Abstract
Excitotoxicity is the pivotal mechanism of neuronal death. Prostaglandins (PGs) produced during excitotoxicity play important roles in neurodegenerative conditions. Previously, we demonstrated that initial burst productions of PGD2, PGE2, and PGF2α are produced by cyclooxygenase-2 (COX-2) in the hippocampus following a single systemic kainic acid (KA) administration. In addition, we showed that blocking of all PG productions ameliorated hippocampal delayed neuronal death at 30 days after KA administration. To investigate the role of individual PGs in the delayed neuronal death, we performed intracerebroventricular injection of PGD2, PGE2, or PGF2α in rats whose hippocampal PG productions were entirely blocked by pretreatment of NS398, a COX-2 selective inhibitor. Administration of PGD2 and PGF2α had a latent contribution to the delayed neuronal death, sustained over 30 days after a single KA treatment. Furthermore, PGD2 enhanced microglial activation, which may be involved in the delayed neuronal death in the hippocampus. These findings suggest that excitotoxic delayed neuronal death is mediated through microglia activated by PGD2.
Keywords: neurons, kainic acid, microglia
Excitotoxicity, the pivotal mechanism of neuronal death, is implicated in a wide range of neurodegenerative diseases, such as hypoxia-ischemia, epilepsy, stroke, and Alzheimer’s disease (1). In animal experiments, excitotoxin kainic acid (KA) has been known to stimulate glutamate receptors and induce hippocampal acute and delayed neuronal death (2). Acute neuronal death is caused by glutamate receptor-mediated mechanisms within a few days after excitotoxic insults (3). Delayed neuronal death develops over several days after excitotoxic insults (4).
Cyclooxygenases (COXs) oxidize arachidonic acid to prostaglandin (PG) G2 and H2, followed by PG synthases that generates several PGs. PGs exert diverse biological activities as intercellular signaling molecules through their cognate G-protein-coupled receptors (5, 6). PGs produced during excitotoxicity play important roles in neurodegenerative conditions (7, 8).
In our previous study, a comprehensive lipidomics analysis demonstrated that large amounts of PGD2, PGE2, and PGF2α are produced in the hippocampus after systemic KA administration (9). These initial burst PG productions are produced by COX-2, which is constitutively expressed in the hippocampus (9). This occurs without upregulation of COX-2 enzyme level (9). We also demonstrated that blockade of all PG productions ameliorated hippocampal delayed neuronal death at 30 days after KA administration, whereas acute neuronal death occurred through a mechanism independent of PG-associated pathways (10). In the present study, we evaluated the role of individual PGs in the delayed neuronal death. We performed intracerebroventricular (i.c.v.) injection of individual PGs (PGD2, PGE2, and PGF2α) in the rats, whose hippocampal PG productions were entirely blocked by pretreatment of NS398, a COX-2 selective inhibitor. Numerous dying neurons were observed in PGD2-treated or PGF2α-treated rats 30 days after treatment, suggesting that PGD2 and PGF2α contributed to the excitotoxicity-induced delayed neuronal death. Enhanced microglial activation was also observed in PGD2-treated rats. These data suggest that microglia, activated by PGD2 after excitotoxicity, lead to delayed neuronal death in the hippocampus.
MATERIALS AND METHODS
Animal procedures
Three-week-old male Wistar rats (Tokyo Laboratory Animals Science, Tokyo, Japan) were housed in appropriate animal care facilities at Saitama Medical University (Saitama, Japan) and were handled according to established international guidelines. Experimental protocols were approved by the Animal Research Committee of Saitama Medical University. Rats were maintained on a 12 h/12 h light/dark cycle with free access to tap water and a regular diet (CE-2, Clea, Tokyo, Japan). KA, NS398, PGD2, PGE2, PGF2α, and PGJ2 were purchased from Cayman Chemical (Ann Arbor, MI). KA (10 mg/kg, dissolved in saline) and NS398 (10 mg/kg, dissolved in saline containing 20% (DMSO) were injected intraperitoneally (i.p.) into the rats. PGD2, PGE2, PGF2α, and PGJ2 (dissolved in saline containing 0.1% ethanol) were administered at a concentration of 500 ng/5 μl by bilateral i.c.v. injections. Rats were reared until after 30 days of KA administration. The administration protocol of the reagents is described in Table 1. For protocol A, rats received saline 30 min after pretreatment with 20% DMSO in saline. After a further 30 min, the rats received 0.1% ethanol in saline (control group). For protocol B, rats received KA 30 min after pretreatment with 20% DMSO in saline. After a further 30 min, the rats received 0.1% ethanol in saline (KA group). For protocol C, rats received KA 30 min after pretreatment with NS398 in saline. After a further 30 min, the rats received 0.1% ethanol in saline (NS + KA group). For protocol D, rats received KA 30 min after pretreatment with NS398 in saline. After a further 30 min, the rats received PGD2 in saline (NS + KA + PGD2 group). For protocol E, rats received KA 30 min after pretreatment with NS398 in saline. After a further 30 min, the rats received PGE2 in saline (NS + KA + PGE2 group). For protocol F, rats received KA 30 min after pretreatment with NS398 in saline. After a further 30 min, the rats received PGF2α in saline (NS + KA + PGF2α group). For protocol G, rats received KA 30 min after pretreatment with NS398 in saline. After a further 30 min, the rats received PGJ2 in saline (NS + KA + PGJ2 group) (Table 1).
TABLE 1.
The reagents administration protocol
| Protocol | Group | 0 (min) | 30 (min) | 60 (min) |
| A | Control group | 20% DMSO/saline (i.p.) | Saline (i.p.) | 0.1% EtOH/saline (i.c.v.) |
| B | KA group | 20% DMSO/saline (i.p.) | KA (i.p.) | 0.1% EtOH/saline (i.c.v.) |
| C | NS + KA group | NS398 (i.p.) | KA (i.p.) | 0.1% EtOH/saline (i.c.v.) |
| D | NS + KA + PGD2 group | NS398 (i.p.) | KA (i.p.) | PGD2 (i.c.v.) |
| E | NS + KA + PGE2 group | NS398 (i.p.) | KA (i.p.) | PGE2 (i.c.v.) |
| F | NS + KA + PGF2α group | NS398 (i.p.) | KA (i.p.) | PGF2α (i.c.v.) |
| G | NS + KA + PGJ2 group | NS398 (i.p.) | KA (i.p.) | PGJ2 (i.c.v.) |
Histology
Rats were intracardially perfused with 4% paraformaldehyde in PBS. Brains were removed and postfixed overnight in 4% paraformaldehyde in PBS. Paraffin embedding and sectioning (6 μm thick coronal sections) of brains were conducted at Bozo Center Inc. (Tokyo, Japan). For histology, sections on slides were deparaffinized and permeabilized with methanol and soaked in 0.3% hydrogen peroxide to block endogenous peroxidase activity. Hematoxylin and eosin (H and E) staining was performed according to standard protocols. Sections were then coverslipped by using Poly-Mount (Polysciences Inc., Boston, MA).
Fluoro Jade C (FJC) staining was performed according to the manufacturer’s instruction [Ready-to-Dilute (RTD) FJC Staining Kit, Biosensis, Temecula, CA]. Slides were incubated in sodium hydroxide for 5 min, then washed with 70% ethanol, followed by distilled water. Slides were then incubated in potassium permanganate for 10 min. Next, slides were washed with distilled water and moved to low light for staining with FJC and 4’,6-diamidino-2-phenylindole (DAPI) for 15 min. Slides were rinsed with distilled water and cleared by brief immersion in xylenes. Slides were then coverslipped by using p-xylenebis(pyridinium)bromide (DPX) (Merck KGaA, Darmstadt, Germany).
For immunofluorescence, sections were incubated for 1 h in a blocking buffer (PBS, 5% BSA, and 0.1% polyoxyethylene sorbitan monolaurate) and incubated with antiionized calcium binding adaptor molecule 1 antibody (anti-Iba-1; 1:1,000; Wako, Osaka, Japan) at 4°C overnight, followed by incubation for 1 h with secondary antibody (Cy3-conjugated AffiniPure goat anti-rabbit IgG; 1:500, Jackson ImmunoResearch, Inc., West Grove, PA) fluorochrome-conjugated in the dark at 25°C. Nuclei were labeled with DAPI mounting medium. Sections were then coverslipped by using DPX. Sections were photographed at 20× magnification on a Keyence BZ-9000 microscope (Keyence Corp., Osaka, Japan). Images were captured by using a Keyence BZ-9000 BZ-II Analyzer.
RNA extraction and quantitative real-time PCR
After rearing for 30 days, rats were euthanized, and hippocampi were collected for RNA extraction. Samples of fresh frozen hippocampus were processed for RNA extraction by using ISOGEN (NIPPON GENE, Tokyo, Japan) following the manufacturer’s instructions. Extracted RNA was resuspended in RNase-free molecular-grade water (TAKARA BIO Inc., Shiga, Japan) and stored at −80°C until analysis. For quantitative real-time PCR (Q-PCR), total RNA (3 μg) was reverse-transcribed by using a PrimeScript RT reagent kit (TAKARA BIO Inc.). Q-PCR was performed by using the 7900 Sequence Detection System (Applied Biosystems, Foster City, CA) with the following gene-specific primers: phosphoglycerate kinase 1 (PGK1; forward: 5′-tccatggtgggtgtgaatctg-3′ reverse: 5′-cagctggatcttgtctgcaactt-3′), glial fibrillary acidic protein (GFAP; forward: 5′-agtggccaccagtaacatgcaa-3′ reverse: 5′-ggactcaaggtcgcaggtcaa-3′), and CD11b (forward: 5′-ccactcattgtgggcagctc-3′ reverse: 5′-caccggcttcattcatcatgtc-3′). Q-PCR conditions were 95°C for 30 s, followed by 40 cycles of 5 s at 95°C and 34 s at 60°C. The amount of target gene expression was calculated by using the ΔΔCT method (11). Data were analyzed by using the relative quantification technique. Q-PCR results were normalized to the expression levels of PGK1. Relative changes in gene expression were reported as a percentage of the expression in control rats.
Western blotting
Hippocampi were homogenized on ice in RIPA [50 mM Tris-HCl pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.1% SDS, and 0.5% deoxycholate] buffer containing 1:1,000 dilution of a protease inhibitor cocktail (CalBiochem, San Diego, CA) with a tissue homogenizer (Brinkmann Instruments, Westbury, NY). Proteins were separated on precast SDS gels and transferred to nitrocellulose membranes. After blocking with 5% skim milk (MEGMILK SNOW BRAND Co. Ltd., Tokyo, Japan) in PBS containing 0.05% Tween 20 (PBS-T), the membranes were incubated with the primary antibodies [anti-GFAP (Abcam, Cambridge, MA) and anti-Iba1 (Wako)] overnight, followed by incubation with HRP-conjugated secondary antibodies (Cell Signaling Technology, Beverly, MA) and washing with PBS-T three times. The membranes were treated with reagent for exposure (Chemi-Lumi One Super, nacalai tesque, Japan; ImmunoStar LD, Wako, Japan). Image of the membranes was captured by using a C-DiGit blot scanner (LI-COR, Lincoln, NE) and subjected to ImageJ analysis.
Statistical analyses
Data were analyzed by one-way ANOVA followed by Newman-Keuls post hoc test. All data were analyzed by using GraphPad Prism (Version 5.01; GraphPad Software, San Diego, CA) and expressed as mean ± SEM. P values < 0.05 were considered statistically significant (* P < 0.05; ** P < 0.01; *** P < 0.001).
RESULTS
Histopathological analyses of the effects of PGs on KA-induced delayed neuronal death in the hippocampus
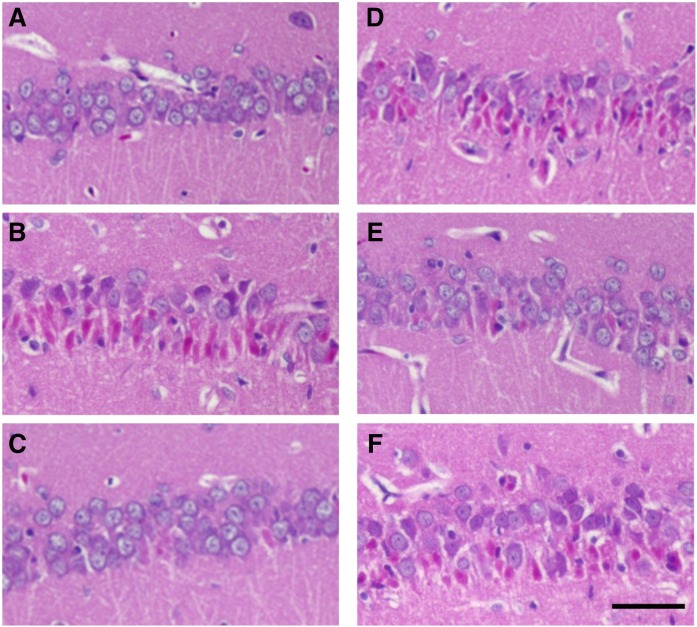
In adult rats, KA induces delayed neuronal death in the hippocampal CA1, CA3, and dentate gyrus via downregulation of GluA2. However, in juvenile rats, GluA2 expression is unchanged in the CA3 pyramidal and dentate gyrus granule cells, which leads to resistance to excitotoxic neuronal death (12). Therefore, we evaluated the delayed neuronal death in the hippocampal CA1 region of 3-week-old juvenile rats, because CA1 pyramidal neurons are particularly vulnerable to excitotoxicity in the rat hippocampal neurons at this age. A single systemic treatment of KA induced delayed neuronal death in the pyramidal layer of the CA1 regions of the hippocampus. H and E staining revealed KA-induced morphological changes in neurons, with the appearance of condensed/pyknotic nuclei (Fig. 1). Histopathological sections of the hippocampus from control rats exhibited intact pyramidal cells (Fig. 1A). Many neurons with pyknotic nuclei and hypereosinophilic cytoplasm appeared 30 days after KA treatment in rats of the KA group (Fig. 1B), whereas the NS + KA treatment resulted in fewer dying neurons (Fig. 1C). Numerous dying neurons were detected in the rats of the NS + KA + PGD2 (Fig. 1D) and NS + KA + PGF2α (Fig. 1F) groups, whereas a lower number of dying neurons were observed in the NS + KA + PGE2 group (Fig. 1E). Similar results were obtained from FJC staining, which selectively identified dying neurons. Numerous FJC-positive neurons were detected in the hippocampus of the KA (Fig. 2B), NS + KA + PGD2 (Fig. 2D), and NS + KA + PGF2α (Fig. 2F) groups. Fewer FJC positive neurons were observed in the NS + KA (Fig. 2C) and NS + KA + PGE2 (Fig. 2E) groups.
Fig. 1.
Histopathological sections (H and E staining) of the hippocampal CA1 region. Representative photomicrographs of coronal brain sections at the level of the fimbria demonstrate neuronal death of the hippocampal CA1 region to 30 days after the reagents administration are shown. H and E staining of control (A), KA (B), NS + KA (C), NS + KA + PGD2 (D), NS + KA + PGE2 (E), and NS + KA + PGF2α (F) groups is shown. Scale bar, 50 μm.
Fig. 2.
Degenerate neuron staining of the hippocampal CA1 region. Representative photomicrographs of coronal brain sections at the level of the fimbria demonstrate degenerate neuron of the hippocampal CA1 region to 30 days after all of the reagent administration are shown. FJC staining of control (A), KA (B), NS + KA (C), NS + KA + PGD2 (D), NS + KA + PGE2 (E), and NS + KA + PGF2α (F) groups is shown. Scale bar, 50 μm.
Glial responses to hippocampal injury
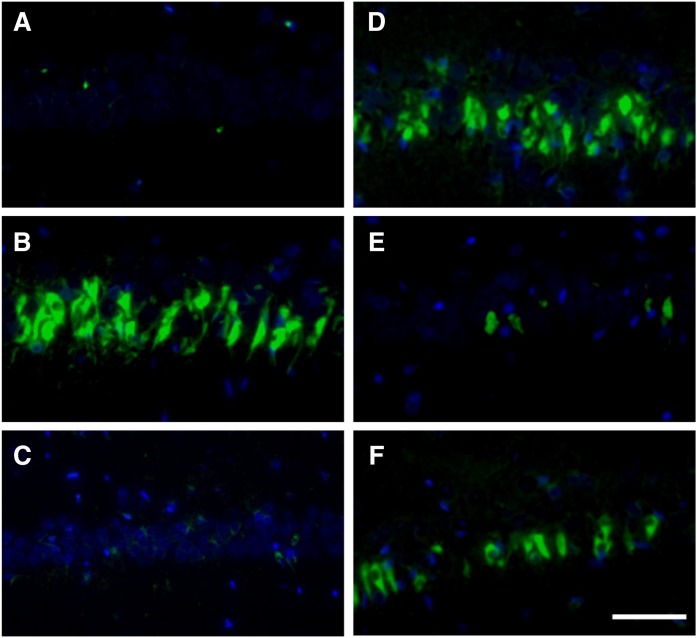
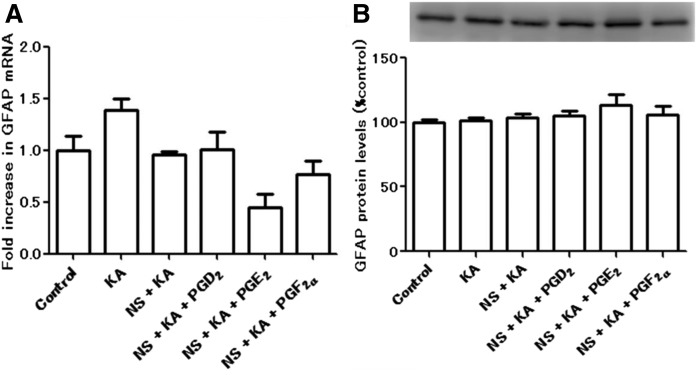
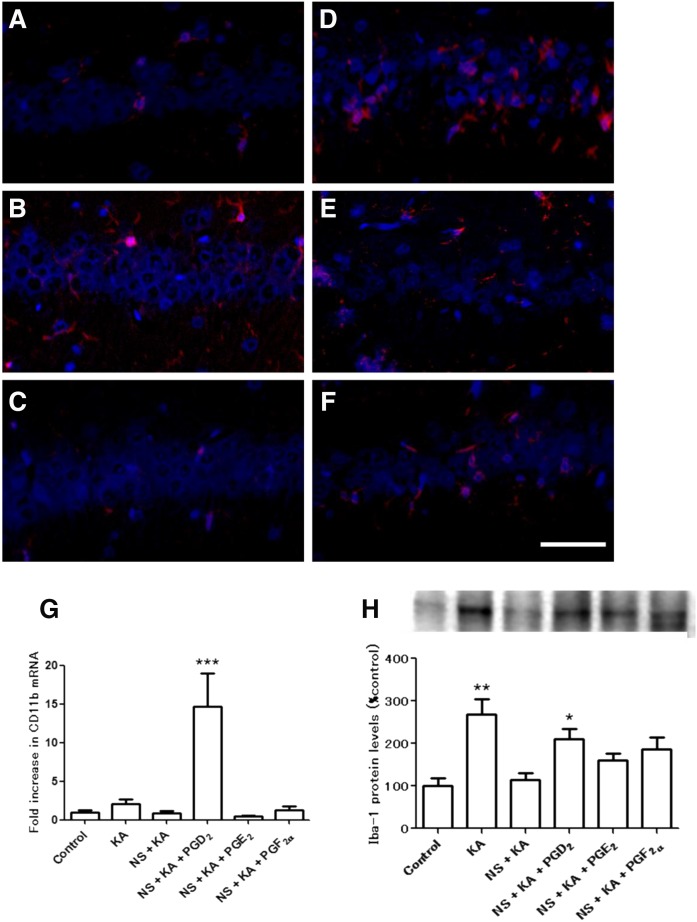
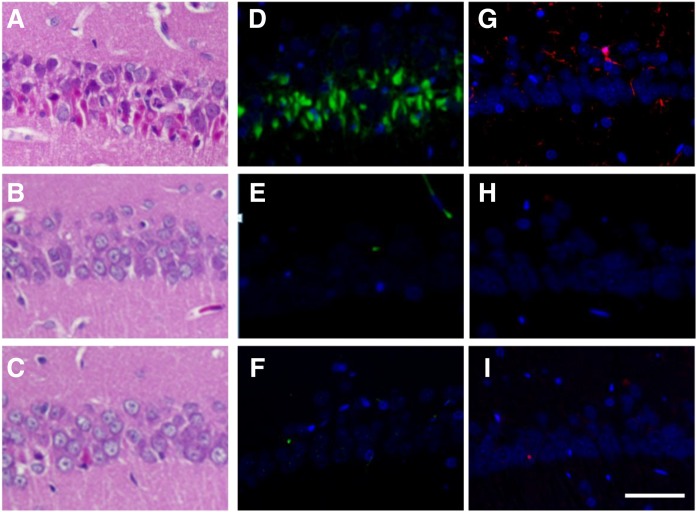
The activation and proliferation of glial cells are known to be involved in excitotoxic insults. We measured gene expression and protein levels of astrocytic (Fig. 3) and microglial (Fig. 4) markers. No significant differences were observed in the GFAP (astrocytic marker) gene expression (Fig. 3A) and protein levels (Fig. 3B). We showed by immunofluorescence staining that Iba-1-positive microglia were detected in the hippocampus of KA (Fig. 4B) and NS + KA + PGD2 (Fig. 4D) groups. Consistent with the microscopy data, a significant increase in the gene expression of CD11b (microglial marker) was observed in the rats of the NS + KA + PGD2 group (Fig. 4G), and protein levels of Iba-1 were increased in the KA and NS + KA + PGD2 groups (Fig. 4H).
Fig. 3.
The mRNA and protein levels of astrocytic marker. A: The mRNA expression levels of GFAP relative to PGK1 in the hippocampus to 30 days after all of the reagent administration, as determined by real-time PCR. B: The protein levels of GFAP in the hippocampus to 30 days after all of the reagent administration. Data are means ± SEM. Statistical analysis was performed by using one-way ANOVA followed by posthoc Newman-Keuls test.
Fig. 4.
Immunofluorescence staining, mRNA expression, and protein levels of microglial marker. Iba-1 staining of control (A), KA (B), NS + KA (C), NS + KA + PGD2 (D), NS + KA + PGE2 (E), and NS + KA + PGF2α (F) groups is shown. G: The mRNA expression levels of CD11b relative to PGK1 in the hippocampus after 30 days of KA exposure, as determined by real-time PCR. H: The protein levels of Iba-1 in the hippocampus to 30 days after all of the reagent administration. Data are means ± SEM. Statistical analysis was performed by using one-way ANOVA followed by posthoc Newman-Keuls test. * P < 0.05; ** P < 0.01; *** P < 0.001 vs. control. Scale bar, 50 μm.
The effects of PGJ2, a downstream metabolite of PGD2, on KA-induced delayed neuronal death
To investigate the effects of PGD2 downstream products, we evaluated delayed neuronal death and microglial activation in the PGJ2-treated rats. KA treatment induced numerous dying and FJC-positive neurons (Fig. 5A, D) and Iba-1 positive microglia (Fig. 5G). Fewer dying and FJC-positive neurons as well as Iba-1 positive microglia were observed in the NS + KA (Fig. 5B, E, H) and NS + KA + PGJ2 (Fig. 5C, F, I) groups.
Fig. 5.
Histopathological sections of the PGJ2-treated rat hippocampal CA1 region. Representative photomicrographs of coronal brain sections at the level of the fimbria demonstrate neuronal death of the hippocampal CA1 region to 30 days after the reagents administration. H and E staining of KA (A), NS + KA (B), and NS + KA + PGJ2 (C) groups is shown. FJC staining of KA (D), NS + KA (E), and NS + KA + PGJ2 (F) groups is shown. Iba-1 staining of KA (G), NS + KA (H), and NS + KA + PGJ2 (I) groups is shown. Scale bar, 50 μm.
DISCUSSION
In this study, we have demonstrated that PGD2 and PGF2α contribute to the excitotoxicity-induced delayed neuronal death in the hippocampus. A single i.c.v. treatment of PGD2 and PGF2α in the acute phase had a latent contribution to delayed neuronal death in the CA1 sustained over 30 days after a single KA treatment. We also found that PGD2 enhanced microglial activation, which may be involved in the delayed neuronal death in the hippocampus.
PGD2 is one of the most abundant PGs in the brain and regulates sleep, modulation of body temperature, hormone release, and nociception (13, 14). It signals through two distinct G-protein-coupled receptors (15), DP1 and DP2 receptor (CRTH2), that have opposing effects on cAMP regulation (16, 17). Signaling through the DP1 receptor stimulates adenylyl cyclase, leading to increased levels of cAMP and neuronal protection against acute neurological conditions such as excitotoxicity, ischemia, and stroke (18, 19). DP1 receptor expresses in the hippocampal neurons and protects neurons from acute excitotoxic injury in a cAMP-dependent manner (20). Our results showed that PGD2 promoted microglial activation and induced delayed neuronal death, sustained more than 30 days after KA-induced excitotoxicity. Supporting our results, DP1 receptor is also expressed in microglia (21) and can therefore respond to PGD2. The microglial PGD2-DP1 pathway is known to mediate neuronal damage though microglial activation (22). Furthermore, microglia upregulate cell proliferation signals in response to PGD2 in the chronic phase of neurodegeneration, such as in Alzheimer’s disease (21). Thus, PGD2 produced by excitotoxicity in the acute phase may enhance microglial activation and lead to persistent activation of microglia, resulting in a consequent exertion of neurotoxic effects in the hippocampus over a long period.
To investigate whether PGD2 itself or PGD2 downstream products exert neuroinflammatory responses, we analyzed the PGJ2-treated rats and found that PGJ2 did not affect microglial activation and delayed neuronal death. PGD2 is initially converted to PGJ2, which can undergo further conversion to Δ12-PGJ2 and then to 15-deoxy-Δ12, 14- PGJ2 (15d-PGJ2). 15-PGJ2 has antiinflammatory properties via PPARγ and proinflammatory properties via DP2 mechanisms (23, 24). One possible reason for our results is the counteracting effects of proinflammatory and antiinflammatory actions of 15d-PGJ2. In addition, the amounts of PGD2 downstream products (PGJ2, Δ12-PGJ2, and 15d-PGJ2) were almost undetectable in our previous comprehensive lipidomics analysis (9), suggesting that they are not major metabolites of PGD2 in the KA-administrated rat hippocampus. These results suggest that PGD2 itself probably contributes to microglial activation and delayed neuronal death.
In our experimental condition, PGE2 did not actively evoke the KA-induced delayed neuronal death. We have speculated that the possible reason is PGE2 receptor subtype diversity, because PGE2 activates four kinds of receptor subtypes (EP1–4) (25). EP1 disrupts Ca2+ homeostasis and acts on brain injury induced by excitotoxicity (26). EP2 mediates neuroprotection in a cAMP-dependent manner (27). Pharmacological studies indicate that EP3 and EP4 mediate neurotoxicity and neuroprotection, respectively (28, 29). Therefore, the contribution of PGE2 to the delayed neuronal death is complicated by its pleiotropic effects.
Prostamide/PGF synthase, which catalyzes the conversion of PGH2 to PGF2α and prostamide H2 to prostamide F2 (30), is expressed in oligodendrocytes (31), and PGF2α receptor (FP) is expressed in oligodendrocytes and astrocytes (32, 33). Several lines of evidence suggest that PGF2α/FP signaling mediates neurotoxicity, and inhibition of PGF2α/FP signaling protects from brain damage in ischemia (34), middle cerebral artery occlusion (35), and demyelination model (32). Similarly, we found that PGF2α contributes to neuronal degeneration. PGF2α/FP signaling in oligodendrocytes and/or astrocytes probably mediates neurotoxicity and is involved in the delayed neuronal death.
Astrocytes are known to have a dual role in neurotoxicity and neuroprotection in neurodegenerative conditions (36). In the present study, none of the PGs examined significantly changed mRNA and protein levels of GFAP in the 30 days after KA treatment. We previously reported that blockade of all PG productions did not affect the astrocyte reactivity after KA-induced excitotoxicity (10). Therefore, astrocyte reactivity in the KA-induced excitotoxicity might be independent of PG-associated pathways.
Our data demonstrated that the excitotoxicity-induced immediate surge of PGD2 and PGF2α productions (30 min after an excitotoxic insult) are a critical acute-phase event that results in delayed neuronal death, sustained over a long period (30 days) in the hippocampus. An instantaneous inhibitory treatment of PGD2 and PGF2α productions is an important therapeutic strategy to reduce brain damage induced by excitotoxic mechanisms.
Acknowledgments
The authors thank the Division of Laboratory Animal Medicine, Biomedical Research Center, Saitama Medical University, for maintaining the rats. We thank Messrs. T. Awaji and S. Suo for various advice. We thank Messrs. D. Yamagishi, T. Matsui, M. Fujita, N. Kubo, H. Takasaki, M. Hayashi, and K. Oryu (Saitama Medical University Faculty of Medicine) for research support.
Footnotes
Abbreviations:
- 15d-PGJ2
- 15-deoxy-Δ12, 14-PGJ2
- COX
- cyclooxygenase
- FJC
- Fluoro Jade C
- GFAP
- glial fibrillary acidic protein
- FP
- prostaglandin F receptor
- Iba-1
- ionized calcium binding adapter molecule 1
- i.c.v.
- intracerebroventricular
- i.p.
- intraperitoneally
- KA
- kainic acid
- PG
- prostaglandin
- PGK1
- phosphoglycerate kinase 1
- Q-PCR
- quantitative real-time PCR
This work was supported by MEXT KAKENHI Grant 25870677; grants from Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics and The Promotion and Mutual Aid Corporation for Private Schools of Japan, Shimabara Science Foundation; Saitama Medical University Internal Grant 24-B-1-19; and an Ochiai Memorial Award Research Grant.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Wang Q., Yu S., Simonyi A., Sun G. Y., and Sun A. Y.. 2005. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol. Neurobiol. 31: 3–16. [DOI] [PubMed] [Google Scholar]
- 2.Noch E., and Khalili K.. 2009. Molecular mechanisms of necrosis in glioblastoma: the role of glutamate excitotoxicity. Cancer Biol. Ther. 8: 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bittigau P., and Ikonomidou C.. 1997. Glutamate in neurologic diseases. J. Child Neurol. 12: 471–485. [DOI] [PubMed] [Google Scholar]
- 4.Domercq M., and Matute C.. 2004. Neuroprotection by tetracyclines. Trends Pharmacol. Sci. 25: 609–612. [DOI] [PubMed] [Google Scholar]
- 5.Hirata T., and Narumiya S.. 2011. Prostanoid receptors. Chem. Rev. 111: 6209–6230. [DOI] [PubMed] [Google Scholar]
- 6.Narumiya S. 2009. Prostanoids and inflammation: a new concept arising from receptor knockout mice. J. Mol. Med. (Berl.). 87: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 7.Liang X., Wu L., Wang Q., Hand T., Bilak M., McCullough L., and Andreasson K.. 2007. Function of COX-2 and prostaglandins in neurological disease. J. Mol. Neurosci. 33: 94–99. [DOI] [PubMed] [Google Scholar]
- 8.Yagami T., Koma H., and Yamamoto Y.. 2016. Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system. Mol. Neurobiol). 53: 4754–4771. [DOI] [PubMed] [Google Scholar]
- 9.Yoshikawa K., Kita Y., Kishimoto K., and Shimizu T.. 2006. Profiling of eicosanoid production in the rat hippocampus during kainic acid-induced seizure: dual phase regulation and differential involvement of COX-1 and COX-2. J. Biol. Chem. 281: 14663–14669. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa K., Kita Y., Furukawa A., Kawamura N., Hasegawa-Ishii S., Chiba Y., Takei S., Maruyama K., Shimizu T., and Shimada A.. 2013. Excitotoxicity-induced immediate surge in hippocampal prostanoid production has latent effects that promote chronic progressive neuronal death. Prostaglandins Leukot. Essent. Fatty Acids. 88: 373–381. [DOI] [PubMed] [Google Scholar]
- 11.Livak K. J., and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H., Grooms S. Y., Bennett M. V., and Zukin R. S.. 2000. The AMPAR subunit GluR2: still front and center-stage. Brain Res. 886: 190–207. [DOI] [PubMed] [Google Scholar]
- 13.Narumiya S. 2007. Physiology and pathophysiology of prostanoid receptors. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 83: 296–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urade Y., and Hayaishi O.. 2011. Prostaglandin D2 and sleep/wake regulation. Sleep Med. Rev. 15: 411–418. [DOI] [PubMed] [Google Scholar]
- 15.Trist D. G., Collins B. A., Wood J., Kelly M. G., and Robertson A. D.. 1989. The antagonism by BW A868C of PGD2 and BW245C activation of human platelet adenylate cyclase. Br. J. Pharmacol. 96: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crider J. Y., Griffin B. W., and Sharif N. A.. 1999. Prostaglandin DP receptors positively coupled to adenylyl cyclase in embryonic bovine tracheal (EBTr) cells: pharmacological characterization using agonists and antagonists. Br. J. Pharmacol. 127: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharif N. A., Williams G. W., and Davis T. L.. 2000. Pharmacology and autoradiography of human DP prostanoid receptors using [(3)H]-BWA868C, a DP receptor-selective antagonist radioligand. Br. J. Pharmacol. 131: 1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dore S., and Shafique Ahmad A.. 2015. Frontiers in neuroengineering cytoprotective role of prostaglandin D2 DP1 receptor against neuronal injury following acute excitotoxicity and cerebral ischemia. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. F. H. Kobeissy, editor. CRC Press/Taylor & Francis, Boca Raton, FL. 97–116. [PubMed] [Google Scholar]
- 19.Ahmad A. S., Ahmad M., Maruyama T., Narumiya S., and Dore S.. 2010. Prostaglandin D2 DP1 receptor is beneficial in ischemic stroke and in acute exicitotoxicity in young and old mice. Age (Dordr.). 32: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang X., Wu L., Hand T., and Andreasson K.. 2005. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J. Neurochem. 92: 477–486. [DOI] [PubMed] [Google Scholar]
- 21.Mohri I., Kadoyama K., Kanekiyo T., Sato Y., Kagitani-Shimono K., Saito Y., Suzuki K., Kudo T., Takeda M., Urade Y., et al. 2007. Hematopoietic prostaglandin D synthase and DP1 receptor are selectively upregulated in microglia and astrocytes within senile plaques from human patients and in a mouse model of Alzheimer disease. J. Neuropathol. Exp. Neurol. 66: 469–480. [DOI] [PubMed] [Google Scholar]
- 22.Bate C., Kempster S., and Williams A.. 2006. Prostaglandin D2 mediates neuronal damage by amyloid-beta or prions which activates microglial cells. Neuropharmacology. 50: 229–237. [DOI] [PubMed] [Google Scholar]
- 23.Forman B. M., Tontonoz P., Chen J., Brun R. P., Spiegelman B. M., and Evans R. M.. 1995. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 83: 803–812. [DOI] [PubMed] [Google Scholar]
- 24.Liu H., Li W., Rose M. E., Pascoe J. L., Miller T. M., Ahmad M., Poloyac S. M., Hickey R. W., and Graham S. H.. 2013. Prostaglandin D2 toxicity in primary neurons is mediated through its bioactive cyclopentenone metabolites. Neurotoxicology. 39: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugimoto Y., and Narumiya S.. 2007. Prostaglandin E receptors. J. Biol. Chem. 282: 11613–11617. [DOI] [PubMed] [Google Scholar]
- 26.Kawano T., Anrather J., Zhou P., Park L., Wang G., Frys K. A., Kunz A., Cho S., Orio M., and Iadecola C.. 2006. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat. Med. 12: 225–229. [DOI] [PubMed] [Google Scholar]
- 27.McCullough L., Wu L., Haughey N., Liang X., Hand T., Wang Q., Breyer R. M., and Andreasson K.. 2004. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J. Neurosci. 24: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad M., Ahmad A. S., Zhuang H., Maruyama T., Narumiya S., and Dore S.. 2007. Stimulation of prostaglandin E2–EP3 receptors exacerbates stroke and excitotoxic injury. J. Neuroimmunol. 184: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Echeverria V., Clerman A., and Dore S.. 2005. Stimulation of PGE receptors EP2 and EP4 protects cultured neurons against oxidative stress and cell death following beta-amyloid exposure. Eur. J. Neurosci. 22: 2199–2206. [DOI] [PubMed] [Google Scholar]
- 30.Moriuchi H., Koda N., Okuda-Ashitaka E., Daiyasu H., Ogasawara K., Toh H., Ito S., Woodward D. F., and Watanabe K.. 2008. Molecular characterization of a novel type of prostamide/prostaglandin F synthase, belonging to the thioredoxin-like superfamily. J. Biol. Chem. 283: 792–801. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa K., Takei S., Hasegawa-Ishii S., Chiba Y., Furukawa A., Kawamura N., Hosokawa M., Woodward D. F., Watanabe K., and Shimada A.. 2011. Preferential localization of prostamide/prostaglandin F synthase in myelin sheaths of the central nervous system. Brain Res. 1367: 22–32. [DOI] [PubMed] [Google Scholar]
- 32.Iwasa K., Yamamoto S., Takahashi M., Suzuki S., Yagishita S., Awaji T., Maruyama K., and Yoshikawa K.. 2014. Prostaglandin F2alpha FP receptor inhibitor reduces demyelination and motor dysfunction in a cuprizone-induced multiple sclerosis mouse model. Prostaglandins Leukot. Essent. Fatty Acids. 91: 175–182. [DOI] [PubMed] [Google Scholar]
- 33.Kitanaka J., Hashimoto H., Gotoh M., Kondo K., Sakata K., Hirasawa Y., Sawada M., Suzumura A., Marunouchi T., Matsuda T., et al. 1996. Expression pattern of messenger RNAs for prostanoid receptors in glial cell cultures. Brain Res. 707: 282–287. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa H., Sasaki T., Kassell N. F., Nakagomi T., Lehman R. M., and Hongo K.. 1987. Immunohistochemical demonstration of increase in prostaglandin F2-alpha after recirculation in global ischemic rat brains. Acta Neuropathol. 75: 62–68. [DOI] [PubMed] [Google Scholar]
- 35.Saleem S., Ahmad A. S., Maruyama T., Narumiya S., and Dore S.. 2009. PGF(2alpha) FP receptor contributes to brain damage following transient focal brain ischemia. Neurotox. Res. 15: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sofroniew M. V. 2015. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 16: 249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]