Abstract
d-Pen2,d-Pen5 enkephalin (DPDPE) is one of the most selective synthetic peptide agonists targeting the δ-opioid receptor. Three cyclic analogues of DPDPE containing a xylene bridge in place of disulfide bond have been synthesized and fully characterized as opioid receptors agonists. The in vitro activity was investigated showing a good affinity of 7a–c for μ- and δ-receptors. In vivo biological assays revealed that 7b is the most potent analogue with the ability to maintain high level of analgesia from 15 to 60 min following intracerebroventricular (i.c.v.) administration, whereas DPDPE was slightly active until 45 min. Compound 7b induced long lasting analgesia also after subcutaneous administration, whereas DPDPE was inactive.
Keywords: Opioids, DPDPE, xylene bridge, antinociception, peptides
The cloning of opioid receptors has provided direct structural evidence of the “multiple opioid receptors” concept as a powerful tool for physiological and pharmacological evaluation of their roles in both normal and acute pain states.1,2
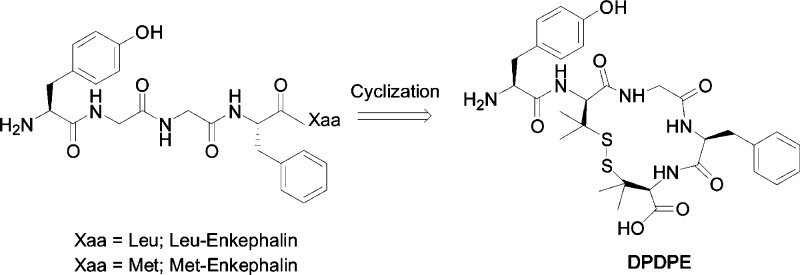
Delta-opioid receptors (DOP) are appealing drug targets for pain relief, due to the lack of unwanted side effects and the strong antinociceptive activity showed by their selective agonists. Ligands possessing dual agonist activities at the δ- and μ-receptors may allow for the effective treatment of pain with lessened μ-receptor-mediated side effects.3 Thus, it is highly desirable the design of “unbalanced” dual-acting opioid drugs as full δ-opioid receptor agonist and only as partial agonist of the μ-opioid receptor, which is strongly related to the development of tolerance and physical dependence.4 Met-Enkephalin and Leu-enkephalin are linear endogenous penta-peptides with high affinity for DOP regulating human nociception; numerous structural modifications have been explored during the last years to investigate the structure–activity relationships (SAR) and to improve their selectivity;5,6 H-Tyr-c[d-Pen-Gly-Phe-d-Pen]-OH (DPDPE) is the first synthetic prototype of highly selective constrained cyclic peptide for this receptor (Figure 1).7
Figure 1.
DPDPE cyclization strategy from Leu/Met-enkephalins.
DPDPE is employed as radiolabeled δ receptor full agonist in the opioid binding assays due to its resistance to proteolytic degradation and permeability to the blood–brain barrier (BBB).8
The X-ray crystal structure of DPDPE published in 19949 has been assumed as starting point in a recent computational study based on MD simulations,10 from which a series of putative bioactive conformations has been provided: gem-dimethyl groups of the d-penicillamine residues are responsible for the rigidified structure and their adverse steric interactions for the low μ-opioid (MOP) receptor affinity. Disulfide bond is prone to reduction following ring opening, so incorporation of different types of linkers could provide conformational diversity involving different cyclization strategies and global and local constrictions;7 the replacement of disulfide bond with more stable bridges can increase metabolic stability and change the overall conformation in order to improve the receptor binding affinity of the novel compounds.11,12 A systematic and synergistic multidisciplinary approach has been used, over the last decades, for the design of novel peptide ligands with unique biological activity profiles.13 In this article we propose the design, synthesis, and biological evaluation of new cyclic DPDPE analogues 7a–c containing o-, m-, p-xylene regioisomers, respectively, as μ/δ mixed opioid receptor agonists, using in vitro and in vivo models to examine the antinociception activity of the new entities. The C-terminal free carboxyl group in DPDPE improves the δ/μ selectivity,14 then it has been maintained in our cyclic peptides. We envisaged that the introduction of different disulfide bridge in DPDPE would lead to different μ/δ affinity, increased stability, and activity of the newly designed peptides; according to this working hypothesis, a side-chain-to-side-chain cyclization involving the two thiols groups and three dibromo-xylene regioisomers has been performed. The xylene-type bridging reaction utilizes the exquisite reactivity of dibromo-xylene scaffolds toward free thiol groups of penicillamine residues of compound 4,15 providing cyclic peptides containing two robust thioether bonds (5a–c). The novel cyclic peptides 7a–c have been also investigated by molecular docking study, to discern the structural influences of different xylene regioisomers on the molecular interactions of the cyclic peptides at the δ-opioid receptors. Other similar organic scaffolds may offer new insights for the preparation of further constrained DPDPE analogues (e.g., N-substituted bromomaleimide motif)16 and could be selected for future development.
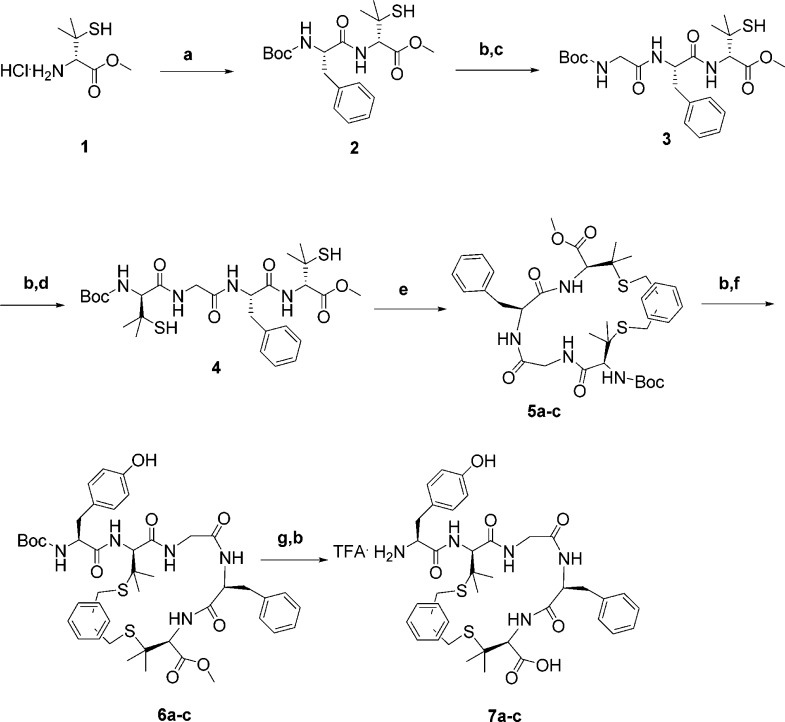
Solution phase peptide synthesis has been performed following the standard method of EDC·HCl/HOBt anhydrous/DIPEA in DMF,17 in accordance with Boc protection strategy to afford the three cyclic peptides 7a–c as TFA salts (for the general procedures, see SI). The commercially available d-penicillamine has been converted in its methyl ester derivative 1 following the well-established literature procedure,18 and then it was submitted to coupling reaction with BocPhe-OH. Coupling reactions have been repeated together with Boc-deprotection cocktail treatment, until to reach the key intermediate compound Boc-(d)Pen-Gly-Phe-(d)Pen-OMe (4), which was used for the subsequent cyclization reaction (Scheme 1).
Scheme 1. Synthesis of Final Compounds 7a–c.
Reagents and conditions: (a) 1.1 equiv of BocPhe-OH, 1.1 equiv of EDC·HCl, 1.1 equiv of HOBt anhydrous, 3.3 equiv of DIPEA, DMF under N2 atmosphere, r.t., overnight; (b) TFA/DCM = 1:1 under N2 atmosphere, r.t., 1 h; (c) 1.1 equiv of BocGly-OH, 1.1 equiv of EDC·HCl, 1.1 equiv of HOBt anhydrous, 3.3 equiv of DIPEA, DMF under N2 atmosphere, r.t., overnight; (d) 1.1 equiv of Boc(d)Pen-OH, 1.1 equiv of EDC·HCl, 1.1 equiv of HOBt anhydrous, 3.3 equiv of DIPEA, DMF under N2 atmosphere, r.t., overnight; (e) 2.1 equiv of o-dibromo-xylene, 6 days for 5a, 1.3 equiv of m-dibromo-xylene, 4 days for 5b, 2.1 equiv of p-dibromo-xylene, 6 days for 5c, 2.6 equiv of DIPEA in DMF under N2 atmosphere, r.t.; (f) 1.1 equiv of BocTyr-OH, 1.1 equiv of EDC·HCl, 1.1 equiv of HOBt anhydrous, 3.3 equiv of DIPEA, DMF under N2 atmosphere, r.t., overnight; (g) 4 equiv of 1 M NaOH in THF, 5 h for 7a, 2 equiv of 1 M NaOH in THF, 2 h for 7b, 3.5 equiv of 1 M NaOH in THF, 3 h for 7c, r.t.
Based on the work of Benito and Meldal on bicyclic organo-peptides19 we applied the same chemoselective cyclization reaction relying on the chemical linkage of peptides onto scaffolds (CLIPS) technology,20 to afford our desired cyclic intermediates 5a–c in 51%, 63%, and 39% yields, respectively, after silica gel chromatography. The reaction between linear intermediate 4 and 1.3 equiv of m-dibromo-xylene is completed after 4 days, while the cyclization reaction to form 5a,c requires 1 week to reach the completeness with 2.1 equiv of alkylation reagent; this is possibly due to the steric hindrance of halogen atoms in o-dibromo-xylene, which makes difficult the attack of the two thiol groups; in the opposite case, the distance between the two bromine atoms in the p-substituted regioisomer hampers the ring closure, thus prolonging further the reaction time and requiring more equivalents of reagent. Then the Boc protecting group has been removed from 5a–c, and Boc-Tyr-OH has been attached to obtain the full cyclic sequences 6a–c. Finally, saponification under basic condition occurred following the same behavior of the side chain-to-side chain cyclization in terms of time and reagents converting the free acid compounds into the corresponding TFA salts. The purification by RP-HPLC chromatography allowed to obtain the desired cyclic peptides 7a–c with a grade of purity ≥95% in 41%, 55%, and 40% overall yields, respectively. Analysis of cyclic penta-peptides incorporating thioether bridge is tricky and complex in order to achieve a rigorous proof of structure; according to a recent paper by Johnson et al.,21 full characterization of the final products 7a–c has been performed through MS-UPLC (Figures S1–S3), analytical RP-HPLC, and 1H NMR.
The method applied resulted to be efficient and straightforward to furnish exhaustive qualitative and structural information on our novel molecular entities, which were used for the competition binding assays versus μ- and δ-opioid receptors;22,23 all the cyclic compounds 7a–c showed good binding profile for δ-opioid receptor, although they displayed a lower affinity than DPDPE (Figure S4, Table 1). However, they showed a considerable higher affinity for μ-opioid receptor compared to DPDPE, indicating that the structural modifications on the parent compound significantly reduced its δ-opioid receptor selectivity (Table 1). This behavior closely resembles that of small cycle enkephalin analogues containing a thiourea bridge.6 In particular, cyclic peptide 7a had the best Ki value for DOR and MOR (Table 1).
Table 1. Affinity Values (logIC50 ± S.E.M. and Ki) and μ/δ Selectivity Ratios of 7a–c Compounds and DPDPE in [3H]IleDelt II and [3H]DAMGO Competition Binding Assays in Rat Brain Membrane Homogenatesa.
| (Ki) |
|||
|---|---|---|---|
| compds | [3H]IleDelt II (δ) (nM) | [3H]DAMGO (μ) (nM) | ratio μ/δ |
| DPDPE | 4.5 | 438.1 | 97.3 |
| 7a | 16.9 | 115 | 6.7 |
| 7b | 34.9 | 292.2 | 8.3 |
| 7c | 546 | 249 | 0.5 |
The logIC50 and Ki values were calculated based on the competition binding curves seen in Figure S4.
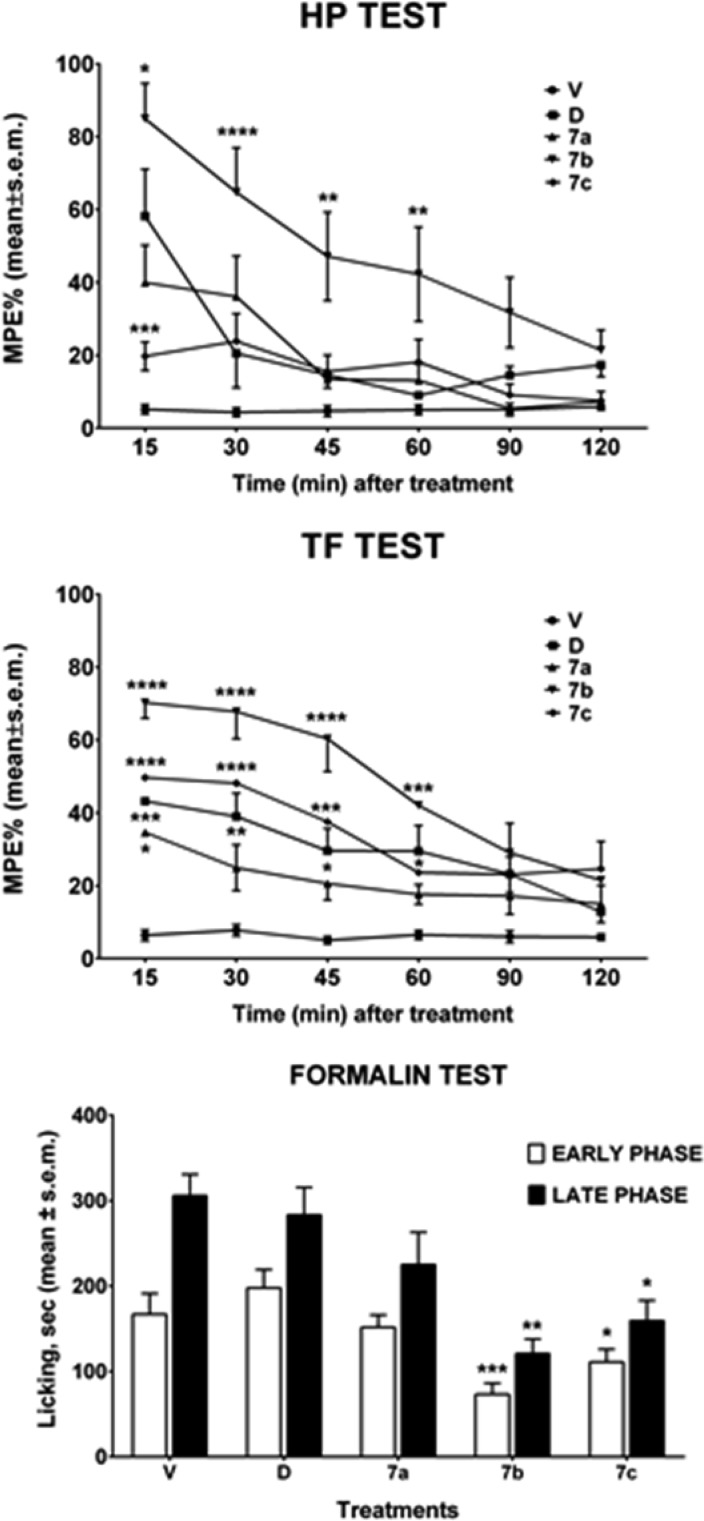
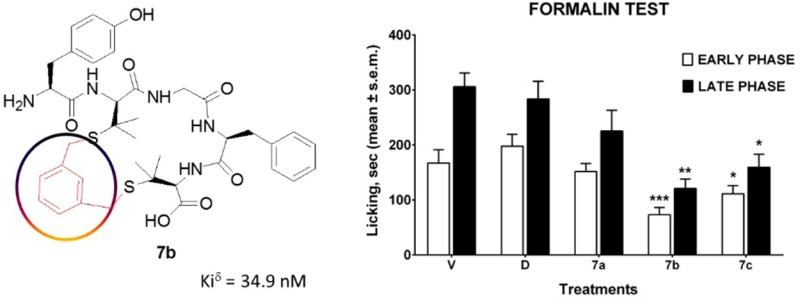
Antinociception assays have been also carried out to investigate the in vivo potential activity of the newly designed compounds.24,25 From the tail flick (TF) and hot plate (HP) tests we observed that compound 7b exerted a potent analgesic effect over 60% MPE, ranging from 15 to 60 min, after i.c.v. administration; this result has been also confirmed by formalin test (Figure 2). The compounds 7a–c and DPDPE were docked to the δ-opioid receptor (PDB: 4RWD) to explore the impact of xylene bridge incorporation on the biological activity profile. This has been carried out by using Glide tool embedded in maestro 9.2 and employing the generated grid around 10 Å from the crystallographic ligand H-Dmt-Tic-Phe-Phe-NH2 (DIPP-NH2).26
Figure 2.
Tail flick, hot plate and formalin test assays on cyclic peptides 7a–c. V is for vehicle; D is for DPDPE. In the HP and TF test, drugs were injected i.c.v. at the dose of 23 nmol/mouse. In the formalin test, drugs were administered s.c. at the dose of 150 nmol/mouse, 15 min before formalin. ****P < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05 vs D. N = 8–10.
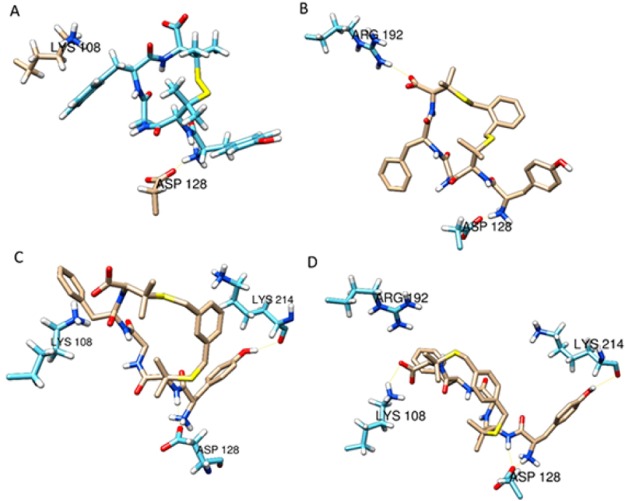
The best docking pose for each ligand was selected based on XP scoring function. The interaction between residue Asp128 and the amino terminus of each ligand is the most important and was preserved in all five models; the orientation of Tyr amino acid is overall very similar (Figure 3).
Figure 3.
Best docking poses of DPDPE (A), 7a (B), 7b (C), and 7c (D) at the DOR (only the involved residues are depicted).
The interaction with residue Arg192 has been also maintained in 7a and 7c, but 7b gained the best docking score; its docking pose is stabilized by hydrogen bonds with residues Asp128, Lys214 and a saline bridge with Lys108 (Figure S5). It is worth noting that the xylene bridge enclosed in 7a–c is topically very similar to the Tic moiety present in DIPP-NH2.26
However, an important difference is the orientation of the fourth aromatic ring, which may discriminate the agonist vs antagonist activity of the DPDPE cyclic analogues vs the crystallographic ligand DIPP-NH2 (Figure S6).26,27
Simple variation of xylene bridge regioisomer in a small cycle greatly impacts the biological activity while maintaining the same peptide sequence. Considering that xylene substitution could influence the bridge orientation rendering the cycle less flexible, and leading to a less favorable conformation, compound 7b incorporating m-xylene bridge represents an exquisite example of well-balanced equilibrium between δ-opioid receptor affinity and analgesic potency. Compound 7b was tested in vivo displaying antinociceptive activity in TF, HP, and formalin test, after i.c.v. and subcutaneous injections. Our data validate the hypothesis that the design of novel chemical entities specifically targeted at known receptors will provide vantages for pain-relief in many pathological situations. Local peripheral administration of opioid endeavored antinociceptive effects in chronic inflammatory conditions,28,29 although this effect was reached at relatively high doses. A previous report showed that DPDPE induced antinociceptive effects after subcutaneous administration in the formalin test, but in the second phase only.30
Our data demonstrate that the novel compounds induced robust and long-lasting antinociceptive effects both after central and local peripheral administration.
Experimental Procedures
Boc-protected amino acids, EDC·HCl, HOBt anhydrous, DIPEA, solvents, and other reagents were purchased from Sigma-Aldrich (Milano) and Iris-Biotech (Germany) and were used without further purification. Solvents for RP-HPLC and ESI-MS were of HPLC grade. All the reactions were conducted under nitrogen atmosphere. Final products 7a–c were purified by RP-HPLC using a Waters XBridge Prep BEH130 C18, 5.0 μm, 250 mm × 10 mm column at a flow rate of 4 mL/min on a Waters Binary pump 1525, using as eluent a linear gradient of H2O/acetonitrile 0.1% TFA ranging from 5% acetonitrile to 90% acetonitrile in 45 min. The purity of the Nα-Boc-protected products was confirmed by NMR analysis on a Varian Inova 300 MHz and mass spectrometry ESI-LRMS. The purity of all final TFA salts was confirmed by NMR analysis, ESI-LRMS, and analytical RP-HPLC (C18-bonded 4.6 mm × 150 mm) at a flow rate of 1 mL/min, using as eluent a gradient of H2O/acetonitrile 0.1% TFA ranging from 5% acetonitrile to 95% acetonitrile in 50 min and was found to be ≥95%. 1H NMR spectra were performed in DMSO-d6 solution on a Varian Inova operating at the 1H frequency of 300 MHz. Chemical shifts were referred to the residual proton signal of DMSO at 2.5 ppm. Peptide structures were also confirmed by UPLC-MS (see SI).
Glossary
ABBREVIATIONS
- Boc
tert-butyloxycarbonyl
- [3H]DAMGO
[3H]-[DAla(2),N-Me-Phe-(4),Gly-ol(5)]enkephalin
- [3H]-U69593
[3H]-(+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-l]benzeneacetamide
- DCM
dichloromethane
- DIPEA
diisopropylethylamine
- DPDPE
[2-d-penicillamine,5-d-penicillamine]enkephalin
- [3H]IleDelt II
ile5,6 deltorphin II
- DMF
N,N-dimethylformamide
- DMSO
dimethyl sulfoxide
- DOR
δ opioid receptor
- EDC
1-ethyl-(3-(dimethylamino)propyl)carbodiimide
- HOBt
1-hydroxybenzotriazole
- MOR
μ opioid receptor
- DIPEA
N,N-diisopropylethylamine
- RP-HPLC
reversed phase high performance liquid chromatography
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- TMS
tetramethylsilane
- MS-UPLC
ultraperformance liquid-chromatography tandem mass spectrometry
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00044.
Details of compounds characterization, synthetic procedures, molecular modeling, and biological assays (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This study was supported in part by the National Research Development and Innovation Office (NKFIH, grant number: OTKA 108518).
The authors declare no competing financial interest.
Supplementary Material
References
- Evans C. J.; Keith D. E.; Morrison H.; Magendzo K.; Edwards R. H. Cloning of a delta opioid receptor by functional expression. Science 1992, 258, 1952–1955. 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- Pasternak G. Opioids and their receptors: are we there yet?. Neuropharmacology 2014, 76, 198–203. 10.1016/j.neuropharm.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar R. J. Endogenous opiates and behavior: 2012. Peptides 2013, 50, 55–95. 10.1016/j.peptides.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Crow J. M. Move over, morphine. Nature 2016, 535, S4–S6. 10.1038/535S4a. [DOI] [PubMed] [Google Scholar]
- Pencheva N.; Milanovc P.; Vezenkove L.; Pajpanovaf T.; Naydenova E. Opioid profiles of Cys2-containing enkephalin analogues. Eur. J. Pharmacol. 2004, 498, 249–256. 10.1016/j.ejphar.2004.07.059. [DOI] [PubMed] [Google Scholar]
- Touati-Jallabe Y.; Bojnik E.; Legrand B.; Mauchauffée E.; Chung N. N.; Schiller P. W.; Benyhe S.; Averlant-Petit M. C.; Martinez J.; Hernandez J. F. Cyclic Enkephalins with a Diversely Substituted Guanidine Bridge or a Thiourea Bridge: Synthesis, Biological and Structural Evaluations. J. Med. Chem. 2013, 56, 5964–5973. 10.1021/jm4008592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica A.; Stefanucci A.; Costante R.; Hruby V. J.. Rational Approach to the Design of Bioactive Peptidomimetics: Recent Developments in Opioid Agonist Peptides. In Studies in Natural Products Chemistry, Vol. 46; Elsevier B.V., 2016; pp 27–68. [Google Scholar]
- Kopra K.; Martikkala E.; Hänninen P.; Petäjä-Repo U.; Härmä H. A homogeneous single-label quenching resonance energy transfer assay for a δ-opioid receptor-ligand using intact cells. Analyst 2013, 138, 4907–4914. 10.1039/c3an00736g. [DOI] [PubMed] [Google Scholar]
- Flippen-Anderson J. L.; Hruby V. J.; Collins N.; George C.; Cudney B. X-ray structure of [D-Pen2-D-Pen5]enkephalin a highly potent, delta opioid receptor selective compound: comparisons with proposed solution conformations. J. Am. Chem. Soc. 1994, 116, 7523–7531. 10.1021/ja00096a008. [DOI] [Google Scholar]
- Scarabelli G.; Provasi D.; Negri A.; Filizola M. Bioactive Conformations of Two Seminal Delta Opioid Receptor Penta-peptides Inferred from Free-Energy Profiles. Biopolymers 2014, 101, 21–27. 10.1002/bip.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk R.; Harris P. W. R.; Brimble M. A.; Callon K. E.; Watson M.; Cornish J. Synthesis and evaluation of disulfide bond mimetics of amylin-(1–8) as agents to treat osteoporosis. Bioorg. Med. Chem. 2012, 20, 2661–2668. 10.1016/j.bmc.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Mollica A.; Carotenuto A.; Novellino E.; Limatola A.; Costante R.; Pinnen F.; Stefanucci A.; Pieretti S.; Borsodi A.; Samavati R.; Zador F.; Benyhe S.; Davis P.; Porreca F.; Hruby V. J. Novel Cyclic Biphalin Analogue with Improved Antinociceptive Properties. ACS Med. Chem. Lett. 2014, 5, 1032–1036. 10.1021/ml500241n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby V. J.; Porreca F.; Yamamura H. I.; Tollin G.; Agnes R. S.; Lee Y. S.; Cai M.; Alves I.; Cowell S.; Varga E.; Davis P.; Salamon Z.; Roeske W.; Vanderah T.; Lai J. New paradigms and tools in drug design for pain and addiction. AAPS J. 2006, 8, E450–E460. 10.1208/aapsj080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica A.; Guardiani G.; Davis P.; Ma S.-W.; Porreca F.; Lai J.; Mannina L.; Sobolev A. P.; Hruby V. J. Synthesis of Stable and Potent μ/δ Opioid Peptides: Analogues of H-Tyr-c[D-Cys-Gly-Phe-D-Cys]-OH by Ring-Closing Metathesis. J. Med. Chem. 2007, 50, 3138–3142. 10.1021/jm061048b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeenk L. E. J.; Dailly N.; Hiemstra H.; van Maarseveen J. H.; Timmerman P. Synthesis of water-soluble scaffolds for peptide cyclization, labeling and ligation. Org. Lett. 2012, 14, 1194–1197. 10.1021/ol203259a. [DOI] [PubMed] [Google Scholar]
- Smith M. E. B.; Schumacher F. F.; Ryan C. P.; Tedaldi L. M.; Papaioannou D.; Waksman G.; Caddick S.; Baker J. R. Protein modification, bioconjugation, and disulfide bridging using bromomaleimides. J. Am. Chem. Soc. 2010, 132, 1960–1965. 10.1021/ja908610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica A.; Pinnen F.; Feliciani F.; Stefanucci A.; Lucente G.; Davis P.; Porreca F.; Ma S.-W.; Lai J.; Hruby V. J. New potent biphalin analogues containing p-fluoro-L-phenylalanine at the 4,4′ positions and non-hydrazine linkers. Amino Acids 2011, 40, 1503–1511. 10.1007/s00726-010-0760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yar M.; McGarrigle E. M.; Aggarwal V. K. An annulation reaction for the synthesis of morpholines, thiomorpholines, and piperazines from beta-heteroatom amino compounds and vinyl sulfonium salts. Angew. Chem., Int. Ed. 2008, 47, 3784–3786. 10.1002/anie.200800373. [DOI] [PubMed] [Google Scholar]
- Benito J. M.; Meldal M. Bicyclic organo-peptides as selective carbohydrate receptors: Design, solid-phase synthesis, and on-bead binding capability. QSAR Comb. Sci. 2004, 23, 117–129. 10.1002/qsar.200320011. [DOI] [Google Scholar]
- Timmerman P.; Beld J.; Puijk W. C.; Meloen R. H. Rapid and quantitative cyclization of multiple peptide loops onto synthetic scaffolds for structural mimicry of protein surfaces. ChemBioChem 2005, 6, 821–824. 10.1002/cbic.200400374. [DOI] [PubMed] [Google Scholar]
- Johnson M.; Liu M.; Struble E.; Hettiarachchi K. Characterization of cyclic peptides containing disulfide bonds. J. Pharm. Biomed. Anal. 2015, 109, 112–120. 10.1016/j.jpba.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyhe S.; Farkas J.; Tóth G.; Wollemann M. Met5-enkephalin-Arg6-Phe7, an endogenous neuropeptide, binds to multiple opioid and nonopioid sites in rat brain. J. Neurosci. Res. 1997, 48, 249–258. . [DOI] [PubMed] [Google Scholar]
- Yung-Chi Y.-C.; Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Colucci M.; Maione F.; Bonito M. C.; Piscopo A.; Di Giannuario A.; Pieretti S. New insights of dimethyl sulfoxide effects (DMSO) on experimental in vivo models of nociception and inflammation. Pharmacol. Res. 2008, 57, 419–425. 10.1016/j.phrs.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Porreca F.; Mosberg H. I.; Omnaas J. R.; Burks T. F.; Cowan A. Supraspinal and spinal potency of selective opioid agonists in the mouse writhing test. J. Pharmacol. Exp. Ther. 1987, 240, 890–894. [PubMed] [Google Scholar]
- Fenalti G.; Zatsepin N. A.; Betti C.; Giguere P.; Han G. W.; Ishchenko A.; Liu W.; Guillemyn K.; Zhang H.; James D.; Wang D.; Weierstall U.; Spence J. C.; Boutet S.; Messerschmidt M.; Williams G. J.; Gati C.; Yefanov O. M.; White T. A.; Oberthuer D.; Metz M.; Yoon C. H.; Barty A.; Chapman H. N.; Basu S.; Coe J.; Conrad C. E.; Fromme R.; Fromme P.; Tourwé D.; Schiller P. W.; Roth B. L.; Ballet S.; Katritch V.; Stevens R. C.; Cherezov V. Structural basis for bifunctional peptide recognition at human δ-opioid receptor. Nat. Struct. Mol. Biol. 2015, 22, 265–268. 10.1038/nsmb.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioja E.; Tourwè D.; Kertèsz I.; Tòth G.; Borsodi A.; Benyhe S. Novel diastereomeric opioid tetrapeptides exhibit differing pharmacological activity profiles. Brain Res. Bull. 2007, 74, 119–129. 10.1016/j.brainresbull.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Carcolé M.; Castany S.; Leánez S.; Pol O. Treatment with a heme oxygenase 1 inducer enhances the antinociceptive effects of μ-opioid, δ-opioid, and cannabinoid 2 receptors during inflammatory pain. J. Pharmacol. Exp. Ther. 2014, 351, 224–232. 10.1124/jpet.114.215681. [DOI] [PubMed] [Google Scholar]
- Hervera A.; Leánez S.; Negrete R.; Pol O. The peripheral administration of a nitric oxide donor potentiates the local antinociceptive effects of a DOR agonist during chronic inflammatory pain in mice. Naunyn-Schmiedeberg's Arch. Pharmacol. 2009, 380, 345–352. 10.1007/s00210-009-0436-6. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N.; Yamamoto T. Involvement of nitric oxide in peripheral antinociception mediated by μ and δ-opioid receptors. Anesth. Analg. 1998, 87, 388–393. 10.1097/00000539-199808000-00028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.