Abstract
T cells with high functional avidity can sense and respond to low levels of cognate Ag, a characteristic that is associated with more potent responses against tumors and many infections, including HIV. Although an important determinant of T cell efficacy, it has proven difficult to selectively induce T cells of high functional avidity through vaccination. Attempts to induce high-avidity T cells by low-dose in vivo vaccination failed because this strategy simply gave no response. Instead, selective induction of high-avidity T cells has required in vitro culturing of specific T cells with low Ag concentrations. In this study, we combined low vaccine Ag doses with a novel potent cationic liposomal adjuvant, cationic adjuvant formulation 09, consisting of dimethyldioctadecylammonium liposomes incorporating two immunomodulators (monomycolyl glycerol analog and polyinosinic-polycytidylic acid) that efficiently induces CD4 Th cells, as well as cross-primes CD8 CTL responses. We show that vaccination with low Ag dose selectively primes CD4 T cells of higher functional avidity, whereas CD8 T cell functional avidity was unrelated to vaccine dose in mice. Importantly, CD4 T cells of higher functional avidity induced by low-dose vaccinations showed higher cytokine release per cell and lower inhibitory receptor expression (PD-1, CTLA-4, and the apoptosis-inducing Fas death receptor) compared with their lower-avidity CD4 counterparts. Notably, increased functional CD4 T cell avidity improved antiviral efficacy of CD8 T cells. These data suggest that potent adjuvants, such as cationic adjuvant formulation 09, render low-dose vaccination a feasible and promising approach for generating high-avidity T cells through vaccination.
Introduction
Novel vaccine candidates have traditionally been evaluated by the quantity of the responding T cells, but recently it has become clear that T cell quality is probably even more important, and strategies to improve T cell quality are now considered crucial for optimizing the potency of novel vaccines (1, 2). Increasing the functional avidity of T cells in vivo through immunization is a promising strategy to increase vaccine efficacy against infectious diseases and tumors (3–9).
T cells of high functional avidity are able to respond to very low levels of cognate Ag, and high functional avidity has been linked with enhanced clearance of viral infections and tumors (5, 10). Functional avidity is highly complex and is regulated by many variables. The strength of binding between a T cell and the APC is crucial and is highly dependent on TCR affinity and structural avidity for the cognate MHC–peptide complex on the APC (10). However, the strength of the immunological synapse and functional avidity between a T cell and the APC are also affected by TCR–coreceptor expression, costimulatory receptor expression levels on T cell/APC, localization of TCR in lipid rafts, TCR signaling efficiency, and the local cytokine/inflammatory milieu among others (11, 12). Despite this complexity, readouts for functional avidity are rather straightforward; they measure the Ag concentration required to activate T cells as assessed by functional assays, including cytokine production, proliferation, and target cell lysis. Importantly, functional T cell avidity is highly dependent on Ag dose. We originally described selective induction of T cells with high functional avidity (5): CD8 T cells cultured in vitro with low levels of Ag displayed higher avidity and antiviral efficacy compared with low-avidity T cells cultured with high Ag concentrations. So far, selectively enhancing functional avidity has mainly been possible through in vitro expansion (5). Priming high-avidity T cells by vaccination in vivo has proved difficult, because vaccination with low vaccine Ag doses in vivo results in no or negligible immune responses (5, 13). Furthermore, it was shown that in vitro–derived high-avidity T cells were very susceptible to clonal deletion through activation-induced cell death, became increasingly susceptible to tolerance induction, and had poor memory capacity (14–16).
Our group has focused on developing cationic liposomal adjuvants for infectious disease targets, and these adjuvants are highly efficient at delivering Ag to and activating dendritic cells (DCs) to prime T cell responses, even at very low Ag doses (17, 18). One such adjuvant, cationic adjuvant formulation (CAF)09, efficiently induces Th and CTL responses (19). Combining novel potent adjuvants with low-dose immunizations has not been done previously; in this study, we investigated this promising strategy for the induction of high-avidity T cells and improved vaccine efficacy.
In this article, we show that immunizing mice with low Ag doses in CAF09 selectively enhances CD4, but not CD8, T cell functional avidity and that this increased functional avidity leads to improved protection in a viral challenge model.
Materials and Methods
Mice
Experiments were performed with 7–10-wk-old BALB/c mice that were immunized three times at 2-wk intervals, unless otherwise stated. For adoptive transfer experiments, we used wild-type (WT) BALB/c or RT1 TCR-transgenic (Tg) mice (20), in which CD8 T cells recognize the H2-Dd–restricted HIV IIIB gp160 envelope aa 318–327 P18-I10 (RGPGRAFVTI) epitope, as donor mice and H2d SCID mice (BALB/c background) as recipient mice. For the IL-15 study, IL-15–knockout (KO) mice (21) on a C57BL/6 background were used with age-matched C57BL/6 WT controls. All mice were bred and purchased from Charles River (Frederick, MD), and experiments were performed at the National Cancer Institute (NCI). All protocols were approved and performed under the guidelines of the NCI’s animal care and use committee; animals were housed in appropriate facilities at the NCI and received water and food ad libitum.
Vaccines, Ags, and immunizations
Ags were mixed in a total volume of 100 μl of 10 mM Tris-HCl (pH 7.4) and mixed 1:1 with 100 μl of the liposomal CAF09 (19), consisting of dimethyldioctadecylammonium bromide (DDA; NCK, Copenhagen, Denmark), synthetic monomycolyl glycerol (MMG; NCK) analog MMG-1, and the TLR-3 agonist polyinosinic-polycytidylic acid (pI:C; Sigma-Aldrich, Copenhagen, Denmark) and formulated by the film method, as previously described (22). Thus, a total of 200 μl of vaccine was given per mouse dose that contained 250 μg of DDA, 50 μg of MMG-1, and 50 μg of pI:C. Vaccines were vortexed for 30 s and left for >10 min before injection. No analgesics or anesthesia were used or needed for immunizations. Three immunizations, spaced at 2-wk intervals, were given i.p., unless otherwise stated. The cluster peptide, PCLUS6.1-P18 (GenScript, Piscataway, NJ), containing a H2d-restricted helper epitope (underlined) in the PCLUS6.1 part of the peptide (DRVIEVVQGAYRAIRHIPRRIRQGLER) and the immune dominant H2-Dd–restricted P18-I10 (underlined) contained within P18 (RIQRGPGRAFVTIGK) (23) were used as vaccine Ags. Doses of PCLUS6.1-P18 are indicated in the figures and legends. The molecular mass = 4.9 kDa; hence, a vaccine dose of 1 nmol per mouse corresponds to ∼4.9 μg. For the IL-15–KO study, mice were immunized with 50 μg of the hepatitis B core Ag aa 128–140 (TPPAYRPPNAPIL; GenScript) (24) formulated in CAF09, as described above.
In vitro cell cultures
Splenocyte cultures were obtained by passage of spleens through a 100-μm nylon cell strainer (BD Pharmingen, San Jose, CA). After washing, cells pooled from three to five mice in each experiment were cultured in tissue culture–treated 96-well round-bottom Costar plates (Corning Life Sciences, Corning, NY) containing 2 × 105 cells in a volume of 200 μl of complete RPMI (RPMI 1640 supplemented with 5 × 10−5 M 2-ME, 1% [v/v] premixed penicillin-streptomycin solution, 1 mM glutamine [all from Life Technologies, Thermo Fischer Scientific, Waltham, MA], and 10% (v/v) FBS [Gemini Bio Products, West Sacramento, CA]). Supernatants from triplicate cultures were harvested from cultures after 5 d of incubation at 37°C and 5% CO2 for the assessment of IFN-γ by ELISA (see below). For intracellular cytokine and surface marker analysis by flow cytometry, 1–2 × 106 cells per well from individual spleens were cultured at 37°C and 5% CO2 in complete RPMI for 1 h in tissue culture–treated 96-well round-bottom plates (Costar) and subsequently for 5 h after the addition of brefeldin A (5 μg/ml; Sigma-Aldrich, St. Louis, MO) or overnight (∼16-h incubation). Cells were kept at 4°C overnight or stained immediately. No differences in responses or avidity were observed in a direct comparison between the two methods. For some in vitro studies, 5 ng/ml recombinant human IL-15 (PeproTech, Rocky Hill, NJ) was added to splenocyte cultures for the stimulation period before intracellular cytokine staining (ICS). PMA (40 ng/ml) and ionomycin (1 μg/ml; both from Sigma-Aldrich) were used as a positive control and induced IFN-γ production in 5–20 and <70% of CD4 and CD8 T cells, as measured by flow cytometry, respectively, or >10,000 pg/ml of IFN-γ per well, as assessed by ELISA (see below).
Cytokine ELISA
A sandwich ELISA was used to determine the concentration of IFN-γ in culture supernatants in microtiter plates (96 well; MaxiSorp; Nunc, Copenhagen, Denmark). The Mouse IFN-γ ELISA Ready-SET-Go! kit (eBioscience, San Diego, CA) was used per the manufacturer’s instructions.
Flow cytometry
A total of 1–2 × 106 splenocytes, obtained as described above, was stained for surface markers, including CD3ε (clone 17A2), CD4 (GK1.5), CD8α (53-6.7), TCR-β (H57-587), PD-1 (CD279; J43 or 29F.1A12), Fas (CD95; Jo2), and CTLA-4 (CD152; UC10-4B9), permeabilized, and subsequently stained for intracellular cytokine expression of IFN-γ (XMG1.2), IL-2 (JES6-5H4), TNF-α (MP6-XT22), and IL-17A (eBio17-B7) using the BD Cytofix/Cytoperm kit, according to the manufacturer’s instructions. Samples stained with T-bet (4B10) were assessed using a Foxp3 intracellular staining kit (eBioscience). All Abs were purchased from eBioscience, BD Biosciences (San Jose, CA), or BioLegend (San Diego, CA). All samples were run on a BD LSR II flow cytometer with three (green, red, and violet) or four (green, red, violet, and UV) lasers, and results were analyzed using FlowJo software v8.87 (TreeStar, Ashland, OR). SPICE and PESTLE software, provided by M. Roederer (National Institutes of Health) Bethesda (25), was used for Boolean gating of T cell subsets producing different combinations of measured cytokines. All cell populations of interest were gated using the following hierarchy: singlets (diagonal of forward scatter-A versus forward scatter-H), live cells, lymphocytes by characteristic forward–side scatter profile, and finally either CD4 or CD8 cells where gating was mutually exclusive. CD3 was not used to only include T cells, because it was strongly downregulated after in vitro stimulation, as in evident in the dot plots. Background levels of media controls (<0.15% of CD4/CD8 T cells for IFN-γ, IL-2, and IL-17A; <0.2% for TNF-α) were subtracted for the Boolean gate analysis of T cell subsets producing different combinations of cytokine but not for the total and normalized responses shown for single cytokines in the remaining graphs. Graphs were prepared using Prism version 5 (GraphPad, La Jolla, CA).
Estimating viral loads by plaque assay
Viral loads in ovaries were estimated as previously described (4, 26), with minor modifications. Briefly, ovaries were homogenized and sonicated at 4°C, and serial 10-fold dilutions were plated and cultured in complete DMEM (2.5% FBS, 200 mM L-Glutamine, 100 U/ml Penicillin, and 100 μg/ml Streptomycin; all from Sigma-Aldrich) on confluent BSC-1 cells in 24-well tissue culture–treated plates (Corning Life Sciences); the volume per well was 0.5 ml. After 2 d of incubation at 37°C/5% CO2, cell monolayers were stained with 0.1% Crystal Violet in 20% ethanol, and plaques were counted.
Statistical analysis
Functional avidity was calculated as log10(EC50), where EC50 was the concentration of peptide needed to induce 50% of the maximal response. EC50 was calculated individually for each mouse. Avidity was also shown as the ratio between high-avidity cells and the total response (high- and low-avidity cells; see the text and figure legends for details). For normalization of responses the highest response (% cytokine producing CD4/CD8 T cells) within each mouse was set to 100% (max response), and the normalized responses at different concentrations of stimulation calculated (normalized response at concentration X) = (% cytokine-producing CD4/CD8 T cells at stimulation X)/(maximum response) × 100%. Statistical differences between vaccine groups with regard to magnitudes of response, functional avidity, and log10 protection were determined by one-way ANOVA and the Newman–Keul posttest for multiple comparisons; a p value < 0.05 was considered significant. Viral loads (log10 PFU) were compared using the Kruskal–Wallis nonparametric test, with the Dunn posttest for multiple comparisons. When comparing two mean fluorescent intensity (MFI) curves over the course of several concentrations of stimulation or Boolean subsets of cytokine-producing cells from different vaccine groups, a two-way repeated-measures ANOVA was used, with comparisons for each stimulation concentration done using the Bonferroni correction for multiple comparisons. The relationship between viral load and immune response was calculated using the Pearson product-moment correlation. All statistical analyses were performed with Prism version 5 (GraphPad).
Results
Immunization with low doses of Ag selectively favors CD4 over CD8 T cell induction
We assessed the relationship between induction of CD4 and CD8 T cells and vaccine Ag dose using a 42-aa multiepitope cluster HIV peptide (PCLUS6.1-P18) comprising helper and CTL epitopes restricted to H-2d. The vaccine Ag was given in the novel cross-priming adjuvant CAF09 (DDA/MMG-1/pI:C) (19), and the adjuvant dose was kept identical for all groups. We hypothesized that with the CAF09 adjuvant, which selectively and efficiently delivers Ag to and activates DCs, we would be able to induce a CTL response using low vaccine Ag doses and, hence, overcome the threshold issues observed with low-dose vaccinations using conventional adjuvants and viral vectors. The ultimate goal was to achieve higher functional avidity of the vaccine-specific CD8 T cells when using low vaccine doses.
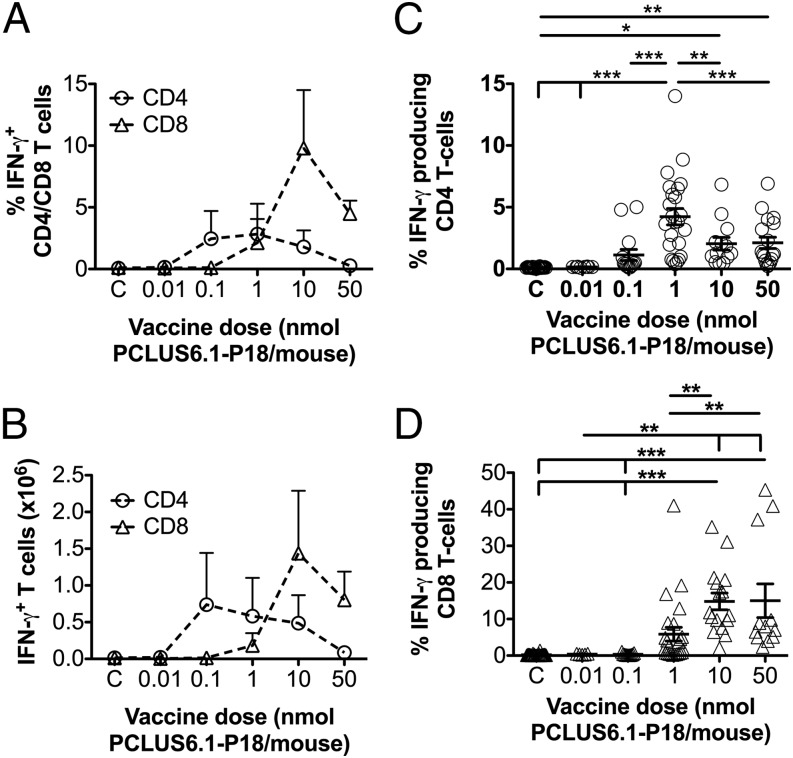
H-2d BALB/c mice were immunized three times 2 wk apart with various doses of the vaccine Ag PCLUS6.1-P18 in CAF09. One week after the final immunization, spleens were harvested. Splenocytes were restimulated in vitro with the vaccine Ag PCLUS6.1-P18, and cytokine production was assessed by flow cytometry and ICS. Higher percentages (Fig. 1A) and absolute numbers (Fig. 1B) of vaccine-specific CD4 IFN-γ+ cells were induced at lower vaccine doses, whereas induction of CD8 T cell responses required a higher dose of Ag. This differential pattern was not specific for any one cytokine; it also held true for TNF, IL-2, and IL-17A (Supplemental Fig. 1A, 1B, 1E). The number of CD4 T cells peaked at a vaccine dose of 0.1–1 nmol PCLUS6.1-P18 in CAF09 per mouse for all three cytokines, whereas an optimal CD8 T cell response was observed at a 10–100-fold higher vaccine dose (10 nmol PCLUS6.1-P18 per mouse) (Fig. 1). Because we were interested in vaccine-specific Th1 and CTL efficacy related to viral challenge, we focused on the canonical Th1 cytokine IFN-γ as the key readout for the remainder of the study. This experiment was repeated multiple times, and we pooled data from eight of the repeated experiments that used the same vaccine doses, timing, and stimulations, confirming a lower-dose optimum for CD4 over CD8 T cells and showing a significant difference in the response magnitudes between vaccine groups (p < 0.05–0.001, Fig. 1C, 1D).
FIGURE 1.
Low-dose immunizations favor induction of CD4 T cells over CD8 T cells. (A and B) BALB/c mice were immunized i.p. three times at 2-wk intervals with different doses of PCLUS6.1-P18 in CAF09, as indicated on the x-axis (C, control group receiving CAF09 only). One week after the third immunization, splenocytes were restimulated in vitro with 5 μM PCLUS6.1-P18 in the presence of brefeldin A and assessed for intracellular IFN-γ production by flow cytometry. The graphs depict the mean (+ SEM) percentages (A) and absolute numbers (B) of CD4 T cells and CD8 T cells producing IFN-γ after stimulation in each vaccine dose group (n = 3 per group). These results are representative of nine experiments with similar results. (C and D) Pooled analysis of eight repeated immunization experiments. Not all experiments included all vaccine doses, and one repeated experiment used different doses and could not be pooled. Mice were immunized, and IFN-γ production was assessed by flow cytometry, as described for (A) and (B). The graphs depict the mean percentages (± SEM) of CD4 T cells (○) and CD8 T cells (△) producing IFN-γ following stimulation with 5 μM PCLUS6.1-P18. n = 28 for CAF09; n = 5, 11, 24, 16, and 12 for vaccine groups dosed at 0.01, 0.1, 1, 10, and 50 nmol PCLUS6.1-P18, respectively. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA and Newman–Keul posttest.
Low-dose Ag vaccination induces CD4, but not CD8, T cells of enhanced functional avidity
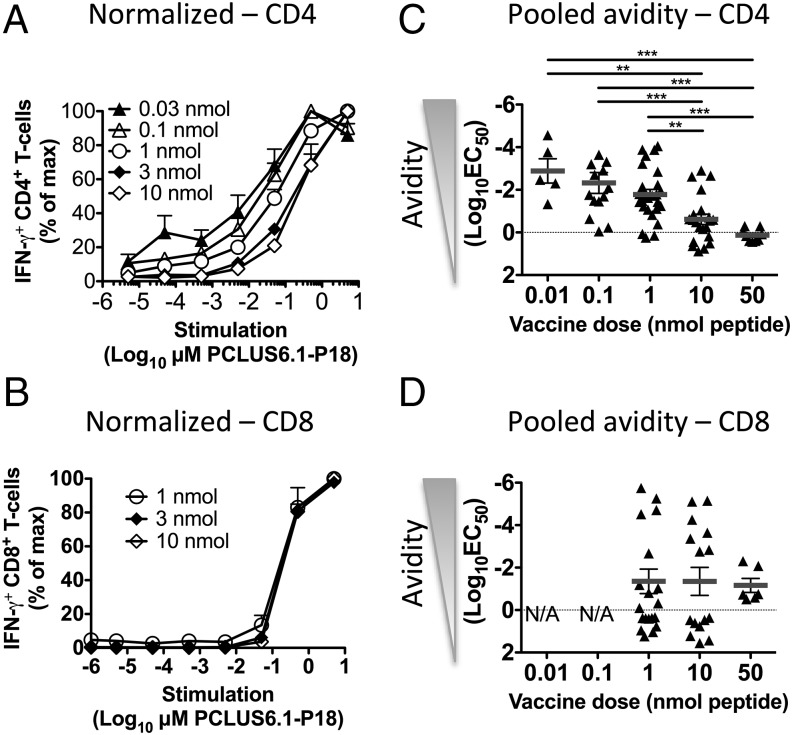
In a follow-up experiment, we immunized mice as described above but included more vaccine doses to measure functional T cell avidity as a function of vaccine Ag dose. Splenocytes from immunized mice were stimulated with a range of vaccine Ag concentrations (5 × 10−5 to 5 μM PCLUS6.1-P18) in vitro after the last immunization, and IFN-γ production was measured by flow cytometry and ICS. Consistent with Fig. 1, we noted that lower vaccine doses (0.03–1 nmol) favored CD4 T cell responses, whereas higher doses (≥1 nmol) were required for CD8 T cell induction (Supplemental Fig. 1C). Higher vaccine doses (50–100 nmol peptide per mouse) did not result in increased Th or CTL responses (data not shown). Importantly, lower vaccine doses (0.03–1 nmol PCLUS6.1-P18 in CAF09 per mouse) resulted in vaccine-specific CD4 T cells responding to lower concentrations of Ag in vitro and, hence, were of higher functional avidity than CD4 T cells from groups given higher vaccine Ag doses (3–10 nmol; Fig. 2A). Functional avidity of CD4 T cells, calculated as log10(EC50), differed significantly among vaccine groups (p < 0.05–0.01; Fig. 2C, Supplemental Fig. 1D). Interestingly, the same trend of higher functional avidity with low vaccine Ag doses in CAF09 was also observed when using TNF, IL-2, and IL-17 as readout (Supplemental Fig. 1E, 1F). Surprisingly, the functional avidity dose-response curves [using IFN-γ and log10(EC50) as readout] for the groups that had induced a significant CD8 T cell response were very similar among vaccine doses (Fig. 2B, 2D, Supplemental Fig. 1C, 1D). Likewise, no differences for dose-response avidity curves were obtained for CD8 T cells by stimulating with various concentrations of the minimal P18-I10 CTL epitope or when the other above-mentioned cytokines were used as readout (data not shown). Because we used relatively small group sizes (n = 3 or 4 mice per group) and had repeated the experiment multiple times, we decided to perform a pooled analysis calculating avidity [log10(EC50)] to confirm the differences observed in T cell avidity. The pooled analysis reflected the data from the experiment shown in Fig. 2A and 2B well and also showed a significant increase in CD4 T cell functional avidity with lower vaccine doses (p < 0.01–0.001), whereas there was no effect of vaccine dose on CD8 T cell avidity (Fig. 2C, 2D). Most likely, a change in the peptide supplier midway through the study resulted in the high variation in functional avidity, especially for CD8 T cells.
FIGURE 2.
Low-dose immunization selectively enhances functional avidity of CD4, but not CD8, T cells. BALB/c mice were immunized i.p. three times at 2-wk intervals with different doses of PCLUS6.1-P18 in CAF09 and euthanized 1 wk later, when functional avidity of splenocytes was assessed by ICS and flow cytometry. Relative percentage of CD4 (A) and CD8 (B) T cells producing IFN-γ after in vitro stimulation with increasing concentrations (5 × 10−6 to 5 μM) of Ag, as indicated on the x-axis. Responses were normalized to the maximum response for each mouse (set to 100%; magnitudes of responses are shown in Supplemental Fig. 1C) and plotted as a function of peptide concentration used for stimulation. Data points represent mean and SEM of n = 3 mice per group from mice immunized with the PCLUS6.1-P18 doses shown. Statistical analyses were performed using two-way repeated-measures ANOVA and the Bonferroni correction for multiple comparisons. Only groups with a response significantly different from the control group are shown. Pooled avidity [log10(EC50)] for CD4 (C) and CD8 (D) T cells calculated based on normalized curves, such as the ones shown in (A) and (B), from repeated experiments. n = 14–26 for 0.1, 1, and 10 nmol vaccine groups; n = 5–9 for the remaining groups. Data points represent functional T cell avidity [log10(EC50)] from individual mice; mean and SEM are shown. The data are representative of 10 experiments with similar results. **p < 0.01, ***p < 0.001, one-way ANOVA and Newman–Keul posttest for multiple comparisons.
Hence, immunization with low doses of PCLUS6.1-P18 in CAF09 selectively induced CD4 T cells, whereas higher doses of Ag were needed to induce a significant CD8 T cell response. To our surprise, lower vaccine doses selectively enhanced functional avidity of vaccine-specific CD4, but not CD8, T cells.
Relative and absolute numbers of high-avidity CD4 T cells increase with low vaccine Ag dose immunizations
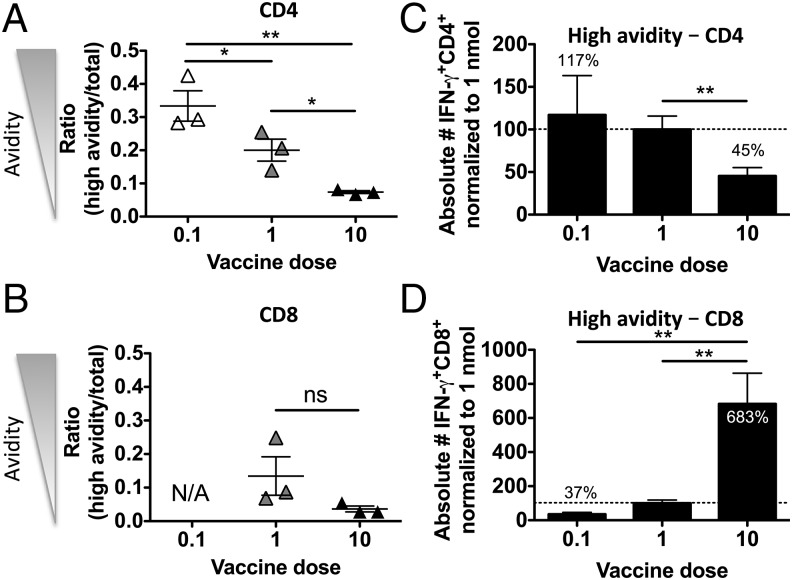
We next examined whether the observed increase in functional avidity after low-dose immunizations was caused by a relative change in the quality of the CD4 T cell population or whether absolute numbers of CD4 T cells of higher functional avidity were increased as well. We immunized mice three times, as described above, with low (0.1 nmol per mouse), medium (1 nmol per mouse), or high (10 nmol per mouse) doses of PCLUS6.1-P18 in CAF09 and stimulated splenocytes in vitro after immunizations with various concentrations of PCLUS6.1-P18, as described before. We then compared avidity by assessing the ratio of high-avidity T cells responding to a low concentration of Ag/total responding T cells as a measure of functional avidity, as previously described (27). Lower vaccine doses significantly increased the relative level of high-avidity CD4 T cells compared with higher vaccine doses (Fig. 3A). In contrast, there was no significant relationship between vaccine Ag dose and the ratio of high-avidity/total responding CD8 T cells (Fig. 3B). We pooled data from five repeated experiments that included the same vaccine doses, timing, and stimulations, and we normalized absolute numbers of high-avidity T cells (as defined in Fig. 3A, 3B) to the 1-nmol vaccine dose group within each experiment to minimize interexperimental variation. We found a significant increase in total numbers of high functional avidity CD4 T cells in the 1-nmol vaccine dose group compared with the high 10-nmol dose group (p < 0.01, Fig. 3C). In contrast, significantly more total high-avidity CD8 T cells were found in the high vaccine dose (10 nmol) group relative to lower doses (0.1 and 1 nmol, p < 0.01), confirming that low vaccine doses did not selectively increase functional avidity of CD8 T cells (Fig. 3D).
FIGURE 3.
Low-dose immunizations favor increased relative, as well as absolute, numbers of high-avidity CD4 T cells but not CD8 T cells. BALB/c mice were immunized i.p. three times at 2-wk intervals, as described, with a low (0.1 nmol), medium (1 nmol), or high (10 nmol) dose of PCLUS6.1-P18 in CAF09. One week later, functional avidity was assessed by ICS and by flow cytometry. (A) CD4 T cells of high functional avidity were defined as cells producing IFN-γ after stimulation with 5 × 10−3 μM PCLUS6.1-P18 (below the EC50 in all groups to assure cells were of high avidity), and the total response was determined as the highest response observed in all groups over all stimulation concentrations. Data points depict the ratio of high avidity/total response from individual mice in the low (0.1 nmol), medium (1 nmol), and high (10 nmol) vaccine dose groups, as indicated on the x-axis. Mean and SEM of n = 3 mice per group are shown. (B) The corresponding ratios of high-avidity/total vaccine–specific CD8 T cells from the same experiments are shown. High-avidity CD8 T cells were defined as CD8 T cells that produced IFN-γ after stimulation with 5 × 10−2 μM PCLUS6.1-P18 (corresponding to <30% of the maximum response in all groups), and total response was defined as the highest response over all stimulation concentrations. Absolute numbers of high-avidity (defined as in A and B) IFN-γ–producing CD4 (C) and CD8 (D) T cells are depicted using data pooled from five similar experiments. Bars depict mean/SEM absolute numbers of high-avidity IFN-γ–producing CD4 (C) and CD8 (D) T cells that were normalized to the response in the 1-nmol vaccine dose group (set to 100%) within each experiment. High avidity was defined as IFN-γ–producing cells responding to the stimulation concentration just below the EC50 concentration. n = 11–14 per group. Amounts of high-avidity CD4 and CD8 T cells are indicated above/within the bars relative to the 1-nmol group. Data are representative of nine experiments showing similar results. *p < 0.05, **p < 0.01, one-way ANOVA and Newman–Keul posttest for multiple comparisons. ns, not significant.
The vaccine Ag dose during priming is essential for functional CD4 T cell avidity and CD8 T cell number
Because functional T cell avidity maturation has been observed during the course of an infection, despite the lack of somatic hypermutations in the TCR (28, 29), we studied avidity maturation during vaccination. CD4 T cell functional avidity was stable and remarkably unaltered after one, two, and three vaccinations and even >1 y after the last vaccination (data not shown). Interestingly, we observed after vaccination with heterologous vaccine Ag doses at the priming and boosting event (low or high priming dose each boosted with low or high doses, respectively) that a low priming dose was essential to elicit high functional CD4 T cell avidity (regardless of booster vaccine dose), whereas the booster dose was the major determinant of the magnitude (low booster dose resulted in the highest CD4 response, Supplemental Fig. 2A, 2C). In contrast to CD4 T cells, the priming dose determined the magnitude of the CD8 T cell response, and, as expected, no significant relationship between functional CD8 T cell avidity and either priming or booster vaccine Ag dose was observed (Supplemental Fig. 2B, 2D). Although these results would indicate a strong selective process favoring T cells with higher intrinsic functional avidity at the priming event, we did not find significant differences between TCR variable α- and β-chains in vaccine-specific CD4 or CD8 T cells (data not shown). In summary, these data do not support the occurrence of avidity maturation during vaccination with CAF09.
Low-dose immunization selectively induces increased levels of polyfunctional T cells, which are not of higher functional avidity
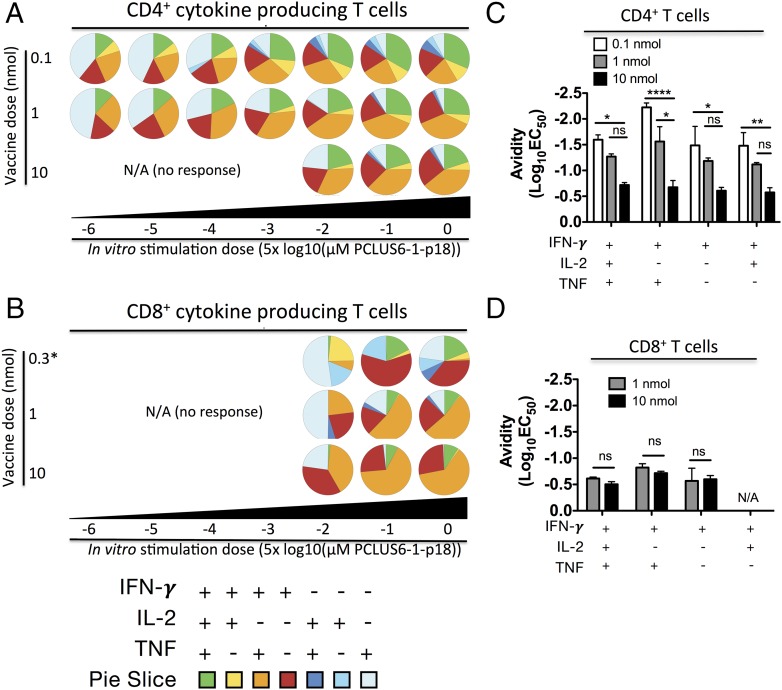
It was reported that T cells of higher functional avidity are also more polyfunctional than their lower-avidity counterparts (7). We examined this in our model by immunizing mice with various doses of PCLUS6.1-P18 in CAF09 and assessed avidity, as well as polyfunctionality, by multicolor flow cytometry and ICS for IFN-γ, TNF, and IL-2 after immunization. Responding T cells were divided into subpopulations producing different combinations of cytokines, as described by Darrah et al. (30), and a color code was assigned to each combination of measured cytokines in pie charts (Fig. 4A, 4B). When comparing pie charts between vaccine doses (indicated to the left of the pie charts, Fig. 4A, 4B) for any given Ag concentration (indicated below the pie charts), lower vaccine doses resulted in a higher proportion of polyfunctional CD4 T cells that produced all three cytokines (green pie slice, Fig. 4A) and fewer effector-like T cells (IFN-γ+ or IFN-γ+TNF+; red and orange pie slices, respectively). To some extent, this also was true for CD8 T cells (Fig. 4B). Representative FACS plots used for SPICE analysis, as well as the magnitudes of CD4 and CD8 T cell responses, are shown in Supplemental Fig. 3A and 3B. Interestingly, larger green (IFN-γ+TNF+IL-2+ T cell) polyfunctional pie slices were observed at higher Ag stimulation concentrations, indicating they were not of higher avidity than other subsets. Furthermore, when comparing functional avidity of the different subpopulations of cytokine producers within one vaccine group, they all seemed to respond equally well to stimulation for both CD4 and CD8 T cells (Fig. 4C, 4D, Supplemental Fig. 3C). If anything, the trend was that effector-like cells producing IFN-γ+, with or without TNF+ but no IL-2, responded better to lower Ag levels compared with polyfunctional cells producing all three cytokines (Supplemental Fig. 3C). A pooled analysis of four experiments (including the experiment shown in Fig. 4A and 4B) also showed that increased CD4 T cell avidity was observed for all investigated subpopulations and that no significant increase in CD8 T cell avidity was evident after lower-dose vaccinations (Supplemental Fig. 3D). Taken together, low vaccine doses selectively enhanced CD4 T cell functional avidity and polyfunctionality; however, overall, polyfunctional T cells were not of higher functional avidity than their monofunctional counterparts.
FIGURE 4.
Low-dose immunizations favor increased polyfunctionality of responding T cells, which, however, is not restricted to T cells with high functional avidity. BALB/c mice (n = 3) were immunized i.p. three times at 2-wk intervals with a low [0.1, 0.3 nmol for (B)], intermediate (1 nmol), or high (10 nmol) dose of PCLUS6.1-P18 in CAF09. One week after the immunizations, splenocytes were stimulated with increasing concentrations of PCLUS6.1-P18 in vitro and assessed for intracellular cytokine production, as described previously. Pie charts represent the relative distribution of CD4 T cell (A) and CD8 T cell (B) subsets producing different cytokine combinations at the concentrations of Ag used for stimulations (indicated below the pie charts for different vaccine doses). Note that the low dose in (B) is 0.3 nmol, because 0.1 nmol did not induce a measurable CD8 T cell response. Responses <0.2% IFN-γ+ or events <50 cytokine-positive were omitted from SPICE pie chart analysis. Functional avidity [shown as log10(EC50)] of different subtypes of CD4 (C) and CD8 (D) T cells producing the various combinations of cytokines indicated on the x-axis. Bars represent log10(EC50) values for n = 3 mice per vaccine dose group calculated from normalized response curves (Supplemental Fig. 4B). Note that the lowest vaccine dose shown for CD8 T cells in (D) is 1 nmol, and not 0.1 nmol, because the latter dose did not induce a measurable CD8 T cell response. Data are representative of five experiments; see Supplemental Fig. 4 for pooled avidity analysis of T cell subset populations. *p < 0.05, **p < 0.01, ****p < 0.0001, two-way repeated-measures ANOVA and Bonferroni correction for multiple comparisons. ns, not significant.
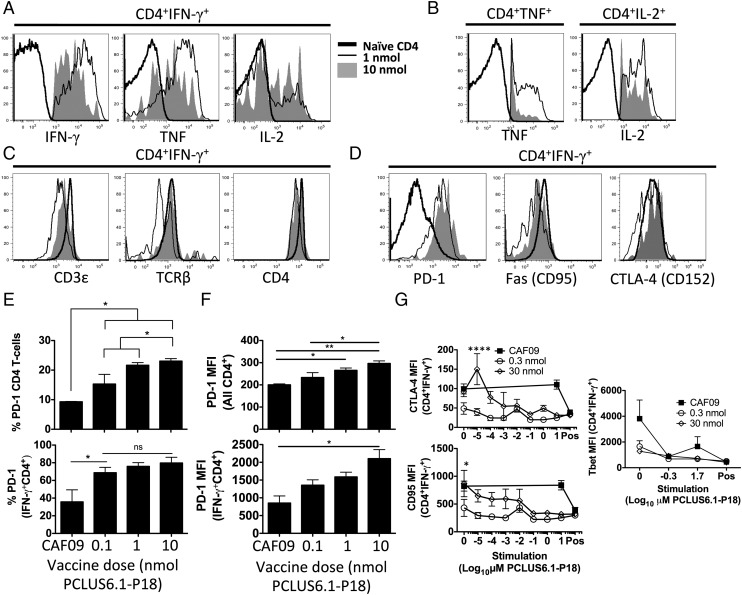
CD4 T cells of high functional avidity display higher cytokine production and greater downregulation of TCR components and inhibitory receptors on a per-cell basis compared with low-avidity cells
We also asked whether high functional avidity CD4 T cells induced by low vaccine doses differed phenotypically from lower-avidity CD4 T cells induced by higher doses. Because high-avidity T cells receive stronger signals through their TCR at a given concentration of stimulation with cognate Ag compared with low-avidity T cells, we speculated that this might lead to stronger activation and more cytokine production per cell among the high-avidity T cells. Therefore, we assessed surface and intracellular expression of effector molecules by flow cytometry and ICS after immunizing mice with various doses of PCLUS6.1-P18 in CAF09. Indeed, cytokine-producing CD4 T cells of higher functional avidity induced by low-dose vaccination (black thin line, Fig. 5A) had higher per-cell expression of IFN-γ (p < 0.001) and TNF (p < 0.05) within IFN-γ+ cells and higher per-cell TNF and IL-2 expression within TNF+ and IL-2+ CD4 T cells, respectively (not significant and p < 0.01, Fig. 5B) compared with lower-avidity T cells induced by high-dose vaccination (filled graphs, Fig. 5A, 5B). Furthermore, after stimulation, IFN-γ–producing high-avidity CD4 T cells expressed lower levels of TCR components (CD3ε, TCRβ, and CD4 coreceptor) than did lower-avidity T cells, indicating more efficient activation-induced downregulation of the TCR machinery following activation (Fig. 5C). T cells of higher functional avidity have lower activation thresholds, indicating a potential role for inhibitory receptors. In fact, higher-avidity IFN-γ+ CD4 T cells expressed lower levels of the inhibitory receptors PD-1 and CTLA-4, as well as the apoptosis-promoting receptor Fas (CD95), indicating less restriction by immune checkpoint regulation through PD-1 and potentially lower susceptibility to Fas-induced apoptosis after stimulation of high-avidity CD4 T cells (Fig. 5D). These differences were observed in stimulated and unstimulated samples (data not shown). In fact, the percentages of PD-1+ CD4 T cells (Fig. 5E), as well as surface expression levels of PD-1 (Fig. 5F), were higher for IFN-γ–producing and total CD4 T cells from groups immunized with a high vaccine Ag dose. In line with this, higher-avidity CD4 T cells selectively expressed lower levels of CD95 (Fas) and the inhibitory receptor CTLA-4 after in vitro stimulation with low Ag concentrations compared with lower-avidity CD4 T cells (Fig. 5G), indicating that CD4 T cells of high and low functional avidity were able to downregulate these receptors but that high-avidity CD4 T cells are able to do so after stimulation with very low levels of cognate Ag. Accordingly, the increased cytokine expression and decreased TCR component levels observed in high- versus low-avidity CD4 T cells was evident at all Ag-stimulation concentrations used (Supplemental Fig. 4). We also speculated that increased T-bet expression in high-avidity CD4 T cells was potentially involved in lowering the activation threshold; however, we did not find different T-bet expression in high- and low-avidity CD4 T cells (Fig. 5G). Interestingly, low-avidity CD4 T cells required ∼10,000-fold greater Ag-stimulation concentrations to produce as much cytokine per cell as high-avidity CD4 T cells (Supplemental Fig. 4A, 4B).
FIGURE 5.
High-avidity CD4 T cells express higher cytokine levels and greater downregulation of TCR and inhibitory receptors than do their low-avidity counterparts. Mice were immunized three times i.p. with the indicated doses of PCLUS6.1-P18 in CAF09, as described previously. One week after immunizations (4 wk for CTLA-4 and Fas analyses), splenocytes were stimulated in vitro and assessed for the surface expression of various markers, as well as intracellularly for cytokine production by flow cytometry. (A) Representative line graphs show intracellular expression of IFN-γ, TNF, and IL-2 gated on IFN-γ–producing CD4 T cells from mice immunized with 0.1 nmol (high avidity; thin black line) or 10 nmol (low avidity; filled graph) PCLUS6.1-P18 in CAF09. IFN-γ expression is shown for CD4 T cells from naive mice that did not produce IFN-γ as a staining control (thick black line). (B) From the same mice in (A), TNF and IL-2 MFI for TNF+ and IL-2+ CD4 T cells, respectively. Data are shown as described in (A). (C) Surface expression of TCR components CD3ε and TCR-β, as well as CD4 coreceptor, on IFN-γ+CD4+ T cells from mice immunized with 0.1 and 10 nmol after stimulation or on naive CD4 T cells; data are shown as described in (A). (D) Surface expression of inhibitory receptor PD-1, death receptor CD95 (Fas), and CTLA-4 on IFN-γ+ CD4 T cells after stimulation. Filled graph (high dose): 30 nmol PCLUS6.1-P18 (low avidity); thin black line (low dose): 0.3 nmol PCLUS6.1-P18 (high avidity), thick black line: naive unstimulated CD4 T cells (control). (E) Percentage of PD-1 expression on all gated CD4 T cells (upper panel) and IFN-γ+ CD4 T cells (lower panel) after stimulation. No upregulation of PD-1 was observed after in vitro stimulation. (F) Bar graphs show MFI of PD-1 on all gated CD4 T cells (upper panel), as well as on IFN-γ+ CD4 T cells (lower panel), from the same experiment shown in (E). Bars represent mean and SEM of n = 3 mice per group immunized as indicated on the x-axis. *p < 0.05, **p < 0.01, one-way ANOVA with Newman–Keul posttest (E and F). (G) In a separate experiment, mice were immunized i.p. with a high (30 nmol) or low (0.3 nmol) dose of PCLUS6.1-P18 in CAF09 three times, as described above. Four weeks later, splenocytes were stimulated in vitro with increasing concentrations of PCLUS6.1-P18, as indicated on the x-axis. Graphs depict surface expression (MFI) of CTLA-4 (upper left panel), CD95 (Fas; lower left panel), and T-bet (right panel) on IFN-γ+ CD4 T cells; data points represent mean and SEM of n = 3 mice per group. Experiments were repeated at least twice with similar results. *p < 0.05, ****p < 0.0001, two-way repeated-measures ANOVA and Bonferroni correction for multiple comparisons. Pos, positive controls (PMA-ionomycin).
Taken together, CD4 T cells of enhanced functional avidity induced by low vaccine doses expressed greater levels of cytokine per cell, as well as lower levels of TCR components and inhibitory receptors, potentially explaining their higher functional avidity.
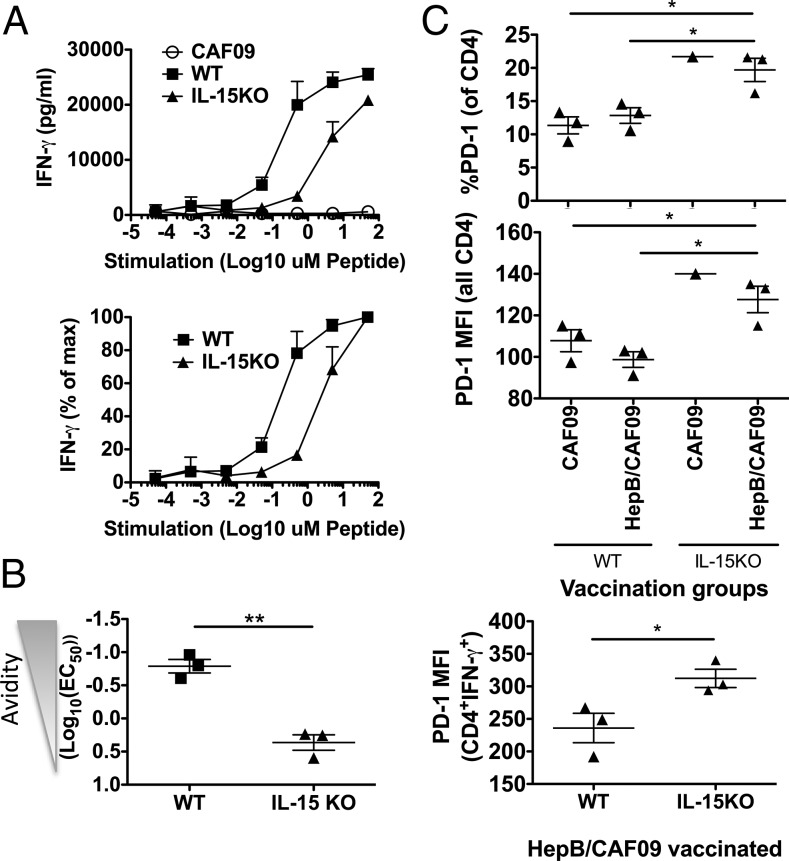
Functional CD4 T cell avidity is dependent on IL-15
It was shown that IL-15 is important for the induction of CD8 T cells of high functional avidity (31); however, less is known regarding the role of IL-15 in CD4 functional T cell avidity. To investigate this, we immunized WT C57BL/6 mice and IL-15–deficient C57BL/6 mice (IL-15−/−) with the immunodominant I-Ab–restricted hepatitis B (hep B) core protein helper epitope (hep B 128–140) in CAF09. After the last immunization, the response magnitude evaluated by flow cytometry and ICS clearly showed lower relative and absolute numbers of IFN-γ+ CD4 T cells in IL-15−/− mice compared with WT mice (Supplemental Fig. 5, p < 0.05 and p < 0.01, respectively). Simultaneously, functional avidity was evaluated by IFN-γ ELISA of splenocyte culture supernatants from WT and IL-15−/− mice stimulated with increasing concentrations of the hep B 128–140 peptide. The results showed greater functional avidity in WT mice than in IL-15−/− mice (Fig. 6A, p = 0.0018, Fig. 6B). Interestingly, we found that more CD4 T cells from IL-15−/− mice expressed the inhibitory PD-1 receptor than did WT CD4 T cells and, furthermore, that CD4 T cells from IL-15−/− mice also expressed higher levels (MFI) of PD-1 per cell (Fig. 6C, top and middle panels). This was also true among IFN-γ+ CD4 T cells from IL-15−/− mice (Fig. 6C, bottom panel). We previously observed that high vaccine doses led to increased PD-1 expression and lower functional avidity of CD4 T cells, and the data from IL-15−/− mice could indicate that the presence of IL-15 during the vaccination phase was important in reducing PD-1 expression, in turn allowing for higher functional avidity of CD4 T cells.
FIGURE 6.
CD4 T cell functional avidity is dependent on the presence of IL-15. WT C57BL/6 mice or IL-15–KO (on B6 background) mice were immunized i.p. with 50 μg per mouse of hep B core 128–140 in CAF09 twice 2 wk apart. Two weeks after the last immunization, splenocytes were stimulated in vitro for immune analyses. (A) Splenocytes were stimulated for 5 d in the presence of increasing concentrations of the hep B core 128–140 helper peptide, and IFN-γ production in the culture supernatant was assessed by IFN-γ ELISA. The curves represent mean and SEM of n = 3 mice per group immunized with hep B core 128–140 in CAF09 (WT and IL-15–KO mice) or WT mice receiving only CAF09 as a control (CAF09). Absolute levels of culture supernatant IFN-γ (pg/ml) (upper panel). IFN-γ production normalized to the maximum production for each mouse (lower panel). (B) From the normalized values in the lower panel in (A), the concentration of peptide needed to induce 50% of the maximum response (EC50) was calculated for each mouse; data points represent avidity shown as log10(EC50) mg/ml hep B 128–140 peptide with SEM. (C) Percentages of PD-1+ CD4 T cells (upper panel) and PD-1 expression per cell (MFI; middle panel) for all CD4 T cells. PD-1 MFI for IFN-γ+ CD4 T cells after stimulation with hep B 128–140 and ICS (lower panel). Data points represent individual mice; mean and SEM are indicated. The data shown are representative of two experiments with similar results. *p < 0.05, **p < 0.01, one-way ANOVA and Newman–Keul posttest.
CD4 T cell avidity is important in protection against viral infection
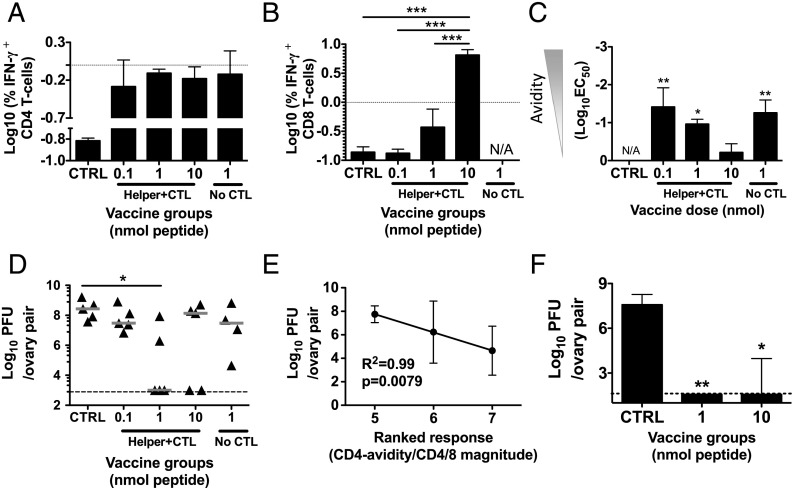
Our original aim was to increase functional avidity of CD8 T cells through low-dose immunizations. However, to our surprise, CD8 T cell functional avidity seemed independent of vaccine Ag dose, whereas CD4 T cell functional avidity was highly dependent on vaccine dose. Because CD4 help is a crucial part of efficient anti-infectious CD8 T cell responses, we wished to examine the role of increased CD4 T cell functional avidity in the antiviral capacity of the induced CD8 T cells.
We immunized mice with various doses of PCLUS6.1-P18 in CAF09, as previously described, and 5 wk after the third immunization we challenged mice i.p. with 2 × 107 PFU a recombinant vaccinia virus (vPE-16) expressing HIV IIIB gp160 (5, 32). Five days after challenge, vaccine protection was evaluated by harvesting ovaries (in which the virus preferentially grows) and estimating viral loads by plaque assay (see Materials and Methods). The results showed that only the intermediate dose of 1 nmol PCLUS6.1-P18 induced significant protection from the viral challenge (Fig. 7D) and that 10 nmol (resulting in higher CD8 T cell responses but lower CD4 avidity) also induced some level of protection, albeit nonsignificant. One week before the challenge (4 wk after the last immunization), we assessed the immune responses of the different vaccine groups. We noted that the 1 nmol per mouse group had the highest CD4 T cell response (Fig. 7A), although it was not significantly greater than the remaining groups. Furthermore, the high vaccine dose group (10 nmol) had a significantly higher CD8 T cell response than did all of the other groups, including the 1-nmol group (p < 0.001; Fig. 7B), indicating that a strong CTL response alone was not enough to induce protection because this group was not significantly protected (Fig. 7D). Furthermore, the immune analysis showed, as seen previously, higher CD4 T cell functional avidity in groups receiving lower (0.1–1 nmol) vaccine doses (p < 0.05–0.01 compared with the adjuvant controls, Fig. 7C). The best correlate observed between viral load and a single immune parameter was log10 absolute numbers of CD4+IFN-γ+ cells (R2 = +0.99, p = 0.08), which approached significance, in stark contrast to the number of IFN-γ+ CD8 T cells, which did not correlate with viral load at all (R2 = +0.006, p = 0.84; data not shown). We then ranked the immune responses among the three vaccine groups (0.1, 1, or 10 nmol PCLUS6.1-P18 in CAF09) with regard to the absolute numbers of IFN-γ–producing CD4 and CD8 T cells and functional CD4 T cell avidity. Within each immune parameter, the group with the highest magnitude of CD4/8 T cell response or CD4 avidity received three rank points, and the group with the lowest response/avidity received 1 rank point. When summing these rank points, the intermediate 1-nmol group ranked highest because it had the highest absolute numbers of IFN-γ–producing CD4 T cells and the highest functional avidity, although not the highest CD8 T cell response, and this group was the only one with significant protection from the viral infection (Fig. 7D). Furthermore, the ranked immune parameter exhibited a significant inverse correlation with viral loads (R2 = +0.99, p = 0.0079, Fig. 7E). A high-quality and high functional avidity CD4 T cell response alone was not enough to confer protection, because immunization with the lowest dose (0.1 nmol PCLUS6.1-P18), as well as with 1 nmol of the PCLUS6.1 helper peptide (that did not contain the CTL epitope), induced similar CD4 T cell responses compared with the protected 1-nmol group but no CD8 T cell response. It should be mentioned that the immune analysis of response magnitude and avidity was measured 1 wk prior to challenge; however, in a separate experiment, we noted a very stable magnitude of CD4/CD8 T cell responses and functional avidity between 4 and 5 wk postvaccination (data not shown). We repeated the challenge experiment using a low and high vaccine dose to perform T cell analysis at the time of viral load assessment and, hence, direct correlations within single animals. We used a lower challenge dose to determine whether a proposed effect of high avidity would be greater with lower pathogen and presumably Ag levels; however, at the lower challenge dose (1 × 107 PFU per mouse), both low and high vaccine doses protected, indicating a lower threshold for protection at this dose (Fig. 7F). Unfortunately, vaccine protection resulted in PFU values below the threshold of detection, thus hindering meaningful correlation analyses between T cell responses and protection at the time of viral load assessment.
FIGURE 7.
A combination of high CD4 T cell functional avidity and the presence of a CD8 T cell response protects against viral vaccinia challenge. Mice were immunized three times, as described previously, with the indicated doses of PCLUS6.1-P18 containing the HIV IIIB gp160 Th and CTL epitopes (Helper+CTL), as well as with PCLUS6.1 that included only the Th epitope (No CTL) in CAF09. CAF09 controls are indicated (CTRL). Five weeks after the last immunization, mice were challenged i.p. with 2 × 107 PFU recombinant vaccinia virus vPE-16 expressing HIV IIIB gp160 (vPE-16), and immune responses were assessed 4 wk after the last immunization. Splenocytes were harvested and restimulated in vitro with 5 μM PCLUS6.1-P18 for CD4 (A) and 0.5 μM P18-I10 for CD8 (B) T cell responses. Bars represent log10 mean and SEM of the percentage of IFN-γ–producing T cells (n = 3 per group). Statistical differences for the responses between groups were assessed by one-way ANOVA and the Newman–Keul posttest for multiple comparisons. ***p < 0.001. (C) Functional avidity of IFN-γ–producing CD4 T cells was assessed by ICS and flow cytometry and calculated as previously described; bars depict mean log10(EC50) with SEM for each vaccine group. No difference in CD8 functional avidity between groups was observed (data not shown). *p < 0.05, **p < 0.01 versus control group, one-way ANOVA and Newman–Keul posttest. (D) In the same experiment, vaccine protection was evaluated by estimating viral load in ovaries 5 d postchallenge by plaque assay (see Materials and Methods). The graph depicts estimated log10 PFU of paired ovaries (both right and left) from individual animals; n = 5 per group (n = 4 in the group vaccinated with PCLUS6.1 without the P18 CTL epitope) with medians indicated. The level of detection (1000 PFU) is indicated by the dashed horizontal line. *p < 0.05, Kruskal–Wallis test with Dunn test for multiple comparisons. (E) Linear regression was performed between estimated log10 PFU levels (± SEM) and a composite ranked immune parameter that was derived by adding ranks among the three vaccine groups (0.1, 1, and 10 nmol PCLUS6.1-P18/CAF09) with regard to the magnitudes of the CD4 and CD8 T cell responses and CD4 T cell functional avidity (rank 3 = highest response/highest avidity, rank 1 = lowest response/lowest avidity). Note that mice were sacrificed prior to challenge to perform the immune analysis shown in (A–C); hence, correlations between immune response and PFU were performed at the group level, with different mice giving rise to immune parameters and PFU, not within paired single animals. (F) Mice were immunized three times i.p. with 1 or 50 nmol PCLUS6.1-P18 in CAF09 or CAF09 alone (control) and were challenged 4 wk later with a low dose (1 × 107 PFU per mouse) of vPE-16 vaccinia virus. Protection was assessed in ovaries by plaque assay at 5 d postchallenge. Bars represent median log10 PFU and interquartile range of n = 5 mice per group. This experiment had a lower detection limit of 100 PFU, as indicated by the dashed line. *p < 0.05, **p < 0.01 versus control, Kruskal–Wallis test with Dunn test for multiple comparisons.
Taken together, the combination of a CD4 T cell response of high avidity and the presence of a functional CD8 T cell response was crucial in obtaining protection from vaccinia virus infection, and neither alone seemed to be sufficient.
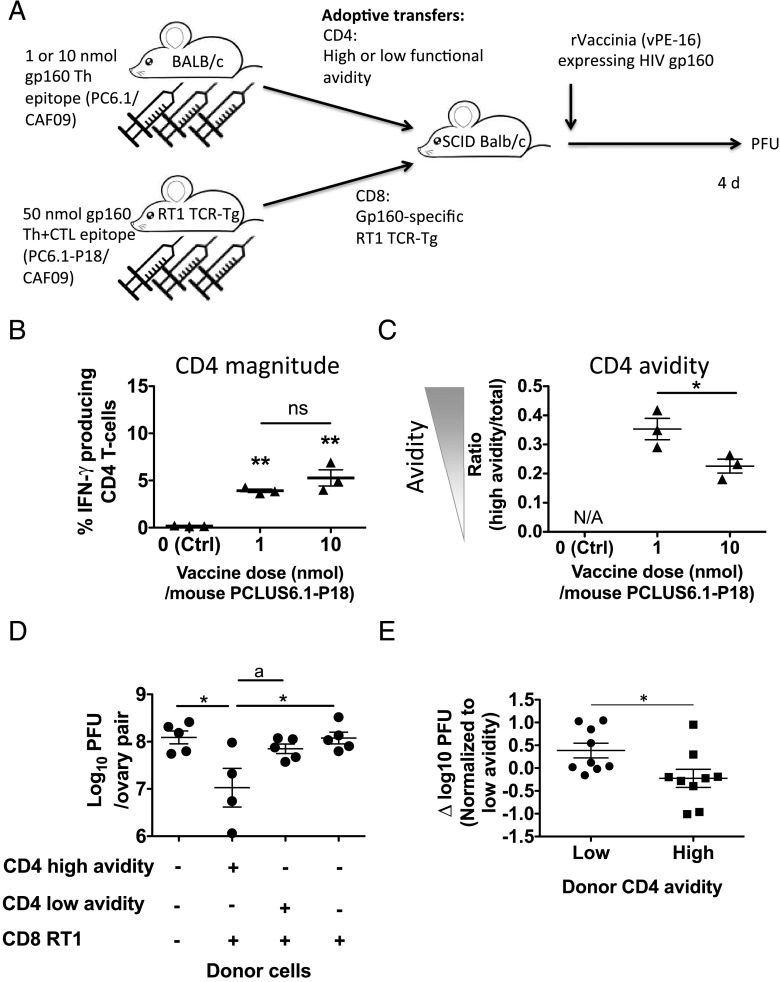
Suboptimal numbers of virus-specific CTLs protect against vaccinia virus challenge only in the presence of CD4 T cells of high functional avidity
In our previous study, it was difficult to separate functional CD4 T cell avidity from CD8 T cell response magnitude because these two parameters seemed to counterbalance each other. Low vaccine dose resulted in high CD4 functional avidity and a lower-magnitude CD8 response; conversely, CD8 responses were highest at high doses at which CD4 functional avidity was low. Therefore, we separated these two factors by adoptively transferring gp160-specific CD4 T cells of either high/low avidity along with identical TCR-Tg gp160-specific CD8 T cells. Thus, we immunized BALB/c mice with a high (10 nmol) or a lower (1 nmol) dose of the HIV IIIB gp160 PCLUS6.1 peptide that did not contain the P18-I10 CTL epitope in CAF09 and, hence, induced gp160-specific Th cells of low or high functional avidity, respectively (but no CTL responses). Simultaneously, we also immunized TCR-Tg RT-1 mice with PCLUS6.1-P18 in CAF09; these mice expressed a Tg TCR specific for the HIV IIIB immunodominant gp160 (P18-I10) H-2Dd–restricted CTL epitope within PCLUS6.1-P18. After immunizations, we harvested splenocytes and purified CD4 T cells from the low- and high-dose PCLUS6.1-immunized BALB/c mice (containing CD4 T cells of higher or lower functional avidity, respectively) and adoptively transferred them along with identical amounts of CD8 T cells from the primed TCR-Tg RT1 mice into immune-deficient H-2d SCID mice. All mice were on a BALB/c background. The SCID mice were subsequently infected with a low dose (0.5 × 107 PFU) of vPE-16 recombinant vaccinia virus expressing HIV IIIB gp160 (see schematic representation in Fig. 8A). We transferred a suboptimal number of CD8 CTLs that, on their own, were not expected to protect against the challenge based on previous data (4) to leave a window for the CD4 T cells to improve protection. Prior to transfers, we assessed responses and found that the avidity of CD4 T cells was higher in the mice receiving the low dose (1 nmol) of PCLUS6.1 compared with mice receiving 10 nmol PCLUS6.1, as expected (data not shown). At the time of transfer, we assessed immune responses of an aliquot of cells used for the transfer and found (as expected) no significant difference in the magnitude of IFN-γ–producing CD4 T cells transferred from the low- and high-avidity groups (Fig. 8B); however, the functional avidity between the groups differed significantly, as observed by the ratio of high avidity/total responding cells (p < 0.05, Fig. 8C). Strikingly, TCR-Tg CD8 T cells adoptively cotransferred with the high-avidity CD4 T cells protected against the subsequent viral challenge to a significant extent (p < 0.05), whereas the same TCR-Tg–specific CD8 CTLs transferred along with the low-avidity CD4 T cells did not (Fig. 8D); CD8 T cells alone also did not protect at this dose. Thus, only the higher functional avidity CD4 T cells led to an improved protective capacity of the transferred CD8 T cells. This experiment was repeated with similar results, and pooled analyses of the two experiments confirmed significantly lower viral loads in recipients of high-avidity CD4 T cells (p = 0.03, Fig. 8E). Our previous experiments had shown that immunization of WT mice with 10 nmol PCLUS6.1-P18 in CAF09 resulted in a higher CD8 T cell response compared with 1 nmol PCLUS6.1-P18 after vaccination (but lower CD4 avidity). In contrast, at 5 d postchallenge (at the time viral loads were assessed), WT mice immunized with 1 nmol had a higher CD8 T cell response than did the 10-nmol group (Supplemental Fig. 6A, 6B), whereas CD4 T cell avidity was still greater in mice vaccinated with the low dose (1 nmol) compared with the high dose (10-nmol group; Supplemental Fig. 6C). This could suggest that the high-avidity CD4 T cells improved CD8 T cell expansion after vaccinia challenge by an unidentified mechanism, leading to increased CD8 T cell antiviral efficacy.
FIGURE 8.
Adoptively transferring CD4 T cells of high, but not low, functional avidity along with primed TCR-Tg RT1 CD8 T cells confers protection against viral vaccinia challenge. BALB/c mice were immunized three times, as described before, with a low (1 nmol) or high (10 nmol) dose of PCLUS6.1 (containing the HIV-IIIB gp160 helper but no CTL epitope) in CAF09. TCR-Tg RT1 mice carrying a TCR specific for the minimal immunodominant HIV IIIB gp160 P18-I10 epitope were immunized three times with 50 nmol PCLUS6.1-P18 in CAF09. Two weeks after immunizations of BALB/c mice and 3 wk after immunizations of TCR-Tg mice, spleens were harvested, and CD4 T cells from BALB/c mice immunized with either a low (1 nmol, high avidity) or high (10 nmol, low avidity) dose of PCLUS6.1 were adoptively transferred i.v. with CD8 T cells from RT1 TCR-Tg mice that were vaccinated with PCLUS6.1-P18 into SCID mice that were subsequently infected i.p. with 0.5 × 107 PFU vPE-16 vaccinia virus within 15 min of T cell transfer. Approximately 1.5 × 106 CD4 T cells of high or low functional avidity specific for PCLUS6.1, along with 5.5 × 106 P18-I10–specific CD8 T cells, were transferred per mouse. (A) Schematic overview of the experiment. (B) At the time of transfer, an aliquot of the transferred CD4 T cells was assessed for immune response, and the percentage (mean and SEM) of transferred CD4 T cells that produced IFN-γ after in vitro restimulation with 5 μM PCLUS6.1, as assessed by flow cytometry and ICS, is shown. (C) At the time of transfer, splenocytes were also restimulated with a range of PCLUS6.1 concentrations, and the ratio of high functional avidity CD4 T cells (defined as CD4 T cells that responded with IFN-γ production after stimulation with 0.05 μM Ag)/total amount of responding CD4 T cells was assessed, as described previously. The graph depicts avidity (mean ± SEM) measured as the ratio of the percentages of high avidity/total responding CD4 T cells for each of n = 3 mice per group. (D) At 4 d postchallenge, ovaries were removed to estimate viral loads, as previously described. The graph depicts estimated log10 PFU values for individual recipient mice receiving the donor cells indicated below the x-axis. Individual log10 PFU values (mean ± SEM) are shown. For (B)–(D), *p < 0.05, **p < 0.01, one-way ANOVA and the Newman–Keul posttest for multiple comparisons. ap < 0.05, Student t test without correction for multiple comparisons (D). (E) The adoptive transfer protection experiment was repeated with similar results; PFU values were pooled from the experiment shown in (D) and the repeated experiment. To bypass the effect of differences in PFU values between the two experiments (∼0.7 log10 PFU difference), we calculated a δ PFU value by normalizing log10 PFU values of individual mice to the mean of mice receiving low-avidity CD4 T cells within each experiment. Thus, each data point represents the δ PFU value (log10 PFU individual mouse − mean log10 PFU low-avidity recipient group in the same experiment); mean and SEM are indicated. *p < 0.05, two-sided t test. ns, not significant.
Discussion
Our main findings were that low vaccine Ag doses selectively primed CD4 T cells over CD8 T cells and that functional avidity of CD4 T cells was increased with lower vaccine doses. Interestingly, adoptive transfer of CD4 T cells of high, but not low, functional avidity along with the same Ag-specific TCR-Tg CD8 CTLs into SCID mice protected against viral vaccinia challenge.
Functional T cell avidity of CD8 T cells is highly dependent on Ag dose during in vitro culturing (5); however, we did not find a clear relationship between vaccine Ag dose in vivo and functional CD8 T cell avidity. We encountered the same problems as previous attempts to induce high-avidity CD8 T cells through low-dose vaccinations, namely, that low vaccine Ag doses (<1 nmol adjuvanted peptide) did not induce a significant CD8 T cell response. It should be mentioned that low-dose immunization with OVA (0.05 μg to 1.1 pmol) in CAF09 induced a significant SIINFEKL-specific CD8 T cell response in C57BL/6 mice but with no increase in functional CD8 T cell avidity compared with higher OVA doses (up to 500 μg OVA; data not shown). A few previous studies observed a relationship between vaccine Ag dose and functional avidity of CD8 T cells (3, 33–35); however, these studies generally observed a decrease in avidity at extremely high Ag doses or one dependent on a heterologous prime-boost regimen with different vaccine vectors (35–37). We did not assess heterologous vectors in our study. Of note, in our experiments the functional avidity of responding CD8 T cells was high, with EC50 for the minimal P18-I10 epitope in the 100–500 pmol range (data not shown). Potentially, an upper limit for functional avidity had been reached, and improvement was not possible, even with altered vaccine doses.
Conversely, CD4 T cell functional avidity was highly dependent on vaccine Ag dose. This is a remarkable and important finding, because CD4 T cell functional avidity has been linked with improved outcome in tumors (16) and infections, such as HIV (38), and it could potentially play an important role in intracellular bacterial infections in which CD4 T cell immunity is crucial. That only CD4, and not CD8, T cell functional avidity was greater after low-dose vaccination was intriguing and likely reflects the major differences between these two cell types. The ability to increase CD4 T cell functional avidity after low-dose vaccination might reflect the great heterogeneity (Th1/2/17 and so forth) and plasticity of CD4 T cells (39). However, we did not find any systematic differences in Th or regulatory cell lineage differentiation (or cytokine skewing) in the different vaccine Ag dose groups (Supplemental Fig. 1; regulatory T cell data not shown). That CD4 T cells generally were primed by lower vaccine Ag doses compared with CD8 T cells could reflect findings in a recent study in which cross-presenting CD8α+ DCs were located more centrally in the T cell zone of draining lymph nodes and required higher Ag levels to access and process the Ag compared with conventional non–cross-presenting DCs, which were located in the periphery of the lymph node closer to Ag drainage from afferent lymphatics (40). Changing the vaccine dose in our study could result in different numbers of Ag+ DCs or the level of Ag on each DC. However, conflicting results have been obtained with regard to which role this plays in T cell functional avidity (3, 41), and we did not assess this in our study.
We also compared the levels of costimulatory receptors on T cells from animals immunized with high and low doses. No major difference in T cell costimulatory receptor expression, such as CD25, CD28, CD44, or CD69, was observed (data not shown); interestingly, however, we found significantly lower expression of the inhibitory receptor PD-1 and CTLA-4, as well as Fas death receptor, on CD4 T cells from mice immunized with low Ag doses (Fig. 5D–G). PD-1 and especially CTLA-4 were proposed to increase activation thresholds on T cells, and decreased expression of these receptors could explain the increased functional CD4 T cell avidity observed after low-dose vaccinations (42).
High Ag concentrations can lead to overstimulation and apoptosis of high-avidity T cells (14), but we did not see increases in active caspase 3 expression following high-dose vaccination compared with low-dose vaccination or differences in viability during in vitro cell cultures from mice immunized with high/low vaccine Ag doses (data not shown). Thus, our data do not support the interpretation that greater functional avidity of CD4 T cells after low vaccine doses was a result of high-dose–dependent elimination of high-avidity T cells; consistent with this, a recent study even found that low-avidity T cells are more susceptible to activation-induced apoptosis than are high-avidity T cells (43). Interestingly, the magnitude of the CD4 and CD8 T cell responses declined with the highest doses (>50 nmol peptide per mouse). Because no increase in activation-induced cell death or viability after stimulation was observed, it could be speculated that higher vaccine doses led to free Ag not bound by liposome-based CAF. Free Ag drains faster to the draining lymph node and is processed and presented by nonactivated DCs, in turn inducing tolerized or even inhibitory regulatory Ag-specific T cells (44). In fact, the 50-nmol PCLUS6.1-P18 vaccine dose equals ∼1.2 mg/ml, and for a similar liposomal adjuvant (CAF01), concentrations > 1 mg/ml Ag led to free unbound Ag (45).
In our studies, the priming dose determined functional CD4 T cell avidity (Supplemental Fig. 2), in contrast to previous studies regarding CD8 avidity (34–37), as well as an older study in which CD4 T cell structural avidity assessed by MHC class II:peptide multimer staining was increased with lower peptide boosting, but not priming, dose (46). This discrepancy could be related to the differences in the experimental setups, adjuvants (CFA versus CAF09), and readout (MHC-multimer stain versus functional assay) used. CAF09 and related CAF adjuvants were shown to imprint effector and central memory phenotypes, as well as Th lineage choice, early after the first or second immunization, and these imprinted phenotypes are stable over prolonged periods of time, even during the course of infection (47–49). This might indicate stable epigenetic regulation of T cell phenotype, as well as functional avidity, which was already imprinted after the first immunization with CAF09. In fact, we found no difference in functional T cell avidity of CD4 or CD8 T cells at different time points (from as early as 3 d up to <12 mo) after one, two, or three immunizations (data not shown).
It was proposed that a correlate of protection in HIV infection is the presence of T cells of high functional avidity and, further, that these high-avidity T cells also were of increased polyfunctionality (7, 8). Interestingly, although we found that low vaccine Ag doses induced CD4 T cells of higher functional avidity and greater polyfunctionality (Fig. 4), cells responding to low Ag concentrations (high functional avidity) displayed less polyfunctionality than did low-avidity T cells responding only to higher Ag concentrations. Importantly, the earlier HIV studies focused on CD8 T cell avidity, in contrast to our findings with CD4 functional avidity.
Finally, because IL-15 was found to be crucial in inducing high-avidity CD8 CTLs (31), we examined the need for IL-15 to induce CD4 T cells of high functional avidity. We found that IL-15 deficiency significantly reduced the functional avidity of responding CD4 T cells after vaccinations, as well as the magnitude of the response (Fig. 6, Supplemental Fig. 5). Interestingly, IL-15−/− CD4 T cells from peptide-vaccinated mice, as well as from adjuvant controls, had much higher PD-1 expression per cell and on a population basis compared with WT cells (Fig. 6C). Vaccination of WT BALB/c mice with high peptide doses led to low functional avidity and high PD-1 expression in CD4 T cells. Low functional avidity and high PD-1 expression were also observed in IL-15–KO mice, and these two findings could support the hypothesis that the presence of IL-15 during vaccination negatively regulates PD-1 expression in responding T cells, and low PD-1 expression, in turn, lowers the T cell Ag threshold, resulting in higher functional avidity.
Importantly, we found that increased CD4 T cell functional avidity was crucial for optimal protection in a viral challenge model using a recombinant nonlethal vaccinia virus expressing HIV IIIB gp160 (32). A possible mechanism for the increased protective capacity is that the high-avidity CD4 T cells are stimulated to provide improved help to CD8 T cells earlier during infection, when Ag loads are still low. In fact, preliminary results showed that the protected 1-nmol vaccine Ag dose group had higher CD8 T cell responses postvaccinia challenge than did the high (10 nmol) group, despite a 10-fold lower CD8 T cell response in the 1-nmol group preinfection (Supplemental Fig. 6). Furthermore, other studies highlighted the importance of CD4 T cell help for CTL activity in improving protection against vaccinia challenge (50, 51); however, to our knowledge, this is the first study to suggest that the avidity of Th cells plays a crucial role.
In conclusion, we found that high functional avidity CD4 T cells specific for HIV IIIB gp160 can be selectively induced by low Ag dose vaccinations when given in the novel liposomal CAF09 adjuvant, and these high-avidity CD4 T cells improved the protective capacity of gp160-specific CD8 T cells in a recombinant gp160-expressing vaccinia challenge model. Because we did not find high-avidity T cells to be tolerogenic, become inducible regulatory T cells, or be highly susceptible to activation-induced cell death, such as was observed for high-avidity CD8 T cells, inducing high-avidity CD4 T cells by low-dose vaccination to improve help for CTL may be an advantageous strategy when designing vaccines against infectious diseases and cancer.
Supplementary Material
Acknowledgments
We thank Rune Fledelius Jensen and Janne Frandsen for excellent technical assistance in formulating adjuvants. The IL-15–KO mice were a kind gift from Cristina Bergamaschi (NCI).
This work was funded by Danish Research Council Grant 12-132230, Carlsberg Foundation Grants 2013_01_0395 and CF14-0420, A.P. Møller Foundation for the Advancement of Medical Sciences Grant 12-24, Director Ib Henriksen Foundation Grant i-5008, and Lundbeck Foundation Grant 2013-14387. This work was also supported in part by intramural funds from the Center for Cancer Research, National Cancer Institute.
The funders had no role in designing experiments, interpreting data, or writing the manuscript. The opinions expressed are those of the authors and are not official United States government opinions.
The online version of this article contains supplemental material.
- CAF
- cationic adjuvant formulation
- DC
- dendritic cell
- DDA
- dimethyldioctadecylammonium bromide
- hep B
- hepatitis B
- ICS
- intracellular staining
- KO
- knockout
- MFI
- mean fluorescent intensity
- MMG
- monomycolyl glycerol
- NCI
- National Cancer Institute
- pI:C
- polyinosinic-polycytidylic acid
- WT
- wild-type.
Disclosures
P.A. and E.M.A. are coinventors of patents regarding the use of CAF09 (patent no. WO2009003474: The use of monomycolyl glycerol (MMG) as an adjuvant). All rights have been assigned to Statens Serum Institut, a state-owned nonprofit research organization. The authors’ coinventorship did not influence the design of studies or preparation of the manuscript. There are no additional patents, products in development, or marketed products to declare. The other authors have no financial conflicts of interest.
References
- 1.Berzofsky J. A. 2012. A push-pull vaccine strategy using Toll-like receptor ligands, IL-15, and blockade of negative regulation to improve the quality and quantity of T cell immune responses. Vaccine 30: 4323–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seder R. A., Darrah P. A., Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8: 247–258. [DOI] [PubMed] [Google Scholar]
- 3.Bullock T. N., Mullins D. W., Engelhard V. H. 2003. Antigen density presented by dendritic cells in vivo differentially affects the number and avidity of primary, memory, and recall CD8+ T cells. J. Immunol. 170: 1822–1829. [DOI] [PubMed] [Google Scholar]
- 4.Derby M., Alexander-Miller M., Tse R., Berzofsky J. 2001. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J. Immunol. 166: 1690–1697. [DOI] [PubMed] [Google Scholar]
- 5.Alexander-Miller M. A., Leggatt G. R., Berzofsky J. A. 1996. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. USA 93: 4102–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallimore A., Dumrese T., Hengartner H., Zinkernagel R. M., Rammensee H. G. 1998. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J. Exp. Med. 187: 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida J. R., Price D. A., Papagno L., Arkoub Z. A., Sauce D., Bornstein E., Asher T. E., Samri A., Schnuriger A., Theodorou I., et al. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204: 2473–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida J. R., Sauce D., Price D. A., Papagno L., Shin S. Y., Moris A., Larsen M., Pancino G., Douek D. C., Autran B., et al. 2009. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood 113: 6351–6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appay V., Iglesias M. C. 2011. Antigen sensitivity and T-cell receptor avidity as critical determinants of HIV control. Curr. Opin. HIV AIDS 6: 157–162. [DOI] [PubMed] [Google Scholar]
- 10.Yee C., Savage P. A., Lee P. P., Davis M. M., Greenberg P. D. 1999. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 162: 2227–2234. [PubMed] [Google Scholar]
- 11.Alexander-Miller M. A. 2005. High-avidity CD8+ T cells: optimal soldiers in the war against viruses and tumors. Immunol. Res. 31: 13–24. [DOI] [PubMed] [Google Scholar]
- 12.Viganò S., Utzschneider D. T., Perreau M., Pantaleo G., Zehn D., Harari A. 2012. Functional avidity: a measure to predict the efficacy of effector T cells? Clin. Dev. Immunol. 2012: 153863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berzofsky J. A., Ahlers J. D., Belyakov I. M. 2001. Strategies for designing and optimizing new generation vaccines. Nat. Rev. Immunol. 1: 209–219. [DOI] [PubMed] [Google Scholar]
- 14.Alexander-Miller M. A., Leggatt G. R., Sarin A., Berzofsky J. A. 1996. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J. Exp. Med. 184: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan D. J., Kurts C., Kreuwel H. T., Holst K. L., Heath W. R., Sherman L. A. 1999. Ontogeny of T cell tolerance to peripherally expressed antigens. Proc. Natl. Acad. Sci. USA 96: 3854–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caserta S., Kleczkowska J., Mondino A., Zamoyska R. 2010. Reduced functional avidity promotes central and effector memory CD4 T cell responses to tumor-associated antigens. J. Immunol. 185: 6545–6554. [DOI] [PubMed] [Google Scholar]
- 17.Aagaard C., Hoang T., Dietrich J., Cardona P. J., Izzo A., Dolganov G., Schoolnik G. K., Cassidy J. P., Billeskov R., Andersen P. 2011. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 17: 189–194. [DOI] [PubMed] [Google Scholar]
- 18.Christensen D., Korsholm K. S., Andersen P., Agger E. M. 2011. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 10: 513–521. [DOI] [PubMed] [Google Scholar]
- 19.Korsholm K. S., Hansen J., Karlsen K., Filskov J., Mikkelsen M., Lindenstrøm T., Schmidt S. T., Andersen P., Christensen D. 2014. Induction of CD8+ T-cell responses against subunit antigens by the novel cationic liposomal CAF09 adjuvant. Vaccine 32: 3927–3935. [DOI] [PubMed] [Google Scholar]
- 20.Yokosuka T., Takase K., Suzuki M., Nakagawa Y., Taki S., Takahashi H., Fujisawa T., Arase H., Saito T. 2002. Predominant role of T cell receptor (TCR)-alpha chain in forming preimmune TCR repertoire revealed by clonal TCR reconstitution system. J. Exp. Med. 195: 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy M. K., Glaccum M., Brown S. N., Butz E. A., Viney J. L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C. R., et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191: 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidsen J., Rosenkrands I., Christensen D., Vangala A., Kirby D., Perrie Y., Agger E. M., Andersen P. 2005. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta 1718: 22–31. [DOI] [PubMed] [Google Scholar]
- 23.Berzofsky J. A., Pendleton C. D., Clerici M., Ahlers J., Lucey D. R., Putney S. D., Shearer G. M. 1991. Construction of peptides encompassing multideterminant clusters of human immunodeficiency virus envelope to induce in vitro T cell responses in mice and humans of multiple MHC types. J. Clin. Invest. 88: 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milich D. R., Hughes J. L., McLachlan A., Thornton G. B., Moriarty A. 1988. Hepatitis B synthetic immunogen comprised of nucleocapsid T-cell sites and an envelope B-cell epitope. Proc. Natl. Acad. Sci. USA 85: 1610–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roederer M., Nozzi J. L., Nason M. C. 2011. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 79: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buller R. M., Wallace G. D. 1985. Reexamination of the efficacy of vaccination against mousepox. Lab. Anim. Sci. 35: 473–476. [PubMed] [Google Scholar]
- 27.Belyakov I. M., Ahlers J. D., Nabel G. J., Moss B., Berzofsky J. A. 2008. Generation of functionally active HIV-1 specific CD8+ CTL in intestinal mucosa following mucosal, systemic or mixed prime-boost immunization. Virology 381: 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busch D. H., Pamer E. G. 1999. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 189: 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slifka M. K., Whitton J. L. 2001. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat. Immunol. 2: 711–717. [DOI] [PubMed] [Google Scholar]
- 30.Darrah P. A., Patel D. T., De Luca P. M., Lindsay R. W., Davey D. F., Flynn B. J., Hoff S. T., Andersen P., Reed S. G., Morris S. L., et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13: 843–850. [DOI] [PubMed] [Google Scholar]
- 31.Oh S., Perera L. P., Burke D. S., Waldmann T. A., Berzofsky J. A. 2004. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc. Natl. Acad. Sci. USA 101: 15154–15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earl P. L., Koenig S., Moss B. 1991. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J. Virol. 65: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lövgren T., Baumgaertner P., Wieckowski S., Devêvre E., Guillaume P., Luescher I., Rufer N., Speiser D. E. 2012. Enhanced cytotoxicity and decreased CD8 dependence of human cancer-specific cytotoxic T lymphocytes after vaccination with low peptide dose. Cancer Immunol. Immunother. 61: 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narayan S., Choyce A., Fernando G. J., Leggatt G. R. 2007. Secondary immunisation with high-dose heterologous peptide leads to CD8 T cell populations with reduced functional avidity. Eur. J. Immunol. 37: 406–415. [DOI] [PubMed] [Google Scholar]
- 35.Kim M., Moon H. B., Kim K., Lee K. Y. 2006. Antigen dose governs the shaping of CTL repertoires in vitro and in vivo. Int. Immunol. 18: 435–444. [DOI] [PubMed] [Google Scholar]
- 36.Hu Z., Wang J., Wan Y., Zhu L., Ren X., Qiu S., Ren Y., Yuan S., Ding X., Chen J., et al. 2014. Boosting functional avidity of CD8+ T cells by vaccinia virus vaccination depends on intrinsic T-cell MyD88 expression but not the inflammatory milieu. J. Virol. 88: 5356–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estcourt M. J., Ramsay A. J., Brooks A., Thomson S. A., Medveckzy C. J., Ramshaw I. A. 2002. Prime-boost immunization generates a high frequency, high-avidity CD8(+) cytotoxic T lymphocyte population. Int. Immunol. 14: 31–37. [DOI] [PubMed] [Google Scholar]
- 38.Vingert B., Perez-Patrigeon S., Jeannin P., Lambotte O., Boufassa F., Lemaître F., Kwok W. W., Theodorou I., Delfraissy J. F., Thèze J., Chakrabarti L. A., ANRS EP36 HIV Controllers Study Group 2010. HIV controller CD4+ T cells respond to minimal amounts of Gag antigen due to high TCR avidity. PLoS Pathog. 6: e1000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J., Paul W. E. 2010. Heterogeneity and plasticity of T helper cells. Cell Res. 20: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerner M. Y., Kastenmuller W., Ifrim I., Kabat J., Germain R. N. 2012. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37: 364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaft N., Wellner V., Wohn C., Schuler G., Dörrie J. 2013. CD8(+) T-cell priming and boosting: more antigen-presenting DC, or more antigen per DC? Cancer Immunol. Immunother. 62: 1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Intlekofer A. M., Thompson C. B. 2013. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 94: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Black C. M., Armstrong T. D., Jaffee E. M. 2014. Apoptosis-regulated low-avidity cancer-specific CD8(+) T cells can be rescued to eliminate HER2/neu-expressing tumors by costimulatory agonists in tolerized mice. Cancer Immunol. Res. 2: 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamath A. T., Mastelic B., Christensen D., Rochat A. F., Agger E. M., Pinschewer D. D., Andersen P., Lambert P. H., Siegrist C. A. 2012. Synchronization of dendritic cell activation and antigen exposure is required for the induction of Th1/Th17 responses. J. Immunol. 188: 4828–4837. [DOI] [PubMed] [Google Scholar]
- 45.Hamborg M., Jorgensen L., Bojsen A. R., Christensen D., Foged C. 2013. Protein antigen adsorption to the DDA/TDB liposomal adjuvant: effect on protein structure, stability, and liposome physicochemical characteristics. Pharm. Res. 30: 140–155. [DOI] [PubMed] [Google Scholar]
- 46.Rees W., Bender J., Teague T. K., Kedl R. M., Crawford F., Marrack P., Kappler J. 1999. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc. Natl. Acad. Sci. USA 96: 9781–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindenstrøm T., Agger E. M., Korsholm K. S., Darrah P. A., Aagaard C., Seder R. A., Rosenkrands I., Andersen P. 2009. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 182: 8047–8055. [DOI] [PubMed] [Google Scholar]
- 48.Lindenstrøm T., Woodworth J., Dietrich J., Aagaard C., Andersen P., Agger E. M. 2012. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect. Immun. 80: 3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Billeskov R., Christensen J. P., Aagaard C., Andersen P., Dietrich J. 2013. Comparing adjuvanted H28 and modified vaccinia virus ankara expressingH28 in a mouse and a non-human primate tuberculosis model. PLoS One 8: e72185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahlers J. D., Belyakov I. M., Thomas E. K., Berzofsky J. A. 2001. High-affinity T helper epitope induces complementary helper and APC polarization, increased CTL, and protection against viral infection. J. Clin. Invest. 108: 1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Z., Cuss S. M., Singh V., Gurusamy D., Shoe J. L., Leighty R., Bronte V., Hurwitz A. A. 2015. CD4+ T cell help selectively enhances high-avidity tumor antigen-specific CD8+ T cells. J. Immunol. 195: 3482–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.