Abstract
1-O-acylceramide is a new class of epidermal ceramide (Cer) found in humans and mice. Here, we report an ESI linear ion-trap (LIT) multiple-stage MS (MSn) approach with high resolution toward structural characterization of this lipid family isolated from mice. Molecular species desorbed as the [M + H]+ ions were subjected to LIT MS2 to yield predominately the [M + H − H2O]+ ions, followed by MS3 to cleave the 1-O-acyl residue to yield the [M + H − H2O − (1-O-FA)]+ ions. The structures of the N-acyl chain and long-chain base (LCB) of the molecule were determined by MS4 on [M + H − H2O − (1-O-FA)]+ ions that yielded multiple sets of specific ions. Using this approach, isomers varied in the 1-O-acyl (from 14:0- to 30:0-O-acyl) and N-acyl chains (from 14:0- to 34:1-N-acyl) with 18:1-sphingosine as the major LCB were found for the entire family. Minor isomers consisting of 16:1-, 17:1-, 18:2-, and 19:1-sphingosine LCBs with odd fatty acyl chain or with monounsaturated N- or O-fatty acyl substituents were also identified. An estimation of more than 700 1-O-acylceramide species, largely isobaric isomers, are present, underscoring the complexity of this Cer family.
Keywords: skin ceramide, lipidomics, multiple-stage tandem mass spectrometry, electrospray ionization, higher collision energy dissociation, sphingolipid, high-resolution mass spectrometry
The outermost layer of the epidermis in mammals is rich in ceramides (Cers), which provide a physical barrier to water loss and proper skin functions (1–3). Epidermal Cers are mainly found in extracellular lipid lamellae and contain various classes of Cers, including those having unusual long-chain amide-linked ω-hydroxyacids, to which the ω-hydroxy group is esterified with an additional long-chain FA, primarily a linoleic acid (4–6).
In addition to the esterified ω-hydroxy FA-containing Cer class, Cers consisting of long to very long N- and 1-O-linked acyl chains were recently identified in humans and mice. This new class of epidermal 1-O-acylceramide derives from classical group I amide-linked nonhydroxy sphingosine Cers and makes up 5% of all esterified Cers. They are hydrophobic and were thought to contribute to the water barrier homeostasis (7).
A large body of literature on the quantitative and qualitative analysis of Cers using mass spectrometric techniques, including GC/MS (8, 9) and ESI tandem MS with or without online HPLC (10–18), has been published; however, very few studies have focused on the structural characterization of complex skin Cers. Among them, Masukawa et al. (15) and t’Kindt et al. (16) conducted the most extensive LC/MS/MS analysis of Cers in human stratum corneum, and revealed hundreds of Cer species in various classes, including many isobaric isomers, but species in the 1-O-acylceramide family were not reported. Rabionet et al. (7) reported the presence of 1-O-acylceramides and a minor 1-O-acylceramide subfamily comprising the N-α-hydroxyacyl group in human and mouse epidermis using UPLC-ESI-tandem quadrupole MS and several isobaric isomers were found. Here, we apply linear ion-trap (LIT) multiple-stage MS (MSn) in combination with high-resolution accurate mass measurements to unveil the structural details of the 1-O-acylceramides isolated from mouse epidermis. Our results reveal the complexity of this 1-O-acylceramide family, which consists of C18-sphingosine as the major long-chain base (LCB) together with minor C16-, C17-, C18-, C19-, and C20-sphingosine LCBs and a C18-dehydrosphingosine LCB to which a whole array of amide-linked fatty acyl substituents (ranging from C14 to C32 with zero to one double bond) and 1-O-linked acyl groups (ranging from C14 to C32 with zero to one double bond) are attached.
MATERIALS AND METHODS
Chemicals
All chemicals and solvents were purchased from Fisher Scientific (Waltham, MA). Standard 1-O-oleoyl-N-heptadecanoyl-D-erythro-sphingosine (18:1-d18:1/17:0-Cer; please refer to the Nomenclature section for an explanation of abbreviations such as this) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). An AMP+ MS kit (50 test) containing N-(4-aminomethylphenyl) pyridinium (AMPP) derivatizing reagent, n-butanol (HOBt), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, and acetonitrile/DMF solution was purchased from Cayman Chemical (Ann Arbor, MI).
Preparation and isolation of 1-O-acylceramides from mouse epidermis
To the lyophilized 10 mg epidermis sample from newborn mice in a centrifuge tube, 0.8 ml water was added. The sample was soaked for 5 min at room temperature and 3 ml chloroform/methanol (1:2, v:v) were added. Following 30 s vortexing and 2 h shaking at room temperature, an additional 1 ml chloroform and 1 ml water were added. The extraction tube was vortexed for another 30 s, centrifuged at 1750 × g at room temperature for 5 min, and the organic layer was transferred to another tube, dried under nitrogen, and stored at −20°C until use. To fractionate, the crude lipids redissolved in 500 ul CHCl3 were loaded to a 3 ml/500 mg Macherey-Nagel (Duren, Germany) amino Chromabond Sep-Pak column, which was prewashed with 2× 3 ml hexane. The column was first eluted with 3 ml hexane:diethyl ether (90:10, v:v) (fraction 1), followed by 3 ml hexane:ethyl acetate (75:25, v:v) (fraction 2), 3 ml chloroform:methanol (15:1, v:v) (fraction 3), 2× 3 ml diisopropyl ether:acetic acid (98:5, v:v) (fractions 4 and 5), and finally eluted with 3 ml acetone:methanol (9:1.4, v:v) (fraction 6) by gravity. The eluants containing 1-O-acylceramide (fraction 2) and minor 1-O-acyl-N-α-OH-acylceramide (fraction 3) lipids were dried under a stream of nitrogen. The dried samples were redissolved in CHCl3:CH3OH (1:2, v:v) before ESI-MS analysis.
Preparation of FA-AMPP derivative for locating the double bonds on the FA chain
To locate the double bond along the 1-O-acyl and N-acyl chains, 1-O-acylceramide was hydrolyzed in 1 ml of solution prepared from 8.6 ml of concentrated HCl and 9.6 ml of water, diluted to 100 ml with methanol as previously described (19). After heating at 80°C for 18 h, the hydrolysate was evaporated to dryness with a stream of nitrogen, and FA-AMPP derivative was prepared with the AMP+ MS kit according to the manufacturer’s instructions, as described previously (20).
MS
Both high-resolution (R = 100,000 at m/z 400) higher collision energy dissociation (HCD) and low-energy collision-induced dissociation (CID) tandem MS experiments were conducted on a Thermo Scientific (San Jose, CA) LTQ Orbitrap Velos mass spectrometer with an Xcalibur operating system. Lipid extracts in chloroform/methanol (2/1) were infused (1.5 μl/min) to the ESI source, where the skimmer was set at ground potential, the electrospray needle was set at 4.0 kV, and temperature of the heated capillary was 300°C. The automatic gain control of the ion trap was set to 5 × 104, with a maximum injection time of 50 ms. Helium was used as the buffer and collision gas at a pressure of 1 × 10−3 mbar (0.75 mTorr). The MSn experiments were carried out with an optimized relative collision energy ranging from 30 to 45% with an activation q value at 0.25 and the activation time at 10 ms to leave a minimal residual abundance of precursor ion (around 20%). The mass selection window for the precursor ions was set at 1 Da wide to admit the monoisotopic ion to the ion-trap for CID for unit resolution detection in the ion-trap or high-resolution accurate mass detection in the Orbitrap mass analyzer. Mass spectra were accumulated in the profile mode, typically for 2–10 min for MSn spectra (n = 2–4).
Nomenclature
For simplicity, 1-O-oleoyl-N-heptadecanoyl-D-erythro-sphingosine is abbreviated as 18:1-d18:1/17:0-Cer to reflect that an oleoyl (18:1) group is attached to the 1-hydroxy LCB of d18:1/17:0-Cer by an ester bond. Likewise, Cers such as 1-O-tetraeicosanoyl-N-tetraeicosenoyl-D-erythro-sphingosine and 1-O-tetraeicosanoyl-N-tetraeicosanoyl-D-erythro-dehydrosphingosine are abbreviated as 24:0-d18:1/24:1-Cer and 24:0-d18:2/24:0-Cer, respectively. The designation of the fragment ions is according to that previously described (12).
RESULTS
The fragmentation pathways of 1-O-acylceramide
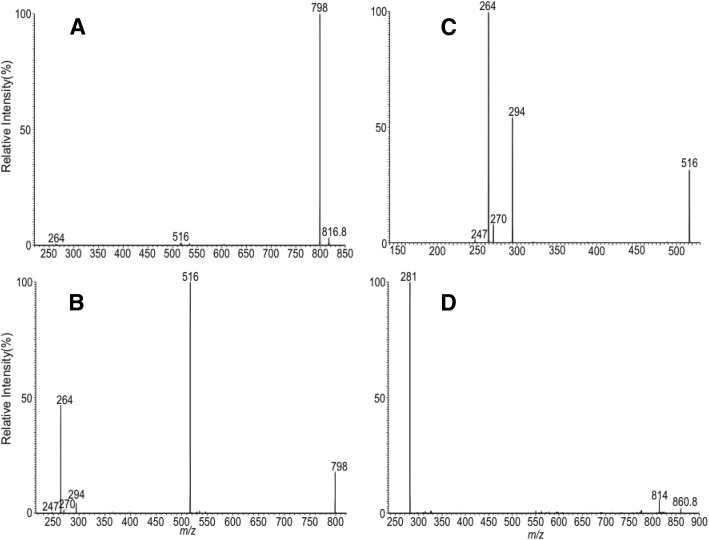
When subjected to ESI in the positive-ion mode, Cers were mainly seen as the [M + H − H2O]+ ions, due to facile loss of a water molecule (10). By contrast, 1-O-acylceramide formed [M + H]+ ions, attributable to the notion that the attachment of the 1-O-acyl group may have deterred the water loss process. For example, the synthetic 18:1-d18:1/17:0-Cer standard was seen at m/z 816.7, corresponding to the [M + H]+ ions, which nevertheless formed the prominent ion of m/z 798.7 (Fig. 1A) by loss of water, when subjected to CID in an ion-trap. The water loss most likely involved the participation of the 3-hydroxy group of LCB (Scheme 1). The speculation was established by the findings that further dissociation of the ion of m/z 798 (816 → 798; Fig. 1B) yielded ions of m/z 516, arising from elimination of the 1-O-oleoyl group as an acid, and of m/z 264 (e3b″), arising from further cleavage of the N-heptaoctanoyl substituent as a ketene (Scheme 1). This fragmentation process was supported by the MS4 spectrum of the ion of m/z 516 (816 → 798 → 516; Fig. 1C), which contained prominent ions of m/z 264 (e3b″) (Scheme 1) that are unique to Cers consisting of C18-sphingosine LCB (10). The spectrum also contained the ions of m/z 294, likely arising from cleavage of the LCB to eliminate a terminally conjugated 1,3-hexadecadiene, and of m/z 270, originated from the highly conjugated ions of m/z 516 that eliminate a C18H30 residue (Scheme 1). This latter fragmentation process also led to the ions of m/z 247, representing the highly conjugated triene cations. The ions of m/z 294 and 270 are equivalent to the c2a (m/z 294) and d1a (m/z 270) ions, as previously described (12). The confirmation of the suggested structures is further supported by the elemental composition extracted by high-resolution MS (data not shown). The observation of the ions of m/z 264 reflecting the presence of d18:1-LCB and the 294/270 ion pair reflecting the N-heptadecanoyl substituents plus the MS3 spectrum of m/z 798 (Fig. 1B) that defines the 1-O-oleoyl group (via loss of oleic acid) led to complete assignment of the 18:1-d18:1/17:0-Cer structure.
Fig. 1.
The MS2 spectrum of the [M + H]+ ion of 18:1-d18:1/17:0-Cer standard at m/z 816.7 (A), its MS3 spectrum of the ion of m/z 798.7 (816.7 → 798.7) (B), and its MS4 spectrum of the ion of m/z 516 (816.7 → 798.7 → 516) (C). D: The MS2 spectrum of the [M + HCO2]− ions of 18:1-d18:1/17:0-Cer at m/z 860.8.
Scheme 1.
The fragmentation pathways proposed for the [M + H]+ ions of 1-O-oleoyl-d18:1/17:0-Cer at m/z 816.7.
In the negative-ion mode in the presence of Cl− or HCO2−, however, ions at m/z 850 and 860 corresponding to the [M + Cl]− and [M + HCO2]− ions, respectively, were observed (data not shown), similar to that previously reported for Cers (11). MS2 on the [M + HCO2]− ions of m/z 860 (Fig. 1D) gave prominent ions of m/z 281, corresponding to the 18:1-carboxylate anion arising from cleavage of the 1-O-fatty acyl substituent, together with the ions of m/z 814 (860 − HCO2H) representing the [M – H]− ions arising from loss of HCO2H. The MS2 spectrum of the [M + Cl]− ions at m/z 850 is similar, mainly consisting of the 18:1-carboxylate anion of m/z 281 (not shown). These MS2 spectra readily recognized the 1-O-oleoyl group, but failed to provide further structural information applicable for complete identification of the molecule and, thus, its utility in the structural identification was not investigated further.
Characterization of 1-O-acylceramide isolated from mouse epidermis
High-resolution ESI mass spectrometric analysis of the 1-O-acylceramides isolated from mouse epidermis (fraction 2) showed an array of abundant [M + H]+ ions (Table 1) with an elemental composition of CnH2n−2O4N1 (n = 53–72), suggesting that the 1-O-acylceramide family mainly consists of sphingosine LCB. A minor ion series with two fewer hydrogens with an elemental composition of CnH2n−4O4N1 (n = 53–72) was also observed, indicating the presence of the minor species with an additional double bond (Table 1). The observation of the analogous of the [M + Cl]− or [M + HCO2]− (not shown) ions in the negative ion mode is consistent with the presence of this 1-O-acylceramide family. In contrast, ions with an elemental composition of CnH2n−4O5N1 were not observed, excluding the presence of the subfamily of 1-O-acylceramides with the N-α-hydroxyacyl group previously reported by Rabionet and colleagues (6, 7) The LIT MSn mass spectrometric approaches together with high-resolution mass measurements toward identification of these mouse epidermis 1-O-acylceramdes are described below.
TABLE 1.
The structures of epidermal 1-O-acylceratnide revealed by LIT MSn and high-resolution MS
| m/z [M + H]+ | Theoretical Mass (Da) | Deviation (mDa) | Composition | Relative Intensity(%) | Major Structure | 1-O-Acyl Substituenta | Isomer Number | |
| Major | Minor | |||||||
| 816.7804 | 816.7803 | 0.05 | C53 H102 04 N | 18:1-d18:1/17:0 (synthetic standard) | 18:1 | — | — | |
| 832.8116 | 832.8116 | 0.01 | C54 H106 04 N | 2.23 | 18:0-d18:1/18:0; 19:0-d18:1/17:0 | 18:0; 19:0 | 16:0 | 7 |
| 860.8431 | 860.8429 | 0.16 | C56 H110 04 N | 4.24 | 16:0-d18:1/22:0; 22:0-d18:1/16:0 | 16:0; 22:0 | 24:0; 14:0; 15:0; 20:0; 19:0; 23:0; 18:0 | 26 |
| 874.8586 | 874.8586 | −0.04 | C57 H112 04 N | 10.50 | 24:0-d17:1/16:0; 16:0-d17:1/24:0 | 24:0; 16:0 | 15:0; 23:0; 14:0; 22:0; 18:0; 17:0; 25:0; 21:0 | 27 |
| 886.8585 | 886.8586 | −0.12 | C58 H112 04 N | 2.95 | 24:0-d18:1/16:1; 16:0-d18:1/24:1 | 24:0; 16:0 | 22:0; 18:0; 23:1; 24:1; 25:0; 20:0; 17:0; | 16 |
| 888.8742 | 888.8742 | 0.01 | C58 H114 04 N | 35.60 | 16:0-d18:1/24:0 | 16:0; | 18:0; 24:0; 22:0 14:0; 20:0; 23:0; 15:0; 17:0; 26:0. | 26 |
| 900.8739 | 900.8742 | −0.33 | C59 H114 04 N | 3.32 | 24:0-d17:1/18:1 | 24:0; | 18:1; 16:1; 23:0; 19:0; 25:0; 25:1; 17:1; 15:0; 22:0; 26:0; | 21 |
| 902.8899 | 902.8899 | 0.03 | C59 H116 04 N | 21.80 | 18:0-d18:1/23:0; 18:0-d16:1/25:0; 16:0-d17:1/26:0 | 18:0; 16:0 | 17:0; 24:0; 15:0; 22:0; 23:0; 25:0; 20:0; 19:0; 21:0; 14:0 | 33 |
| 904.8688b | 904.8692 | −0.39 | C58 H114 05 N | detected | 24:0-d18:1/h16:0 | 24:0 | — | 1 |
| 912.8750 | 912.8749 | 0.01 | C60 H114 04 N | 4.33 | 24:0-d18:1/18:2 | 24:0; | 18:2; 18:1; 24:1; 14:0; 16:1; 25:0; 26:0; | 15 |
| 914.8900 | 914.8899 | 0.14 | C60 H116 04 N | 8.98 | 24:0-d18:1/18:1 | 24:0; | 18:1; 16:1; 16:0; 18:0; 25:0; 26:0; 23:0; | 17 |
| 916.9055 | 916.9055 | −0.04 | C60 H118 04 N | 44.75 | 18:0-d18:1/24:0; 16:0-d18:1/26:0 | 18:0; 16:0 | 24:0; 22:0; 20:0; 26:0 | 15 |
| 928.9058 | 928.9055 | 0.30 | C61 H118 04 N | 2.09 | 18:1-d17:1/26:0 | 18:1; | 25:0; 24:0; 17:1; 16:0; 16:1; 20:0; 20:1; 18:0; 26:0 | 24 |
| 930.9212 | 930.9212 | 0.00 | C61 H120 04 N | 17.21 | 18:0-d18:1/25:0 | 18:0; | 24:0; 17:0; 19:0; 20:0; 16:0; 22:0; 23:0; 25:0; 21:0; 15:0; 26:0 | 33 |
| 942.9211 | 942.9212 | −0.04 | C62 H120 04 N | 5.63 | 18:0-d18:1/26:1 | 18:0; | 18:1; 20:0; 20:1; 22:0; 16:0; 24:0 | 18 |
| 944.9368 | 944.9368 | −0.06 | C62 H122 04 N | 35.40 | 18:0-d18:1/26:0 | 18:0; | 20:0; 24:0; 22:0; 16:0; 14:0; 26:0 | 18 |
| 956.9368 | 956.9368 | −0.08 | C63 H122 04 N | 2.12 | 22:0-d17:1/24:1 | 22:0; | 24:0; 18:1; 24:1; 20:1; 20:0; 21:0; 23:0; 19:1; 25:0; 25:1; 26:1; 26:0; 16:0; 16:1 | 40 |
| 958.9523 | 958.9525 | −0.15 | C63 H124 04 N | 17.20 | 22;0-d18:1/23:0 | 22:0; | 24:0; 20:0; 18:0; 23:0; 21:0;19:0; 16:0; 25:0; 17:0; 26:0; 15:0 | 35 |
| 970.9525 | 970.9525 | 0.04 | C64 H124 04 N | 6.40 | 22:0-d18:1/24:1; 22:1-d18:1/24:0 | 22:0; 22:1 | 24:1; 24:0; 18:0; 20:0; 20:1; 16:0; 26:1; 14:0; 30:1; 28:1 | 34 |
| 972.9680 | 972.9681 | −0.10 | C64 H126 04 N | 48.40 | 22:0-d18:1/24:0; 24:0-d18:1/22:0 | 22:0; 24:0 | 20:0; 18:0; 23:0; 16:0 | 15 |
| 984.9681 | 984.9681 | 0.00 | C65 H126 04 N | 8.15 | 24:0-d17:1/24:1; 24:1-d17:1/24:0 | 24:0; 24:1 | 22:0; 25:0; 22:1; 23:1; 25:0; 25:1; 21:0; 26:1 | 33 |
| 986.9838 | 986.9838 | 0.03 | C65 H128 04 N | 48.45 | 24:0-d17:1/24:0 | 24:0; | 22:0; 23:0; 21:0; 18:0; 25:0; 20:0; 16:0; 26:0; 17:0; 15:0 | 30 |
| 996.9679 | 996.9681 | −0.19 | C66 H126 04 N | 2.28 | 24:0-d18:1/24:2; 24:1-d18:1/24:1 | 24:0; 24:1 | 24:2; 22:0; 22:1; 22:2; 26:1; 26:2; 20:1; 20:0; 25:0 | 24 |
| 998.9836 | 998.9838 | −0.14 | C66 H128 04 N | 21.02 | 24:0-d18:1/24:1; 24:1-d18:1/24:0 | 24:0; 24:1 | 22:0; 16:0; 18:0; 26:0; 20:0; 32:0; 30:0; 28:0; 14:0 | 24 |
| 1,000.9994 | 1,000.9994 | −0.07 | C66 H130 04 N | 100.00 | 24:0-d18:1/24:0 | 24:0; | 22:0; 23:0; 25:0; 26:0 | 14 |
| 1,012.9993 | 1,012.9994 | −0.11 | C67 H130 04 N | 13.23 | 24:0-d18:1/25:1; 24:0-d17:1/26:1; 24:1-d18:1/25:0 | 24:0; 24:1 | 23:0; 25:1; 25:0; 26:1; 22:0; 18:0; 16:0; 17:0; 15:0; 20:0; 21:0; 19:0 | 33 |
| 1,015.0150 | 1,015.0151 | −0.11 | C67 H132 04 N | 58.83 | 24:0-d18:1/25:0; 24:0-d17:1/26:0 | 24:0; | 23:0; 25:0; 22:0; 26:0; 18:0; 16:0; 20:0 | 20 |
| 1,027.0149 | 1,027.0151 | −0.16 | C68 H132 04 N | 22.43 | 24:0-d18:1/26:1; 24:1-d18:1/26:0 | 24:0; 24:1 | 26:1; 18:0; 16:0; 22:0; 20:0 | 15 |
| 1,029.0308 | 1,029.0307 | 0.06 | C68 H134 04 N | 87.02 | 24:0-d18:1/26:0 | 24:0; | 26:0; 21:0; 18:0 | 10 |
| 1,043.0457 | 1,043.0457 | −0.08 | C69 H136 04 N | 10.52 | 25:0-d18:1/26:0; 24:0-d18:1/27:0; 26:0-d18:1/25:0 | 25:0,24:0, 26:0; | 23:0; 22:0; 28:0; 27:0 | 20 |
| 1,053.0307 | 1,053.0307 | −0.08 | C70 H134 04 N | 2.29 | 26:1-d18:1/26:1; 26:2-d 18:1/26:0 | 26:1; 26:2 | 24:0; 24:1; 26:0, 28:1; 24:2; 22:0; 28:2; 22:1; 28:0 | 27 |
| 1,055.0464 | 1,055.0464 | 0.04 | C70 H136 04 N | 5.14 | 26:1-d18:1/26:0; 24:0-d18:1/28:1 | 26:1; 24:0 | 26:0; 28:1; 25:0; 30:1; 27:1 | 21 |
| 1,057.0623 | 1,057.0620 | 0.23 | C70 H138 04 N | 9.11 | 26:0-d18:1/26:0; 24:0-d18:1/28:0 | 26:0; 24:0 | 22:0; 25:0; 28:0; 27:0; 30:0 | 18 |
| Total | 710 | |||||||
Abundances in descending order.
Detected in fraction 3.
Revelation of isobaric isomers in the molecular species
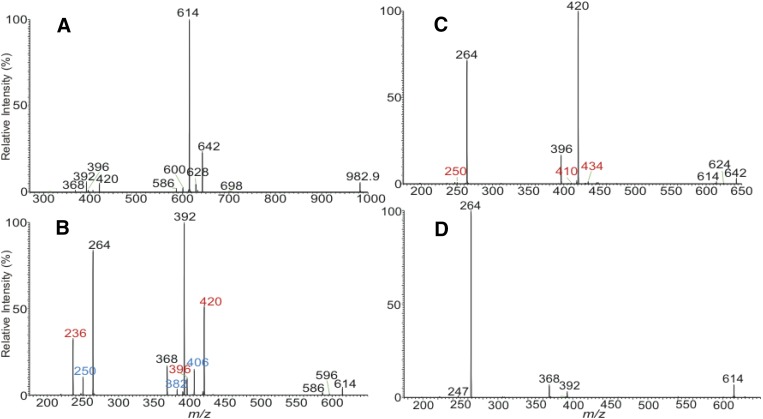
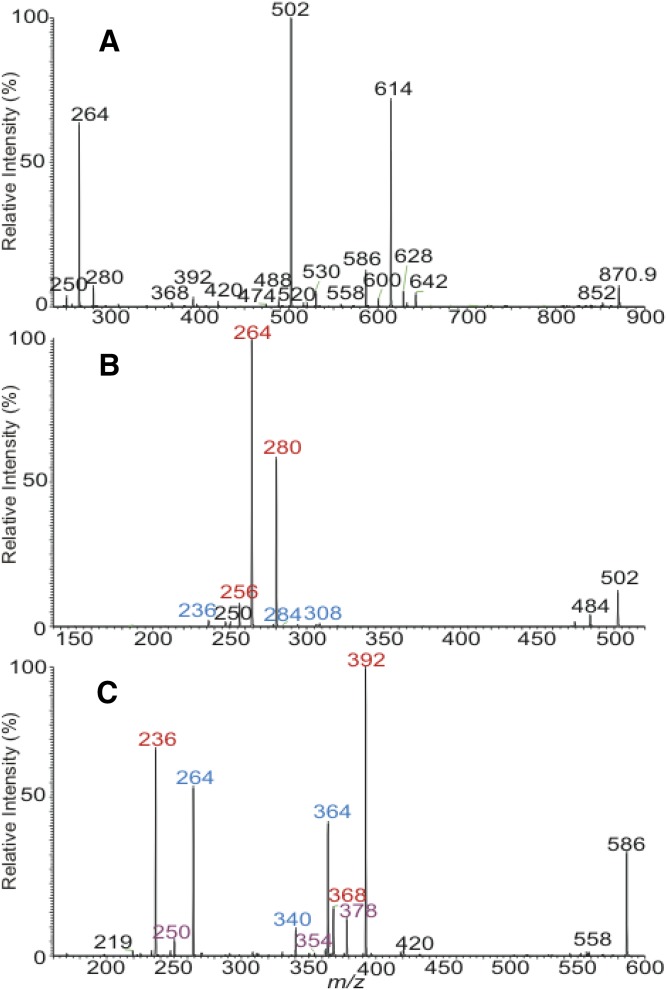
Multiple isobaric isomers were observed for all the ions that appeared in the ESI-MS spectrum (Table 1). For example, the MS2 spectrum of the ions of m/z 1,001.1 is dominated by the ion of m/z 982.9 arising from loss of water (not shown). MS3 on the ions of m/z 982.9 (1,001.1 → 982.9; Fig. 2A) gave rise to the prominent ions of m/z 614 arising from loss of the 1-O-24:0-FA substituent, along with minor ions at m/z 642, 628, 600, and 586 arising from losses of 1-O-22:0-, -23:0-, -25:0-, and -26:0-FA substituents, respectively. The MS4 spectrum of m/z 614 (1,001.1 → 982.9 → 614; Fig. 2B) contained prominent ions at m/z 392 (c2a) and 368 (d1a) arising from cleavages of the LCB, along with the ions of m/z 264 (e3b″), indicating the presence of the major d18:1/24:0-Cer structure (12) arising from the major 24:0-d18:1/24:0-Cer isomer. The spectrum (Fig. 2B) also contained the homologous ions at m/z 420 (c2a), 396 (d1a), and 236 (e3b″) and at m/z 406 (c2a), 382 (d1a), and 250 (e3b″), indicating the presence of 24:0-d16:1/26:0-Cer and 24:0-d17:1/25:0-Cer minor isomers, respectively. Similarly, the MS4 spectrum of the ions of m/z 642 (1,001.1 → 982.9 → 642; Fig. 2c) is dominated by the ions of m/z 420 (c2a), 396 (d1a), and 264 (e3b″), pointing to the presence of d18:1/26:0 structure, along with a minor ion set of m/z 434 (c2a), 410 (d1a), and 250 (e3b″) that identifies the d17:1/27:0 substituent. The combined structural information from MS3 (Fig. 2B) and MS4 (Fig. 2C) resulted in the assignment of 22:0-d18:1/26:0- and 22:0-d17:1/27:0-Cer minor isomers. The minor isomeric LCB/N-acyl-FAs were also seen by the MS4 spectra of the ions of m/z 628, 600, and 586 (Table 2) that resulted in the identification of 23:0-d17:1/26:0, 23:0-d18:1/25:0, 23:0-d16:1/27:0 (1-O-23:0 series); 25:0-d17:1/24:0, 25:0-d18:1/23:0, 25:0-d16:1/25:0 (1-O-25:0 series); and of 26:0-d16:1/24:0, 26:0-d18:1/22:0, 26:0-d17:1/23:0 (1-O-26:0 series) isobaric structures. These analyses resulted in the assignment of 14 isomers (Table 2).
Fig. 2.
The MS3 spectrum of the ions of m/z 982.9 (1,001.1 → 982.9) (A) originated from the ion species of m/z 1,001, its MS4 spectra of the ions of m/z 614 (1,001.1 → 982.9 → 614) (B), and of m/z 642 (1,001.1 → 982.9 → 642) (C). The MS4 spectrum of the ions of m/z 614 (650 → 632 → 614) originated from [M + H]+ ions of d18:1/24:0-Cer at m/z 650 is shown in (D).
TABLE 2.
Structures of the isobaric isomers deduced from MSn on the ions of m/z 1001.1
| MS3 (1,001.1 → 982.99)a | MS4 (1,001.1 → 982.99 → F2) | Structural Assignment | ||||
| Fragment Ions (F2) | 1-O-Acyl Group (from loss of) | LCB | N-Acyl | LCB/N-Acyl Moieties | ||
| e3b″ | c2a | d1a | ||||
| 642 | 22:0 | 264 | 420 | 396 | d18:1/26:0 | 22:0-d18:1/26:0 |
| 250 | 434 | 410 | d17:1/27:0 | 22:0-d17:1/27:0 | ||
| 628 | 23:0 | 250 | 420 | 396 | d17:1/26:0 | 23:0-d17:1/26:0 |
| 264 | 406 | 382 | d18:1/25:0 | 23:0-d18:1/25:0 | ||
| 278 | 392 | 368 | d19:1/24:0 | 23:0-d19:1/24:0 | ||
| 614b | 24:0 | 264 | 392 | 368 | d18:1/24:0 | 24:0-d18:1/24:0 |
| 236 | 420 | 396 | d16:1/26:0 | 24:0-d16:1/26:0 | ||
| 250 | 406 | 382 | d17:1/25:0 | 24:0-d17:1/25:0 | ||
| 600 | 25:0 | 250 | 392 | 368 | d17:1/24:0 | 25:0-d17:1/24:0 |
| 264 | 378 | 354 | d18:1/23:0 | 25:0-d18:1/23:0 | ||
| 236 | 406 | 382 | d16:1/25:0 | 25:0-d16:1/25:0 | ||
| 586 | 26:0 | 236 | 392 | 368 | d16:1/24:0 | 26:0-d16:1/24:0 |
| 264 | 364 | 340 | d18:1/22:0 | 26:0-d18:1/22:0 | ||
| 250 | 378 | 354 | d17:1/23:0 | 26:0-d17:1/23:0 | ||
| The main isomer is indicated by boldface type. | ||||||
Ions of m/z 982.99 are due to loss of water from ions of m/z 1,001.1 via MS2.
Major isomer.
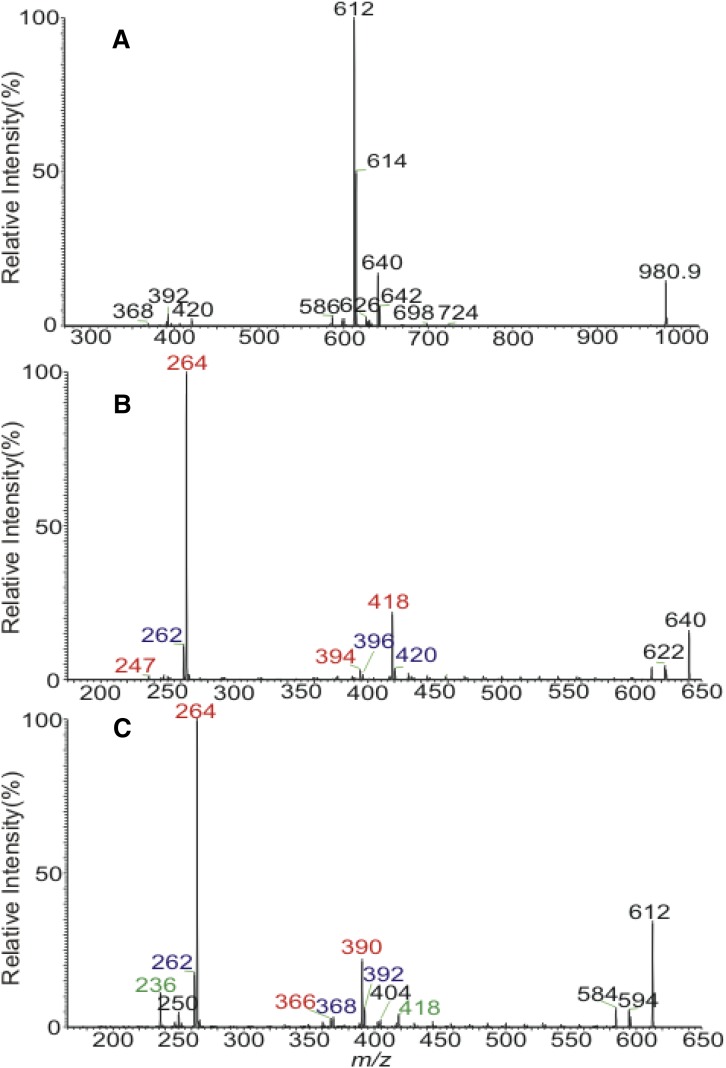
Identification of 1-O-acylceramides with unsaturated bonds
In addition to the major species containing multiple isobaric isomers, minor species consisting of 1-O-acylceramides with additional double bonds located at 1-O-acyl, N-acyl substituent, or LCB are present (Table 1). For example, the MS2 spectrum of the [M + H]+ ions of m/z 998.9 is dominated by the ions of m/z 980.9 (loss of water) (data not shown) as seen earlier. MS3 on the ions of m/z 980.9 (998.9 → 980.9; Fig. 3A) yielded ions at m/z 724, 698, 642, 640, 626, 614, 612, 600, and 586, arising from elimination of 1-O-16:0-, -18:1-, -22:1-, -22:0-, -23:0-, -24:1-, -24:0-, -25:1-, and -26:1-fatty acyl substituents, respectively. The MS4 spectrum of the major ions of m/z 614 (998 → 980 → 614; data not shown) is similar to that shown in Fig. 2C, indicating that the molecules consisted of isomeric d18:1/24:0-, d16:1/26:0-, and d17:1/25:0-Cer substituents, leading to the assignment of 24:1-d18:1/24:0, 24:1-d16:1/26:0, 24:1-d17:1/25:0 isomers. MS4 on the ions of m/z 640 (998→ 980 → 640; Fig. 3B) yielded the predominant ions of m/z 264 (e3b″), suggesting the presence of d18:1-LCB and the 418(c2a)/394(d1a) ion pair that recognize the 26:1-fatty acyl substituent. The results gave assignment of the major 22:0-d18:1/26:1 isomer. The spectrum (Fig. 3B) also contained the minor ions of m/z 262 (e3b″), which signifies the presence of d18:2-LCB, a dehydrosphingosine LCB, and the companion ions of m/z 420(c2a)/396(d1a) that identify the N-26:0 acyl group, indicating that a minor 22:0-d18:2/26:0-Cer isomer is also present.
Fig. 3.
The MS3 spectrum of the ion of m/z 980.9 (998.9 → 980.9) (A), its MS4 spectra of the ions of m/z 640 (998.9 → 980.9 → 640) (B), and of m/z 612 (998.9 → 980.9 → 612) (C).
Similar results were also observed for the MS4 spectrum of the ions of m/z 612 (Fig. 3C), which is dominated by the ions of m/z 264 (e3b″) along with the ions of m/z 390 (c2a) and 366 (d1a), indicating the presence of 24:0-d18:1/24:1-Cer isomer; together with ions of m/z 262 and 392/368 that identify the 24:0-d18:2/24:0 isomer; as well as the minor ion sets of m/z 236 (e3b″) and of 418(c2a)/394(d1a), arising from the 24:0-d16:1/26:1 isomer, and of m/z 250 (e3b″) and 404(c2a)/380(d1a) arising from the 24:0-d17:1/25:1 isomer.
The MS4 spectra of the ions of m/z 642 (not shown) and 614 (not shown) are identical to those shown in Fig. 2, and the MS4 spectra of the ions of m/z 626, 600, and 586 (not shown) all contain the ions that identify the LCB and N-acyl substituents of the molecules (supplemental Table S1). The combined information arising from MS2, MS3, and MS4 revealed the presence of 24 isobaric structures (see supplemental Table S1).
Identification of minor 1-O-acylceramide species in the mixture
The number of the isobaric isomers appears to grow even bigger for the minor ion species, similar to those seen for other lipid classes (21). For example, the MS2 spectrum of the ion of m/z 888.9 (data not shown) is dominated by the ion of m/z 870 (loss of water), which gives rise to the MS3 spectrum (888 → 870; Fig. 4A) consisting of ions of m/z 642, 628, 614, 600, 586, 558, 530, 516, 502, 488, and 474 arising from losses of the 1-O-linked 14:0-, 15:0-, 16:0-, 17:0-, 18:0-, 20:0-, 22:0-, 23:0-, 24:0-, 25:0-, and 26:0-FA substituents, respectively. The MS4 spectrum of the major ions of m/z 614 (888 → 870 → 614; data not shown) is nearly identical to that shown in Fig. 2B, signifying the molecule mainly consists of d18:1/24:0-Cer, along with minor d16:1/26:0- and d17:1/25:0-Cer moieties, resulting in the assignment of 16:0-d18:1/24:0-Cer major isomer, together with minor 16:0-d16:1/26:0-Cer and 16:0-d17:1/25:0-Cer. Similarly, further dissociation of the ions of m/z 502 (888 → 870 → 502; Fig. 4B) gives rise to the ion set of m/z 280 (c2a), 256 (d1a), and 264 (e3b″) that identify the d18:1/16:0 moiety, and the assignment of 24:0-d18:1/16:0-Cer. The MS4 spectrum of the ions of m/z 642 (888 → 870 → 642; data not shown) is similar to that shown in Fig. 2C that recognizes the main d18:1/26:0- and minor d17:1/27:0-Cer substituents, and results in the assignment of 14:0-d18:1/26:0- and 14:0-d17:1/27:0-Cer isomers. Further MS4 on the ions of m/z 586 (888 → 870 → 586; Fig. 4C) gives rise to the ions of m/z 392 (c2a), 368 (d1a), and 236 (e3b″) arising from 18:0-d16:1/24:0-Cer; of m/z 364 (c2a), 340 (d1a), and 264 (e3b″) arising from 18:0-d18:1/22:0; and of m/z 378 (c2a), 354 (d1a), and 250 (e3b″) arising from the minor isomer of 18:0-d17:1/23:0-Cer. MS4 on the ions of m/z 628, 600, 558, 530, 516, 488, and 474 reveals the LCB/N-acyl substituents of the molecule (supplemental Table S2). The combined structural information from MSn (n = 2–4) reveals 26 isobaric isomers (supplemental Table S2) in which 16:0-d18:1/24:0 and 24:0-d18:1/16:0-Cer are dominant. The fragment ions from MS4 (MH+ → [MH+ − H2O] → [MH+ − H2O − 1-O-FA] and the representative Cer core structures found in this study are listed in Table 3. In total, an estimate of 710 species were found in this lipid family.
Fig. 4.
The MS3 spectrum of the ion of m/z 870.9 (888.9 → 870.9) (A), its MS4 spectra of the ions of m/z 502 (888.9 → 870.9 → 502) (B) and of m/z 586 (888.9 → 870.9 → 586) (C).
TABLE 3.
The MS4 [MH+] → [MH+ − H2O] → [MH+ − H2O − 1-O-FA] fragment ions and the representative LCB/N-acyl strictures
| Precursor ion (from MS3) [MH+ − H2O − 1-O-FA] | Fragment ions from MS4 | Structures (LCB/N-Acyl Moieties) | ||
| LCB | N-Acyl | |||
| e3b″ | c2a | d1a | ||
| 752 | 264 | 530 | 506 | d18:1/34:1 |
| 738 | 250 | 530 | 506 | d17:1/34:1 |
| 726 | 264 | 504 | 480 | d18:1/32:0 |
| 724 | 264 | 502 | 478 | d18:1/32:1 |
| 712 | 250 | 504 | 480 | d17:1/32:0 |
| 698 | 264 | 476 | 452 | d18:1/30:0 |
| 250 | 490 | 466 | d17:1/31:0c | |
| 696 | 264 | 474 | 452 | d18:1/30:1 |
| 236 | 502 | 478 | d16:1/32:1b | |
| 694 | 262 | 474 | 450 | d18:2/30:1 |
| 236 | 502 | 478 | d16:1/32:2a | |
| 684 | 264 | 462 | 438 | d18:1/29:0 |
| 250 | 476 | 452 | d17:1/30:0c | |
| 278 | 448 | 424 | d19:1/28:0b | |
| 306 | 420 | 396 | d21:1/26:0c | |
| 670 | 264 | 448 | 424 | d18:1/28:0 |
| 292 | 420 | 396 | d20:1/26:0c | |
| 668 | 264 | 446 | 422 | d18:1/28:1 |
| 236 | 474 | 450 | d16:1/30:1c | |
| 262 | 448 | 424 | d18:2/28:0c | |
| 666 | 262 | 446 | 422 | d18:2/28:1 |
| 264 | 444 | 420 | d18:1/28:2a | |
| 656 | 264 | 434 | 410 | d18:1/27:0 |
| 278 | 420 | 396 | d19:1/26:0b | |
| 250 | 448 | 424 | d17:1/28:0b | |
| 654 | 264 | 432 | 408 | d18:1/27:1 |
| 250 | 446 | 422 | d17:1/28:1a | |
| 276 | 420 | 396 | d19:2/26:0b | |
| 278 | 418 | 394 | d19:1/26:1c | |
| 642 | 264 | 420 | 396 | d18:1/26:0 |
| 250 | 434 | 410 | d17:1/27:0c | |
| 640 | 264 | 418 | 394 | d18:1/26:1 |
| 262 | 420 | 396 | d18:2/26:0b | |
| 252 | 430 | 406 | d17:0/27:2c | |
| 638 | 262 | 418 | 394 | d18:2/26:1 |
| 264 | 416 | 392 | d18:1/26:2a | |
| 628 | 250 | 420 | 396 | d17:1/26:0 |
| 264 | 406 | 382 | d18:1/25:0b | |
| 236 | 434 | 410 | d16:1/27:0c | |
| 626 | 250 | 418 | 394 | d17:1/26:1 |
| 264 | 404 | 380 | d18:1/25:1a | |
| 262 | 406 | 382 | d18:2/25:0b | |
| 614 | 264 | 392 | 368 | d18:1/24:0 |
| 236 | 420 | 396 | d16:1/26:0a | |
| 250 | 406 | 382 | d17:1/25:0b | |
| 612 | 264 | 390 | 366 | d18:1/24:1 |
| 262 | 392 | 368 | d18:2/24:0a | |
| 236 | 418 | 394 | d16:1/26:1b | |
| 250 | 404 | 380 | d17:1/25:1c | |
| 610 | 264 | 388 | 364 | d18:1/24:2 |
| 262 | 390 | 366 | d18:2/24:1 | |
| 250 | 402 | 378 | d17:1/25:2c | |
| 600 | 250 | 392 | 368 | d17:1/24:0 |
| 264 | 378 | 354 | d18:1/23:0b | |
| 236 | 406 | 382 | d16:1/25:0b | |
| 598 | 250 | 390 | 366 | d17:1/24:1 |
| 264 | 376 | 352 | d18:1/23:1c | |
| 586 | 236 | 392 | 368 | d16:1/24:0 |
| 264 | 364 | 340 | d18:1/22:0c | |
| 250 | 378 | 354 | d17:1/23:0b | |
| 584 | 264 | 362 | 338 | d18:1/22:1 |
| 262 | 364 | 340 | d18:2/22:0a | |
| 236 | 390 | 366 | d16:1/24:1a | |
| 582 | 236 | 388 | 364 | d16:1/24:2 |
| 572 | 250 | 364 | 340 | d17:1/22:0 |
| 264 | 350 | 326 | d18:1/21:0a | |
| 222 | 392 | 368 | d15:1/24:0a | |
| 236 | 378 | 354 | d16:1/23:0b | |
| 278 | 336 | 312 | d19:1/20:0b | |
| 570 | 250 | 362 | 338 | d17:1/22:1 |
| 264 | 348 | 324 | d18:1/21:1b | |
| 236 | 376 | 352 | d16:1/23:1c | |
| 558 | 264 | 336 | 312 | d18:1/20:0 |
| 236 | 364 | 340 | d16:1/22:0 | |
| 250 | 350 | 326 | d17:1/21:0b | |
| 556 | 250 | 348 | 324 | d17:1/21:1 |
| 544 | 264 | 322 | 298 | d18:1/19:0 |
| 250 | 336 | 312 | d17:1/20:0b | |
| 530 | 264 | 308 | 284 | d18:1/18:1 |
| 278 | 294 | 270 | d19:1/17:0a | |
| 250 | 322 | 298 | d17:1/19:0c | |
| 528 | 264 | 304 | 280 | d18:1/18:2 |
| 526 | 264 | 304 | 280 | d18:1/18:2 |
| 516 | 264 | 294 | 270 | d18:1/17:0 |
| 514 | 250 | 306 | 282 | d17:1/18:1 |
| 264 | 292 | 268 | d18:1/17:1c | |
| 512 | 250 | 304 | 280 | d17:1/18:2 |
| 502 | 264 | 280 | 256 | d18:1/16:0 |
| 500 | 264 | 278 | 254 | d18:1/16:1 |
| 236 | 306 | 282 | d16:1/18:1 | |
| 498 | 236 | 304 | 280 | d16:1/18:2 |
| 488 | 250 | 280 | 256 | d17:1/16:0 |
| 264 | 266 | 242 | d18:1/15:0c | |
| 474 | 236 | 280 | 256 | d16:1/16:0 |
| 264 | 252 | 228 | d18:1/14:0a | |
| 250 | 266 | 242 | d17:1/15:0c | |
| 460 | 250 | 252 | 228 | d17:1/14:0 |
| 236 | 266 | 242 | d16:1/15:0c | |
| The main isomer (100%) is indicated by boldface type. | ||||
| a Secondary isomer (<80%). | ||||
| b Minor isomer (<50%). | ||||
| c Trace isomer (<10%). | ||||
HCD tandem mass spectrometric analysis of FA-AMPP derivative for locating the double bond(s) on the fatty acyl chain
High-resolution mass measurements on the hydrolysate-AMPP reaction products revealed that the presence of the FA-AMPP ion series ranged from C14 to C32:0 with zero or one double bond (supplemental Table S3) in which the molecules with the saturated fatty acyl chain were the major species; while minor species with unsaturated bond and with odd chain acyl substituents were also present (supplemental Table S1). HCD MS2 on the M+ ions of the FA-AMPP derivatives indicated that the double bond position of the FA substituents were all located at n-9 (ω-9). For example, the HCD MS2 spectrum of the ions of m/z 449 (see supplemental Fig. S1a) contained ions of m/z 349/351 and 295, 281, 253, 239, etc. together with major ions of m/z 183 and 169 that are signature ions of the FA-AMPP derivative, showing that the double bond is located at n-9 and that the ion represents a Δ918:1-FA (ω-9) (20, 22, 23). Another example is shown for the ions at m/z 533 (supplemental Fig. S1b), which yielded the analogous ions of m/z 433/435 and 379, 365, 351, etc., representing a Δ1524:1-FA. Interestingly, the HCD MS2 spectra of the ions of m/z 409 (supplemental Fig. S1c) and 421 (supplemental Fig. S1d) show that the species represent an anteoiso-15:0-FA and a Δ616:1-FA, respectively.
DISCUSSION
It appears to be that the compositions of the Cer core with the same m/z in all the 1-O-acylceramide family are identical, regardless of the chain length or the unsaturation status of the 1-O-acyl substituent. For example, the ions of m/z 642 arising from the MH+ ions of m/z 1,001 by consecutive losses of water and 22:0-fatty acyl residues (Fig. 2C), of m/z 998.9 by the analogous losses of water and 1-O-22:1-FA residues (998.9 – water – 338), and of m/z 888.8 via losses of water and 1-O-14:0-FA (888.8 – water − 228) all consist of a major d18:1/26:0-Cer and a minor d17:1/27:0-Cer core structure; while ions of m/z 614 (Fig. 2B) represent mainly the d18:1/24:0-Cer and minor isomers of d16:1/26:0- and d17:1/25:0-Cer. These findings are consistent with the notion that the 1-O-acylceramide was synthesized from Cer by 1-O-acylation with the participation of diacylglycerol acyltransferase (6, 7). Another important finding in this study is that the abundant species, such as the ions of m/z 888.9, 916.9, 972.9, and 1,000.1, all contain d18:1/24:0-Cer as the major core structure, and the isobaric isomers are seen by the variation of the 1-O-acyl chain, rather than by variation of the Cer core. More than 20 isobaric isomers are present in more than half of the molecular species, and more than 700 structures (Table 1), including a minor subfamily consisting of dehydrosphingosine LCB, are present. Rabionet and colleagues (6, 7) reported the presence of the 1-O-acyl N-α-hydroxyacylceramide subfamily in mouse and human epidermis. Interestingly, this Cer subfamily was found as a very minor species, and was eluted separately (fraction 3) from the major 1-O-acyl N-acylceramides (fraction 2). The presence of the vast number of structures in the 1-O-acylceramide family, as seen in this study, underscores the complexity of epidermal Cers, which consist of more than 10 Cer families (15, 16, 18, 24). Thus, this study highlights the utility of high-resolution LIT MS in the structural elucidation of complex lipid structures and the application of this technique in the complete characterization and differentiation of various Cer classes, including the location of the double bond(s) and hydroxyl group(s) on the fatty acyl chain and LCB has been reported recently (25).
There is a stark difference in the profiles of the MS4 spectra of m/z 612 (Fig. 3C), of which the fatty acyl chain contains a double bond, and of m/z 614 (Fig. 2B), whose fatty acyl chain is saturated. The former spectrum is dominated by the ions of m/z 264 (e3b″), and the ions of m/z 390 (c2a) and 366 (d1a) are of low abundance; while the ions of m/z 264 (e3b″) in the MS4 spectrum of the ions of m/z 614 are less prominent, and the analogous c2a ions at m/z 392 (Fig. 3C) and m/z 368 are abundant. The apparent differences in the MS4 profiles were also observed for m/z 640 (Fig. 3B) and m/z 642 (Fig. 2C) pairs. Whether these differences in the abundances of the analogous fragment ions are attributable to the presence or absence of a double bond in the fatty acyl chain remains unclear. Interestingly, the MS4 spectra of the ions of m/z 612 (648 → 630 →612) (data not shown) arising from the [M + H]+ ions of d18:1/24:1-Cer (with a double bond in 24:1-FA substituent) and of m/z 614 (650 → 632 →614) (Fig. 2D) arising from the [M + H]+ ions of d18:1/24:0-Cer (with no double bond in the 24:0-FA substituent) standards are all dominated by the ions of m/z 264, and the profiles of the spectra are similar to Fig. 3C, indicating that the fragmentation processes (supplemental Scheme S1) leading to the ion formation may be similar. The formation of the ions of m/z 612 (Fig. 3C) and m/z 614 (Fig. 2B) from 24:0-d18:1/24:1 and 24:0-d18:1/24:0-Cer, respectively, arising from the first loss of a water molecule may involve the participation of the 3-OH group of the LCB, followed by elimination of the 1-O-acyl group (Scheme 1). In contrast, the formation of the ions of m/z 612 (648 → 630 →612) originated from the [M + H]+ ions of d18:1/24:1-Cer and of m/z 614 (650 → 632 → 614) originated from the [M + H]+ ions of d18:1/24:0-Cer involves the consecutive losses of water, in which the first water loss may involve the participation of the 1-hydroxy group (supplemental Scheme S1). However, whether this difference in the sequence of the elimination processes leading to these precursor ions (i.e., the third generation ions) contributes to the differential cleavage of fatty acyl groups to form the ions of m/z 264 upon being further subjected to CID is also unclear. The prominence of the ions of m/z 264, as seen in Fig. 1C, may also reflect the fact that loss of a shorter 17:0-fatty acyl chain as a ketene in 18:1-d18:1/17:0-Cer is probably more facile than the analogous loss of the longer 26:0-fatty acyl chain in 22:0-d18:1/26:0-Cer, as supported by the notion that ions of m/z 264 in the MS4 spectrum of m/z 642 (Fig. 2c) is less prominent (than m/z 420).
The observation of the 1-O-acyl and N-acyl substituents ranging from C14 to C32 with zero or one double bond in this Cer family (Table 1, 3) is consistent with the results from the high-resolution mass measurement of the AMPP derivatives of the acid hydrolysate that yielded the M+ ion series ranging from C14 to C32 with zero or one double bond (supplemental Table S3). There are several minor species possessing two double bonds in the 1-O-acyl (e.g., 24:2 and 26:2; Table 1) or N-acyl (e.g., 24:2, 26:2, and 28:2; Table 3) chain, and the very long N-34:1-fatty acyl chain-containing species are also seen (Table 3). However, the corresponding FA species were not observed in the hydrolysate (supplemental Table S3). These FAs may be lost or too low to be detected after hydrolysis and derivatization steps. Nevertheless, the realization of the position of the double bond of the unsaturated FA substituents at n-9 for all the FA substituents, excepting 16:1-FA of which the double bond appears to be located at C6, is consistent with the notion of, for example, the presence of sphingolipids with d18:1/Δ1524:1 (n-9) core structure as reported previously (26). The 16:1-FA appears to be a Δ616:1-FA, which is a sapienic acid exclusively found in sebum (27).
Results from our preliminary study indicate that there is a significantly higher abundance (greater than three times) of the 1-O-acylceramide family in the epidermis of FA transport protein 4 (FATP4)-deficient newborn mice (Fatp4−/− mice) compared with heterozygous control newborn mice (Fatp+/−), while the level of the 1-O-acylceramide family is restored in the rescued null Fatp4 newborn mice (Fatp4−/−; Tg(IVL-Fatp1) (28) (data not shown). Mutations in SLC27A4, the gene encoding FATP4, are known to cause ichthyosis prematurity syndrome in humans (29, 30). FATP4 is critical for development of fetal skin (31) and it has been hypothesized that lack of FATP4 in epidermal keratinocytes induces abnormal lipid metabolism in the epidermis that initiates the observed alterations in epidermal signaling pathways (32, 33). It is still unclear whether the FATP4 gene plays a role in the synthesis of this complex Cer family. Studies toward understanding the mechanism underlying the increase of this 1-O-acylceramide family in Fatp4 mutant fetal epidermis are currently in progress in our laboratory.
Supplementary Material
Footnotes
Abbreviations:
- AMPP
- N-(4-aminomethylphenyl) pyridinium
- Cer
- ceramide
- CID
- collision-induced dissociation
- FATP4
- FA transport protein 4
- HCD
- higher collision energy dissociation
- LCB
- long-chain base
- LIT
- linear ion-trap
- MSn
- multiple-stage MS
This research was supported by United States Public Health Service Grants P41GM103422, P30DK020579, P30DK056341, R21HL120760, and R01AR049269 (to J.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Siegenthaler U., Laine A., and Polak L.. 1983. Studies on contact sensitivity to chromium in the guinea pig. The role of valence in the formation of the antigenic determinant. J. Invest. Dermatol. 80: 44–47. [DOI] [PubMed] [Google Scholar]
- 2.Imokawa G., Akasaki S., Hattori M., and Yoshizuka N.. 1986. Selective recovery of deranged water-holding properties by stratum corneum lipids. J. Invest. Dermatol. 87: 758–761. [DOI] [PubMed] [Google Scholar]
- 3.Imokawa G., Akasaki S., Minematsu Y., and Kawai M.. 1989. Importance of intercellular lipids in water-retention properties of the stratum corneum: induction and recovery study of surfactant dry skin. Arch. Dermatol. Res. 281: 45–51. [DOI] [PubMed] [Google Scholar]
- 4.Wertz P. W., and Downing D. T.. 1983. Ceramides of pig epidermis: structure determination. J. Lipid Res. 24: 759–765. [PubMed] [Google Scholar]
- 5.Ponec M., Weerheim A., Lankhorst P., and Wertz P.. 2003. New acylceramide in native and reconstructed epidermis. J. Invest. Dermatol. 120: 581–588. [DOI] [PubMed] [Google Scholar]
- 6.Rabionet M., Gorgas K., and Sandhoff R.. 2014. Ceramide synthesis in the epidermis. Biochim. Biophys. Acta. 1841: 422–434. [DOI] [PubMed] [Google Scholar]
- 7.Rabionet M., Bayerle A., Marsching C., Jennemann R., Gröne H-J., Yildiz Y., Wachten D., Shaw W., Shayman J. A., and Sandhoff R.. 2013. 1-O-acylceramides are natural components of human and mouse epidermis. J. Lipid Res. 54: 3312–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raith K., Darius J., and Neubert R. H. H.. 2000. Ceramide analysis utilizing gas chromatography–mass spectrometry. J. Chromatogr. A. 876: 229–233. [DOI] [PubMed] [Google Scholar]
- 9.Polito A. J., Akita T., and Sweeley C. C.. 1968. Gas chromatography and mass spectrometry of sphingolipid bases. Characterization of sphinga-4,14-dienine from plasma sphingomyelin. Biochemistry. 7: 2609–2614. [DOI] [PubMed] [Google Scholar]
- 10.Gu M., Kerwin J. L., Watts J. D., and Aebersold R.. 1997. Ceramide profiling of complex lipid mixtures by electrospray ionization mass spectrometry. Anal. Biochem. 244: 347–356. [DOI] [PubMed] [Google Scholar]
- 11.Hsu F-F., and Turk J.. 2002. Characterization of ceramides by low energy collisional-activated dissociation tandem mass spectrometry with negative-ion electrospray ionization. J. Am. Soc. Mass Spectrom. 13: 558–570. [DOI] [PubMed] [Google Scholar]
- 12.Hsu F. F., Turk J., Stewart M. E., and Downing D. T.. 2002. Structural studies on ceramides as lithiated adducts by low energy collisional-activated dissociation tandem mass spectrometry with electrospray ionization. J. Am. Soc. Mass Spectrom. 13: 680–695. [DOI] [PubMed] [Google Scholar]
- 13.Sullards M. C., and Merrill A. H. Jr. 2001. Analysis of sphingosine 1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Sci. STKE. 2001: pl1. [DOI] [PubMed] [Google Scholar]
- 14.Han X. 2002. Characterization and direct quantitation of ceramide molecular species from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal. Biochem. 302: 199–212. [DOI] [PubMed] [Google Scholar]
- 15.Masukawa Y., Narita H., Shimizu E., Kondo N., Sugai Y., Oba T., Homma R., Ishikawa J., Takagi Y., Kitahara T., et al. 2008. Characterization of overall ceramide species in human stratum corneum. J. Lipid Res. 49: 1466–1476. [DOI] [PubMed] [Google Scholar]
- 16.t’Kindt, R., Jorge L., Dumont E., Couturon P., David F., Sandra P., and Sandra K.. 2012. Profiling and characterizing skin ceramides using reversed-phase liquid chromatography-quadrupole time-of-flight mass spectrometry. Anal. Chem. 84: 403–411. [DOI] [PubMed] [Google Scholar]
- 17.Liebisch G., Drobnik W., Reil M., Trümbach B., Arnecke R., Olgemöller B., Roscher A., and Schmitz G.. 1999. Quantitative measurement of different ceramide species from crude cellular extracts by electrospray ionization tandem mass spectrometry (ESI-MS/MS). J. Lipid Res. 40: 1539–1546. [PubMed] [Google Scholar]
- 18.van Smeden J., Hoppel L., van der Heijden R., Hankemeier T., Vreeken R. J., and Bouwstra J. A.. 2011. LC/MS analysis of stratum corneum lipids: ceramide profiling and discovery. J. Lipid Res. 52: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robson K. J., Stewart M. E., Michelsen S., Lazo N. D., and Downing D. T.. 1994. 6-Hydroxy-4-sphingenine in human epidermal ceramides. J. Lipid Res. 35: 2060–2068. [PubMed] [Google Scholar]
- 20.Hsu F-F. 2016. Characterization of hydroxyphthioceranoic and phthioceranoic acids by charge-switch derivatization and CID tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 27: 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu F-F., Soehl K., Turk J., and Haas A.. 2011. Characterization of mycolic acids from the pathogen Rhodococcus equi by tandem mass spectrometry with electrospray ionization. Anal. Biochem. 409: 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang K., Dilthey B. G., and Gross R. W.. 2013. Identification and quantitation of fatty acid double bond positional isomers: a shotgun lipidomics approach using charge-switch derivatization. Anal. Chem. 85: 9742–9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M., Han R. H., and Han X.. 2013. Fatty acidomics: global analysis of lipid species containing a carboxyl group with a charge-remote fragmentation-assisted approach. Anal. Chem. 85: 9312–9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kováčik A., Roh J., and Vávrová K.. 2014. The chemistry and biology of 6-hydroxyceramide, the youngest member of the human sphingolipid family. ChemBioChem. 15: 1555–1562. [DOI] [PubMed] [Google Scholar]
- 25.Hsu F-F. 2016. Complete structural characterization of ceramides as [M-H]- ions by multiple-stage linear ion trap mass spectrometry. Biochimie. 130: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poulos A. 1995. Very long chain fatty acids in higher animals–a review. Lipids. 30: 1–14. [DOI] [PubMed] [Google Scholar]
- 27.Pappas A. 2009. Epidermal surface lipids. Dermatoendocrinol. 1: 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin M. H., and Khnykin D.. 2014. Fatty acid transporters in skin development, function and disease. Biochim. Biophys. Acta. 1841: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin M. H., and Miner J. H.. 2015. Fatty acid transport protein 1 can compensate for fatty acid transport protein 4 in the developing mouse epidermis. J. Invest. Dermatol. 135: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klar J., Schweiger M., Zimmerman R., Zechner R., Li H., Törmä H., Vahlquist A., Bouadjar B., Dahl N., and Fischer J.. 2009. Mutations in the fatty acid transport protein 4 gene cause the ichthyosis prematurity syndrome. Am. J. Hum. Genet. 85: 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gimeno R. E., Hirsch D. J., Punreddy S., Sun Y., Ortegon A. M., Wu H., Daniels T., Stricker-Krongrad A., Lodish H. F., and Stahl A.. 2003. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J. Biol. Chem. 278: 49512–49516. [DOI] [PubMed] [Google Scholar]
- 32.Lin M-H., Hsu F-F., and Miner J. H.. 2013. Requirement of fatty acid transport protein 4 for development, maturation, and function of sebaceous glands in a mouse model of ichthyosis prematurity syndrome. J. Biol. Chem. 288: 3964–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moulson C. L., Martin D. R., Lugus J. J., Schaffer J. E., Lind A. C., and Miner J. H.. 2003. Cloning of wrinkle-free, a previously uncharacterized mouse mutation, reveals crucial roles for fatty acid transport protein 4 in skin and hair development. Proc. Natl. Acad. Sci. USA. 100: 5274–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.