found in granulomas. Thus, current data have clearly shown an association between defects in T-cell immunity, granulomas, and RV.
One of the important findings of this study is the identification of RV-positive cells in granulomas as M2 macrophages. Proinflammatory M1 macrophages play an essential role in eliminating pathogens, whereas anti-inflammatory M2 macrophages are crucial for maintaining tissue homeostasis. RV can infect most cell types and can persistently infect a macrophage-like cell line,5 but it is currently unknown whether this occurs in vivo. Another novel finding is persisting RV antigen in epidermal keratinocytes, in all epidermal layers except the basal layer. In contrast, in acute postnatal rubella cases, rubella antigen was found only in the deep dermis (cell types not defined) in skin biopsies from rubella rashes, whereas the epidermis was negative.6
Wild-type RV can establish persistent infections and cause disease in immunologically normal individuals if infection occurs in immune-privileged sites, for example, Fuchs’ uveitis or fetal development. A role for vaccine virus in Fuchs’ uveitis is also suspected.7,8 Granulomas have been reported only once in CRS.9 We hypothesize that the immune deficiency allows persistence of the attenuated virus, polarization of macrophages to M2 occurs, compromising viral clearance, mutations accrue, and complete viral escape occurs upon acquisition of CD8 epitope mutations. As the virus persists, damage to the keratinocytes occurs and ulcers appear. One patient exhibited persistence over 23 years, defined by sequential biopsies. Our finding that mutations occurred in cytotoxic T lymphocytes epitopes is consistent with CD8+ T-cell–selective forces playing a dominant role. Whether RV is the cause of the granulomas or a passenger in the presence of absent cellular immune responses to the virus can be determined only if antiviral therapy results in resolution of the granulomas.
The inability to isolate live virus from well-preserved skin tissue was unexpected because RV can generally be isolated from the skin with similar amounts of RNA. One conceivable explanation for our failure to recover infectious RV would be if the persisting virus is defective, analogous to the defective measles viruses in subacute sclerosing panencephalitis cases.
In summary, our results demonstrate that RV can establish chronic infection in M2 macrophages and keratinocytes in patients with diverse T-cell deficiencies. Our study suggests that individuals with cellular immune deficiencies may be at risk for persistent RV infections that stimulate nonprotective immune response associated with chronic M2-type granuloma formation.
Supplementary Material
Acknowledgments
We thank Dr Min-hsin Chen for providing genomic sequences of clinical RV strains for phylogenetic analysis, Drs Laura S. Finn and Sejal Shah for expert pathologic support, and Dr Mikko Seppänen for critical insights. We thank, remember, and honor the patients who have contributed.
This study was supported by the Wallace Chair of Pediatrics and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
S. Plotkin serves as a vaccine consultant. P. Russo receives royalties from Springer Francisco Bonilla, serves as a consultant for CSL Behring, received payments for lectures from Pediatric Update, received royalties from UpToDate, received travel support from the Immune Deficiency Foundation, and serves on the Blood Product Advisory Committee (US Food and Drug Administration). K. E. Sullivan serves as a consultant for the Immune Deficiency Foundation and receives grant support from Baxter and royalties from UpToDate.
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Sillevis Smitt JH, Kuijpers TW. Cutaneous manifestations of primary immunodeficiency. Curr Opin Pediatr. 2013;25:492–497. doi: 10.1097/MOP.0b013e3283623b9f. [DOI] [PubMed] [Google Scholar]
- 2.Nanda A, Al-Herz W, Al-Sabah H, Al-Ajmi H. Noninfectious cutaneous granulomas in primary immunodeficiency disorders: report from a national registry. Am J Dermatopathol. 2014;36:832–837. doi: 10.1097/DAD.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 3.Bodemer C, Sauvage V, Mahlaoui N, Cheval J, Couderc T, Leclerc-Mercier S, et al. Live rubella virus vaccine long-term persistence as an antigenic trigger of cutaneous granulomas in patients with primary immunodeficiency. Clin Microbiol Infect. 2014;20:O656–O663. doi: 10.1111/1469-0691.12573. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Folmer J, Coulombe PA. Increased expression of keratin 16 causes anomalies in cytoarchitecture and keratinization in transgenic mouse skin. J Cell Biol. 1994;127:505–520. doi: 10.1083/jcb.127.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarmiento RE, Tirado R, Gomez B. Reinfection-induced increase of rubella persistently infected cells in a macrophage-like cell line. Virus Res. 1997;50:15–22. doi: 10.1016/s0168-1702(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi H, Umino Y, Sato TA, Kohama T, Ikeda Y, Iijima M, et al. Detection and comparison of viral antigens in measles and rubella rashes. Clin Infect Dis. 1996;22:36–39. doi: 10.1093/clinids/22.1.36. [DOI] [PubMed] [Google Scholar]
- 7.Islam SM, El-Sheikh HF, Tabbara KF. Anterior uveitis following combined vaccination for measles, mumps and rubella (MMR): a report of two cases. Acta Ophthalmol Scand. 2000;78:590–592. doi: 10.1034/j.1600-0420.2000.078005590.x. [DOI] [PubMed] [Google Scholar]
- 8.Kreps EO, Derveaux T, De Keyser F, Kestelyn P. Fuchs’ uveitis syndrome: no longer a syndrome? Ocul Immunol Inflamm. 2016;24:348–357. doi: 10.3109/09273948.2015.1005239. [DOI] [PubMed] [Google Scholar]
- 9.Hancock MP, Huntley CC, Sever JL. Congenital rubella syndrome with immunoglobulin disorder. J Pediatr. 1968;72:636–645. doi: 10.1016/s0022-3476(68)80006-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.