Abstract
Two series of novel LOXL2 enzyme inhibitors are described: benzylamines substituted with electron withdrawing groups at the para-position and 2-substituted pyridine-4-ylmethanamines. The most potent compound, (2-chloropyridin-4-yl)methanamine 20 (hLOXL2 IC50 = 126 nM), was shown to be selective for LOXL2 over LOX and three other amine oxidases (MAO-A, MAO-B, and SSAO). Compound 20 is the first published small molecule inhibitor selective for LOXL2 over LOX.
Keywords: LOXL2, lysyl oxidase-like 2, fibrosis, (2-chloropyridin-4-yl)methanamine, inhibitor
Lysyl oxidases (LOXs) are a family of five copper-containing amine oxidases that oxidize the terminal ε-amine of specific lysine or hydroxylysine residues present in the telopeptide regions of collagen (or elastin) to generate the corresponding allysine.1,2 These aldehydes can then condense with other lysines and hydroxylysines to form a cross-linked collagen matrix.3 Pathological tissue fibrosis results from excessive collagen deposition as well as increased cross-linking leading to pro-fibrotic signaling changes within resident cells in the ECM network. The accumulation of extracellular matrix within a disease tissue leads to scarring and destruction of the normal tissue architecture and ultimately to organ failure.4 Treatments for fibrosis are limited; however, two drugs (pirfenidone and nintedanib) were recently approved for IPF though they offer only modest benefits.5 One approach to treating fibrosis is to block the cross-linking of collagen fibrils through inhibition of a LOX-family enzyme.6 The LOX family consists of five isozymes, LOX and LOXL1–4, all of which are secreted enzymes that are proposed to act on collagen molecules within the ECM. They all contain a Cu2+ ion coordinated to three conserved histidines and a lysyl-tyrosine quinone (LTQ) cofactor in the catalytic domain; however, they can be differentiated into two subgroups based on the structure of their N-termini. LOX and LOXL1 contain a basic propeptide at their N-termini which requires cleavage in order to generate a catalytically active enzyme. Mice deficient in either LOX or LOXL1 show cardiovascular and other organ defects.7,8 LOXL2, 3, and 4 all possess four scavenger receptor cysteine-rich (SRCR) domains in their N-termini which are hypothesized to mediate cell-adhesion and protein–protein interactions. LOX and LOXL2 have been well studied and shown to be upregulated in patients with fibrosis and cancer.9−12 In addition to its activity on the ECM, LOX and LOXL2 have proposed intracellular roles involving regulation of chromatin structure and gene transcription.13−15 An anti-LOXL2 antibody (Simtuzumab) that binds to the fourth SRCR domain but does not fully inhibit the catalytic activity recently failed in a number of clinical trials in lung and liver disease.9,16 However, the negative clinical data does not reflect a robust test of the efficacy of a molecule capable of fully inhibiting LOXL2 enzymatic activity. Thus, it was envisioned that a small molecule capable of fully inhibiting the catalytic activity of extracellular, and potentially intracellular, LOXL2 while maintaining selectivity over LOX would be desirable.
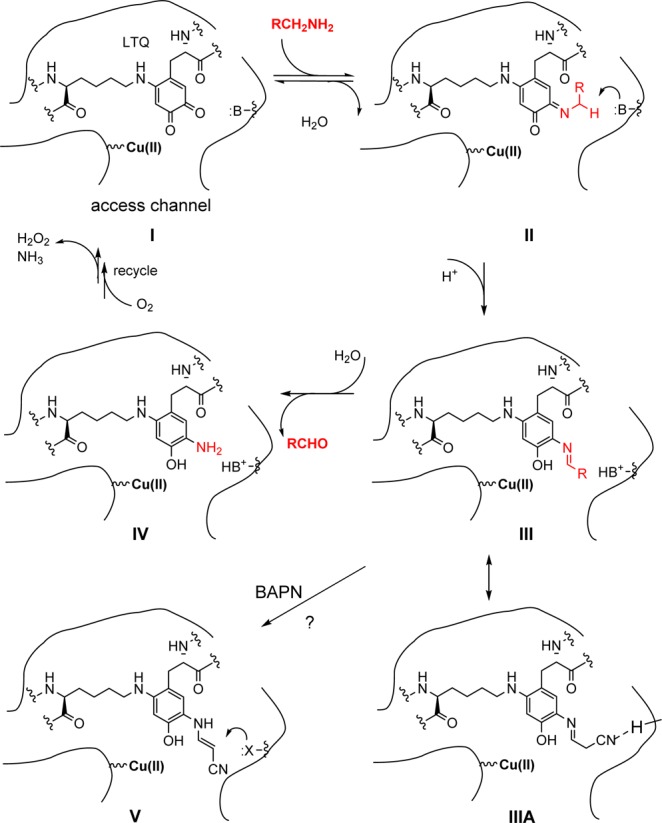
To date, no selective LOXL2 inhibitor structure has been published and no NMR or X-ray structures for any of the LOX(L) enzymes are known. Therefore, the design of our inhibitors started with an examination of the enzyme mechanism and knowledge of the prototypical pan-LOX(L) inhibitor, β-aminopropionitrile (BAPN; 1), a mechanism-based, irreversible inhibitor.17 A simplified proposed mechanism for the oxidation of primary amines by LOXL2 is shown in Scheme 1 and is based on the previously postulated mechanism for LOX/LOXL2.6 Briefly, in the oxidation of lysine, the ε-amino group is first reversibly reacted with the LTQ of I to afford the intermediate substrate Schiff base II. A nearby basic residue is postulated to catalyze the irreversible rearrangement of II by proton abstraction, leading to a reduction of the quinoid ring to form the product Schiff base, phenol III. Hydrolysis of III yields the aldehyde (allysine) and intermediate IV, which is then recycled via a number of steps involving O2 and Cu to I with the release of H2O2 and NH3.18 The role of Cu(II) in the catalytic cycle has been studied for LOX and is controversial.6 In the case of LOX, when BAPN binds, the intermediate III has been proposed to isomerize to a more stable product V, potentially capable of forming a copper chelated species, or a nearby nucleophilic residue forms a covalent adduct to irreversibly inactivate the enzyme.19 Another simpler hypothesis to explain the inhibition is that the nitrile from BAPN in intermediate III makes an interaction with the LOX(L) protein which stabilizes a conformation (e.g., IIIA) resistant to hydrolysis. Such interactions involving a nitrile group forming a hydrogen bond with an enzyme to stabilize an enzyme inhibitor complex are well precedented.20 A two-pronged approach was used to identify lead inhibitors: (i) a focused rational screen evaluating known inhibitors of other AOs (in particular LOX) and (ii) modifications of weak substrates in order to identify derivatives that could bind to the LTQ-quinone and generate the postulated stable enzyme–inhibitor complex (c.f. IIIA). It was hypothesized that the differences in the active site between LOX and LOXL2 (the latter has an N-glycosylated Asn644 in the catalytic domain) would then allow for the design of selective inhibitors.6
Scheme 1. Proposed Mechanism for the Oxidation of Primary Amines by LOXL2 and the Inhibition by BAPN.
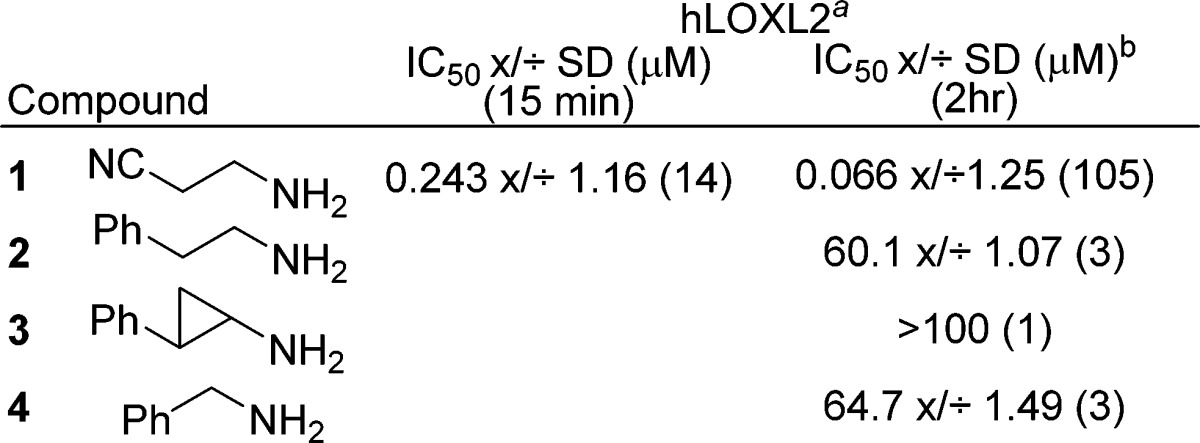
All compounds were obtained from commercial vendors, and the assay methods employed are described in the Supporting Information. The human LOXL2 activity assay was carried out using an Amplex Red assay to measure the inhibition of H2O2 produced following the oxidative deamination of 1,5-diaminopentane (DAP).21 Serum-free concentrated conditioned media (CCM) from CHO cells stably expressing human LOXL2 was used as the source of enzyme (Supporting Information Figure 1). BAPN 1 (Table 1) shows time-dependent inhibition in this assay with the IC50 shifting from 243 nM to 66 nM in going from a 15 min to a 2 h preincubation (Supporting Information Figure 2). There was no significant change in potency with a 4 h pretreatment; therefore, a 2 h preincubation time was used for compound screening. Phenethylamine 2 (wherein the nitrile of BAPN is replaced by phenyl) was tested and was shown to be a weak inhibitor of LOXL2 (IC50 = 60 μM), in contrast to its effect on LOX, where it is a substrate.22 2-Phenyl cyclopropylamine 3, a weak inhibitor and substrate of LOX, as well as a potent mechanism-based inhibitor of the FAD-containing AOs LSD-1 and MAO-A and B, did not show any inhibition of LOXL2 activity up to 100 μM.23,24 Benzylamine 4 (a weak substrate of LOX and a weak inhibitor of the oxidation of elastin by LOX) also showed weak inhibition of LOXL2 with an IC50 of ∼65 μM. Therefore, 4 was used as a starting point for structure–activity relationship (SAR) studies.25 The effect of substituting the phenyl ring of 4 was then evaluated (Table 2). Electron-donating groups in the para position 4-OMe (5) and 4-NH2 (6) had little effect. In contrast, electron-withdrawing substituents had a beneficial effect on potency. The 4-fluoro derivative 7 (26 μM) was about 2-fold more potent than 4, while the 4-nitro 8 and 4-cyano 9 derivatives were significantly more potent with IC50’s of ∼700 nM. The 4-CO2Me analog 10 showed reasonable potency with an IC50 of ∼3.6 μM, and the regioisomer 3-CO2Me 11 was equally potent. For the amidine substituents, para substitution 12 (4-C(NH)NH2; IC50 ∼ 1.1 μM) was more potent when compared to meta substitution 13 (3-C(NH)NH2; IC50 = ∼ 5 μM). In the results obtained by Williamson and Kagan for the inhibition of bovine aorta LOX, electron-withdrawing substituents on benzylamine decreased the substrate potential while increasing the ability to inhibit the enzyme.25 Further, they postulate that the developing anion generated in the proton abstraction step (Scheme 1, II to III) is stabilized and the enzyme–substrate (ES) complex III is lower in energy in the presence of a para-electron-withdrawing group, which would raise the activation energy in the hydrolysis step (III to IV).
Table 1. Inhibition of Human LOXL2 Activity Following Either 15 min or 2 h Incubation with Amines 1–4.
Amplex Red assay. Numbers in parentheses are N-values.
IC50 values shown as geometric mean x/÷ geometric standard deviation
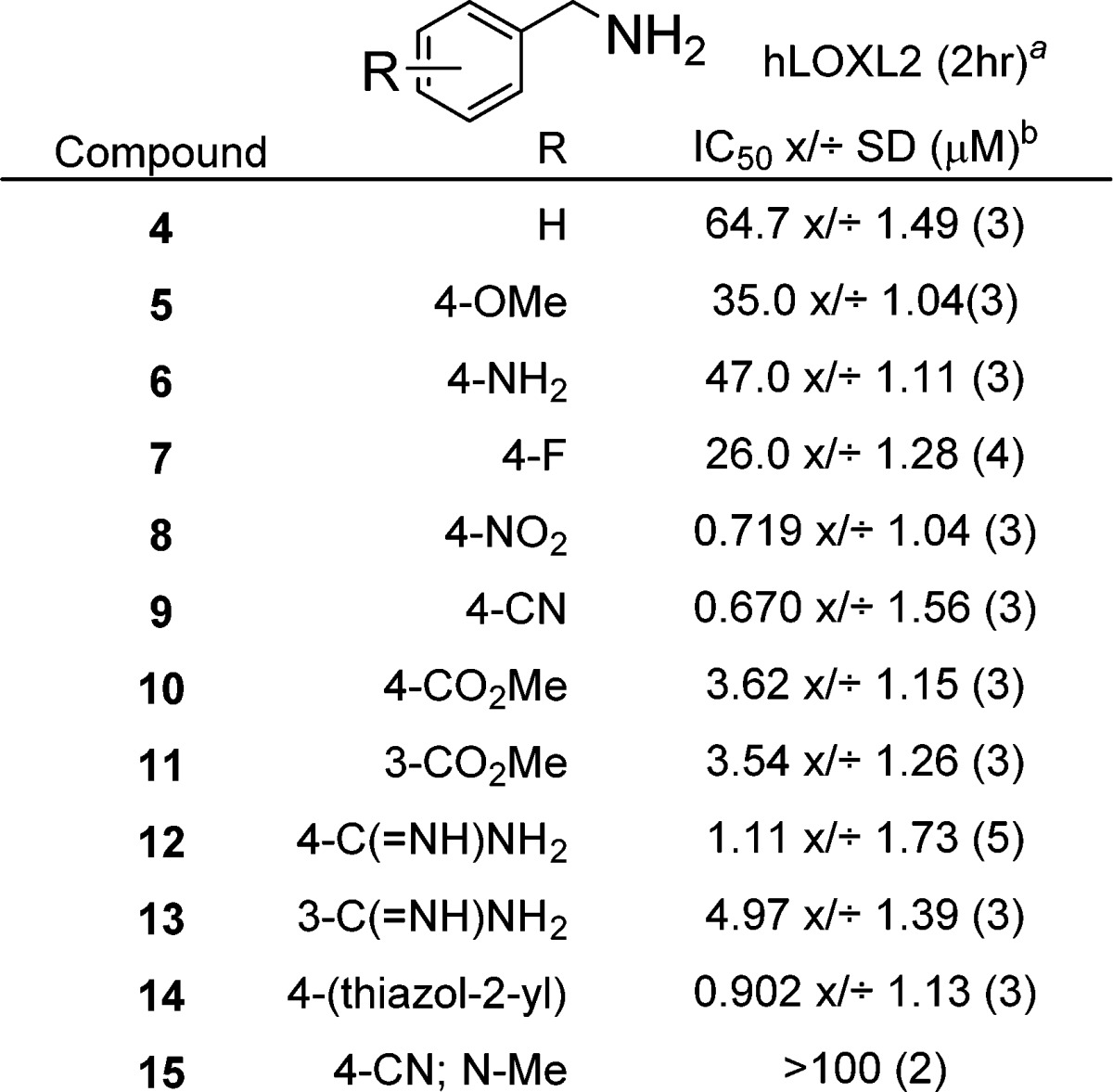
Table 2. SAR of Benzylamines.
Amplex Red assay. Numbers in parentheses are N-values.
IC50 values shown as geometric mean x/÷ geometric standard deviation
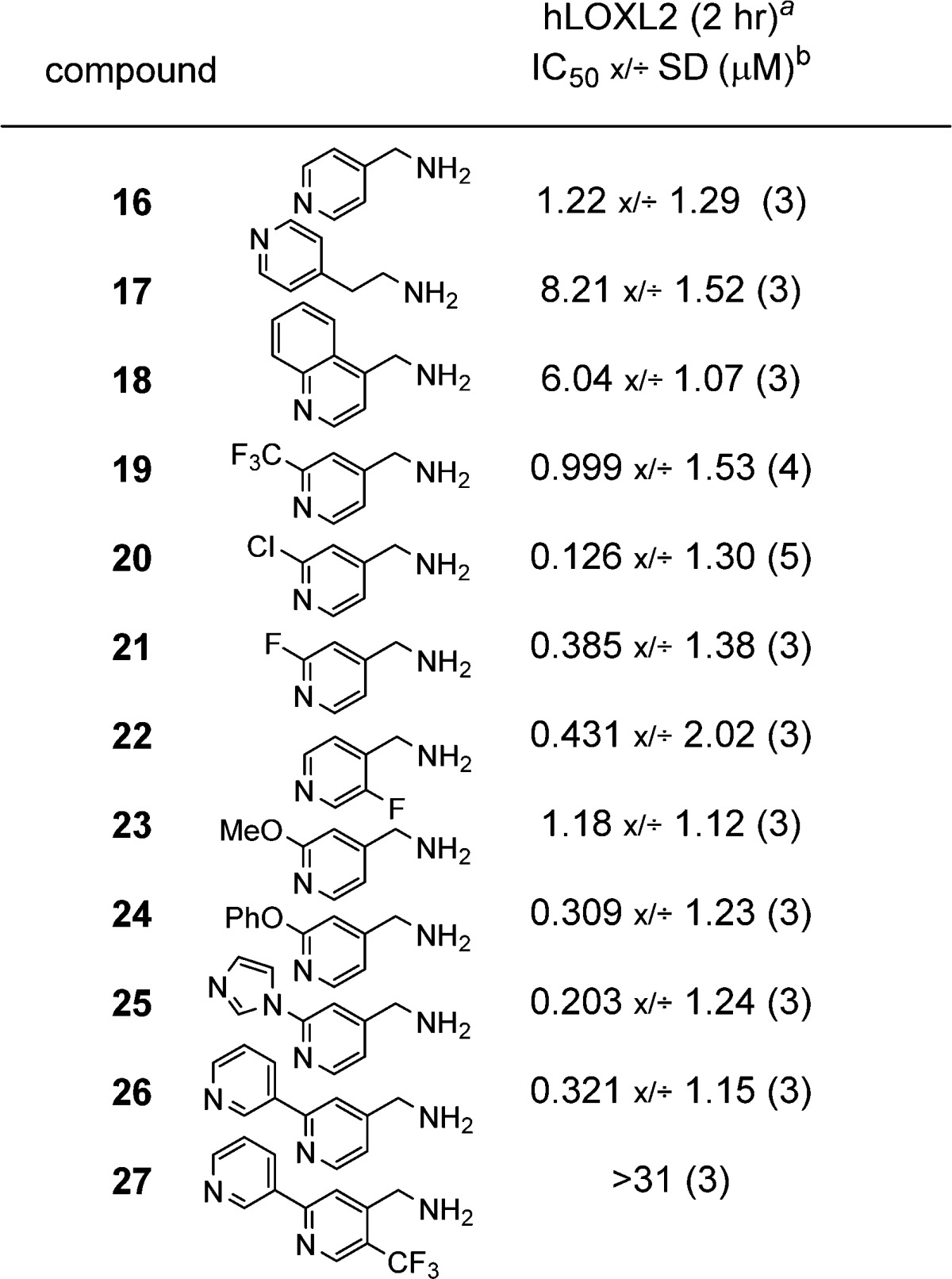
More elaborate para-substituents were explored, and these also show a range of potencies, with the 4-(thiazol-2-yl) derivative 14 being one of the more potent compounds at ∼0.9 μM. To confirm that a primary amine is required for activity, compound 15 was screened (the N-Me analog of 9), and this showed no inhibition up to 100 μM. While some of the substituted benzylamines described in Table 2 showed interesting activity, these compounds also inhibit LOX.25 The lack of selectivity prompted us to evaluate related heterocyclic methylamines as potential selective LOXL2 inhibitors. Pyridine-based inhibitors of copper-containing AO’s have been described and were screened against porcine aorta LOX. Only two of four compounds showed inhibition at high concentrations (0.1 mM), and inhibition of LOXL2 was not reported.26 In our hands, the pyridin-4-yl methanamine 16, when tested against LOXL2, was found to be a much more effective LOXL2 inhibitor than benzylamine 4 (Table 3; 1.22 μM vs 64.7 μM). Based on this, we decided to probe the SAR of related substituted pyridines. Extension of the amine chain to the ethylamine analog 17 led to a reduction in potency indicating that the methylamine is preferred. The quinoline analog 18 was less active than 16, perhaps due to the added steric bulk ortho to the methylamine group. The addition of electron withdrawing groups in the 2-position of the pyridine ring had a positive effect on the inhibitory potency. Such substitution significantly reduces the basicity of the pyridine nitrogen. Thus, the 2-CF3 (19), 2-Cl (20), and 2-F (21) derivatives all showed good inhibition with the 2-Cl analog 20 being the most potent at 126 nM. Moving the fluorine from the 2- to the 3-position (21 to 22) did not have a major effect, since the 3-F analog was only slightly less active. The electron donating 2-methoxy substituent of 23 did not alter the potency compared to the unsubstituted pyridine 16. However, the 2-phenoxy derivative 24 was 4-fold more potent with an IC50 of 309 nM. Presumably the phenyl group is picking up an additional interaction with the enzyme. Compounds containing heteroaryls, e.g. the 2-(imidazole-1-yl) analog 25 and 2-(pyridin-3-yl) analog 26, were also found to be potent inhibitors, with IC50 values of 203 nM and 321 nM, respectively. Bulky substituents ortho to the methylamine are not tolerated (compare 26 and 27), although the small fluorine atom in the ortho position is beneficial (16 versus 22). In summary, pyridine-based inhibitors are generally more potent and selective than the benzylamines.
Table 3. SAR of Pyridine Methanamines.
Amplex Red assay. Numbers in parentheses are N-values.
IC50 values shown as geometric mean x/÷ geometric standard deviation
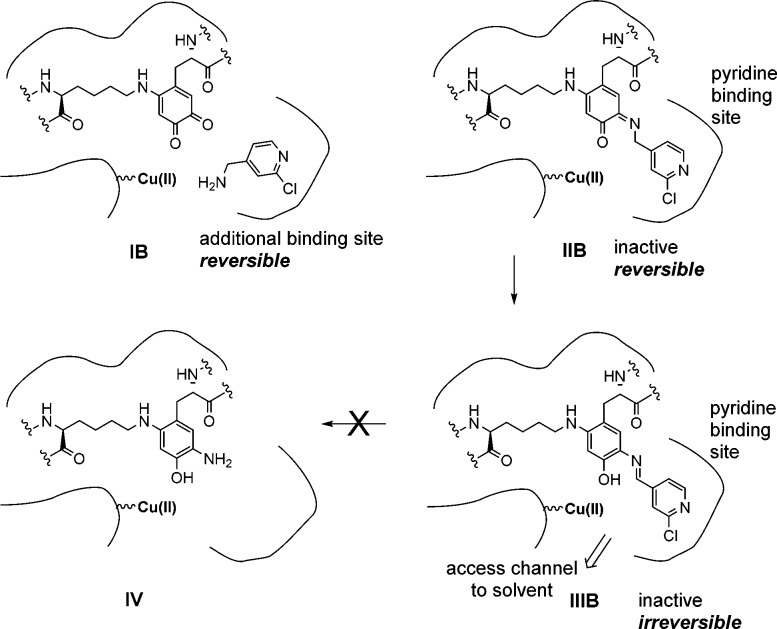
Unlike BAPN (an irreversible inhibitor), compound 20 was found to be largely a reversible inhibitor (using a washout method; see Supporting Information Figure 3); however, ∼35% of the inhibition by 20 was irreversible at 2 h, and this was maintained out to 24 h. This implies that there are at least two different populations of bound inhibitor–LOXL2 complexes: a reversible population and an irreversible population. Additional analogs of 20 containing bulky substituents were shown to be mostly irreversible inhibitors (data not shown). These inhibitors would not be able to form additional covalent bonds with the enzyme as proposed in V (Scheme 1), so this hypothesis seems unlikely. In an attempt to rationalize these data, we propose that the inhibitors lock down the enzyme into two populations (Scheme 2). The reversible population could be intermediate IIB (substrate Schiff base), or it is possible that the inhibitor is initially bound noncovalently to LOXL2 (intermediate IB) either prior to the formation of the Schiff base or it is bound to a secondary, reversible binding site. Such secondary binding sites are known for other AOs.27−29 The irreversible population is potentially IIIB; the product Schiff base may occupy a pyridine binding site that confers resistance to hydrolysis. For our inhibitors, the pyridine nitrogen is potentially involved in stabilizing the enzyme–inhibitor complex and the pyridine ring may be involved in a π-stacking interaction. Presumably, the active site of LOX is different and lacks the pyridine binding site, hence the selectivity for LOXL2 that is observed. Large substituents are tolerated and, since substrates for LOXL2 contain lysines residues in protein molecules, it is reasonable to assume that the active site is large enough to allow access.30 This is the case for the topaquinone (TPQ)-containing enzyme, Pichia pastoris lysyl oxidase, which has a broad funnel leading to the active site.31
Scheme 2.
In AOs containing the topaquinone (TPQ) cofactor, the TPQ can readily rotate in the active site, resulting in two enzyme conformations, both observed by X-ray analysis.32 The “on-copper” (inactive) conformation has the quinone oxygen acting as a copper ligand, whereas the “off-copper” orientation is not copper ligated and is catalytically competent. For LOX(L) enzymes, the LTQ cofactor is further tethered through the lysine adduct and it is not free to rotate, leading to a more restricted catalytic site.
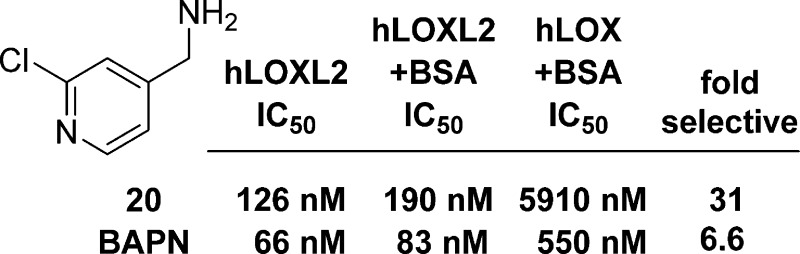
The most potent LOXL2 inhibitor, (2-chloropyridin-4-yl) methanamine 20, was selected for further evaluation and comparison to BAPN (Table 4.) In the human whole blood LOXL2 assay (using the Amplex Red assay to measure the oxidative deamination of DAP by human recombinant LOXL2 spiked into human whole blood), the amine 20 had an IC50 of 1.45 μM compared to 126 nM in the absence of blood proteins. BAPN was 2–4-fold more potent than 20 in both assays (396 nM and 66 nM in the blood and CCM assays, respectively), and thus, both compounds are modestly shifted by ∼6 to 10-fold in the presence of human blood proteins. To assess the selectivity versus the related LTQ-containing human LOX, a modified assay system was used, as LOX required the addition of BSA to the serum-free media from HEK cells stably expressing hLOX in order to obtain measurable activity. The corresponding plus BSA assay was also run for LOXL2 to generate an accurate head-to-head comparison. The results show a 31-fold selectivity of amine 20 for LOXL2 + BSA (IC50 = 190 nM) over LOX + BSA (IC50 = 5.91 μM) whereas BAPN is relatively nonselective (6-fold difference). This selectivity shown by 20 is a significant advantage over BAPN. We also measured the potency of 20 against LOXL3 and found it to have an IC50 of 214 nM, almost equipotent to LOXL2 (data not shown). Attempts to generate stable cell lines for LOXL1 and 4 were unsuccessful, so the potencies against these two enzymes were not determined. The potencies of 20 and BAPN were evaluated against rat and mouse LOXL2 using identical assay conditions (CCM + BSA) and were found to be similar (range 127–227 nM.) Against a panel of non-LTQ-containing AO enzymes (MAO-A, MAO-B, and SSAO), amine 20 was found to be inactive at 30 μM. Lastly, amine 20 was profiled for the inhibition of three different CYP enzymes (CYPs 3A4, 2C9, and 2D6), and in each case the IC50 was >30 μM.33 Thus, amine 20 is a selective LOXL2 inhibitor and may be suitable for in vitro testing.34
Table 4. Inhibition of Selected LOX(L) Enzymes.
| IC50 x/÷ SD (μM)g |
||
|---|---|---|
| 20 | BAPN, 1 | |
| hLOXL2a | 0.126 x/÷ 1.30 (5) | 0.066 x/÷ 1.25 (104) |
| hLOXL2 bloodb | 1.45 x/÷ 1.57 (4) | 0.396 x/÷ 1.38 (43) |
| hLOXL2 + BSAc | 0.19 x/÷ 1.40 (3) | 0.083 x/÷ 1.53 (40) |
| hLOX + BSAd | 5.91 x/÷ 1.84 (3) | 0.550 x/÷ 1.36 (36) |
| mLOXL2 + BSAe | 0.227 x/÷ 1.46 (3) | 0.128 x/÷ 1.29 (3) |
| rLOXL2 + BSAf | 0.136 x/÷ 1.13(3) | 0.127 x/÷ 1.15(3) |
Amplex Red assay.
hLOXL2.
hLOXL2 spiked into human blood.
hLOXL2 with BSA.
hLOX with BSA.
mLOXL2.
rLOXL2.
IC50 values shown as geometric mean x/÷ geometric standard deviation.
In summary, we have identified two series of novel LOXL2 enzyme inhibitors: (a) para-substituted benzylamines and (b) 2-substituted pyridin-4-ylmethanamines. The most potent compound, (2-chloropyridin-4-yl)methanamine 20, was shown to be a mostly reversible inhibitor on LOXL2 and selective for LOXL2 over LOX and three other AOs. It has similar potency in inhibiting LOXL2 as the pan-LOX(L) inhibitor BAPN. Compound 20 is commercially available and may be a useful tool compound to probe LOXL2. We will describe the design of more potent analogs in future publications.
Glossary
Abbreviations
- AO
amine oxidase
- BAPN
β-aminopropionitrile
- ECM
extracellular matrix
- IPF
idiopathic lung fibrosis
- LOX
lysyl oxidase
- LOXL2
lysyl oxidase-like 2
- LTQ
lysyl-tyrosine quinone
- TPQ
topaquinone
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00014.
In vitro assay methods and figures (PDF)
Author Contributions
All authors contributed to the manuscript and have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): All authors are employees of PharmAkea Inc.
Supplementary Material
References
- Finney J.; Moon H. J.; Ronnebaum T.; Lantz M.; Mure M. Human copper-dependent amine oxidases. Arch. Biochem. Biophys. 2014, 546, 19–32. 10.1016/j.abb.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau-Bové X.; Ruiz-Trillo I.; Rodriguez-Pascual F. Origin and evolution of lysyl oxidases. Sci. Rep. 2015, 5, 10568. 10.1038/srep10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M.; Sricholpech M. Lysine post-translational modifications of collagen. Essays Biochem. 2012, 52, 113–133. 10.1042/bse0520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. L.; Carruthers A. M.; Mustelin T.; Murray L. A. Matrix regulation of idiopathic pulmonary fibrosis: the role of enzymes. Fibrog. Tissue Repair 2013, 6 (1), 20. 10.1186/1755-1536-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter M.; Bonella F.; Wijsenbeek M.; Maher T. M.; Spagnolo P. Pharmacological Treatment of Idiopathic Pulmonary Fibrosis: Current Approaches, Unsolved Issues, and Future Perspectives. BioMed Res. Int. 2015, 2015, 329481. 10.1155/2015/329481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. J.; Finney J.; Ronnebaum T.; Mure M. Human lysyl oxidase-like 2. Bioorg. Chem. 2014, 57, 231–241. 10.1016/j.bioorg.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Zhao Y.; Gao J.; Pawlyk B.; Starcher B.; Spencer J. A.; Yanagisawa H.; Zuo J.; Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 2004, 36 (2), 178–182. 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Pischon N.; Maki J. M.; Weisshaupt P.; Heng N.; Palamakumbura A. H.; N’Guessan P.; Ding A.; Radlanski R.; Renz H.; Bronckers T. A.; Myllyharju J.; Kielbassa A. M.; Kleber B. M.; Bernimoulin J. P.; Trackman P. C. Lysyl oxidase (lox) gene deficiency affects osteoblastic phenotype. Calcif. Tissue Int. 2009, 85 (2), 119–126. 10.1007/s00223-009-9252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry-Hamilton V.; Spangler R.; Marshall D.; McCauley S.; Rodriguez H. M.; Oyasu M.; Mikels A.; Vaysberg M.; Ghermazien H.; Wai C.; Garcia C. A.; Velayo A. C.; Jorgensen B.; Biermann D.; Tsai D.; Green J.; Zaffryar-Eilot S.; Holzer A.; Ogg S.; Thai D.; Neufeld G.; Van Vlasselaer P.; Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 2010, 16 (9), 1009–1017. 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- Xiao Q.; Ge G. Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer Microenviron. 2012, 5 (3), 261–273. 10.1007/s12307-012-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanoki M.; Ishii M.; Kobayashi H.; Fushida H.; Yashiro N.; Hamada T.; Ooshima A. Increased expression of lysyl oxidase in skin with scleroderma. Br. J. Dermatol. 1995, 133 (5), 710–715. 10.1111/j.1365-2133.1995.tb02743.x. [DOI] [PubMed] [Google Scholar]
- Murawaki Y.; Kusakabe Y.; Hirayama C. Serum lysyl oxidase activity in chronic liver disease in comparison with serum levels of prolyl hydroxylase and laminin. Hepatology 1991, 14 (6), 1167–1173. 10.1002/hep.1840140635. [DOI] [PubMed] [Google Scholar]
- Herranz N.; Dave N.; Millanes-Romero A.; Pascual-Reguant L.; Morey L.; Díaz V. M.; Lórenz-Fonfría V.; Gutierrez-Gallego R.; Jerónimo C.; Iturbide A.; Di Croce L.; García de Herreros A.; Peiró S. Lysyl oxidase-like 2 (LOXL2) oxidizes trimethylated lysine 4 in histone H3. FEBS J. 2016, 283 (23), 4263–4273. 10.1111/febs.13922. [DOI] [PubMed] [Google Scholar]
- Iturbide A.; García de Herreros A.; Peiró S. A new role for LOX and LOXL2 proteins in transcription regulation. FEBS J. 2015, 282 (9), 1768–1773. 10.1111/febs.12961. [DOI] [PubMed] [Google Scholar]
- Peinado H.; Iglesias-de la Cruz M. C.; Olmeda D.; Csiszar K.; Fong K. S.; Vega S.; Nieto M. A.; Cano A.; Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005, 24 (19), 3446–3458. 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner E. G.; McLaughlin M.; Matthews L.; Gharib A. M.; Wood B. J.; Levy E.; Sinkus R.; Virtaneva K.; Sturdevant D.; Martens C.; Porcella S. F.; Goodman Z. D.; Kanwar B.; Myers R.; Subramanian M.; Hadigan C.; Masur H.; Kleiner D. E.; Heller T.; Kottilil S.; Kovacs J. A.; Morse C. G. Simtuzumab treatment of advanced liver fibrosis in HIV and HCV-infected adults: Results of a 6-month open-label safety trial. Liver Int. 2016, 36 (12), 1783–1792. 10.1111/liv.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnell S. R.; Martin G. R. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc. Natl. Acad. Sci. U. S. A. 1968, 61 (2), 708–716. 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. J.; Yukl E. T.; Klema V. J.; Klinman J. P.; Wilmot C. M. Structural snapshots from the oxidative half-reaction of a copper amine oxidase: implications for O2 activation. J. Biol. Chem. 2013, 288 (39), 28409–28417. 10.1074/jbc.M113.501791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S. S.; Trackman P. C.; Kagan H. M. Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J. Biol. Chem. 1983, 258 (7), 4331–4338. [PubMed] [Google Scholar]
- Fleming F. F.; Yao L.; Ravikumar P. C.; Funk L.; Shook B. C. Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore. J. Med. Chem. 2010, 53 (22), 7902–7917. 10.1021/jm100762r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamakumbura A. H.; Trackman P. C. A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal. Biochem. 2002, 300 (2), 245–251. 10.1006/abio.2001.5464. [DOI] [PubMed] [Google Scholar]
- Tang S. S.; Simpson D. E.; Kagan H. M. beta-Substituted ethylamine derivatives as suicide inhibitors of lysyl oxidase. J. Biol. Chem. 1984, 259 (2), 975–979. [PubMed] [Google Scholar]
- Schmidt D. M.; McCafferty D. G. trans-2-Phenylcyclopropylamine is a mechanism-based inactivator of the histone demethylase LSD1. Biochemistry 2007, 46 (14), 4408–4416. 10.1021/bi0618621. [DOI] [PubMed] [Google Scholar]
- Shah M. A.; Trackman P. C.; Gallop P. M.; Kagan H. M. Reaction of lysyl oxidase with trans-2-phenylcyclopropylamine. J. Biol. Chem. 1993, 268 (16), 11580–11585. [PubMed] [Google Scholar]
- Williamson P. R.; Kagan H. M. Electronegativity of aromatic amines as a basis for the development of ground state inhibitors of lysyl oxidase. J. Biol. Chem. 1987, 262 (30), 14520–14524. [PubMed] [Google Scholar]
- Bertini V.; Buffoni F.; Ignesti G.; Picci N.; Trombino S.; Iemma F.; Alfei S.; Pocci M.; Lucchesini F.; De Munno A. Alkylamino derivatives of 4-aminomethylpyridine as inhibitors of copper-containing amine oxidases. J. Med. Chem. 2005, 48 (3), 664–670. 10.1021/jm0408316. [DOI] [PubMed] [Google Scholar]
- Bligt-Lindén E.; Pihlavisto M.; Szatmári I.; Otwinowski Z.; Smith D. J.; Lázár L.; Fülöp F.; Salminen T. A. Novel pyridazinone inhibitors for vascular adhesion protein-1 (VAP-1): old target-new inhibition mode. J. Med. Chem. 2013, 56 (24), 9837–9848. 10.1021/jm401372d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovaara H.; Kidron H.; Parkash V.; Nymalm Y.; Bligt E.; Ollikka P.; Smith D. J.; Pihlavisto M.; Salmi M.; Jalkanen S.; Salminen T. A. Identification of two imidazole binding sites and key residues for substrate specificity in human primary amine oxidase AOC3. Biochemistry 2011, 50 (24), 5507–5520. 10.1021/bi200117z. [DOI] [PubMed] [Google Scholar]
- McGrath A. P.; Hilmer K. M.; Collyer C. A.; Shepard E. M.; Elmore B. O.; Brown D. E.; Dooley D. M.; Guss J. M. Structure and inhibition of human diamine oxidase. Biochemistry 2009, 48 (41), 9810–9822. 10.1021/bi9014192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. M.; Kim E. C.; Kim Y. The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol. Biol. Rep. 2011, 38 (1), 145–149. 10.1007/s11033-010-0088-0. [DOI] [PubMed] [Google Scholar]
- Duff A. P.; Cohen A. E.; Ellis P. J.; Kuchar J. A.; Langley D. B.; Shepard E. M.; Dooley D. M.; Freeman H. C.; Guss J. M. The crystal structure of Pichia pastoris lysyl oxidase. Biochemistry 2003, 42 (51), 15148–15157. 10.1021/bi035338v. [DOI] [PubMed] [Google Scholar]
- Klema V. J.; Wilmot C. M. The role of protein crystallography in defining the mechanisms of biogenesis and catalysis in copper amine oxidase. Int. J. Mol. Sci. 2012, 13 (5), 5375–5405. 10.3390/ijms13055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsky R. L.; Obach R. S. Validated assays for human cytochrome P450 activities. Drug Metab. Dispos. 2004, 32 (6), 647–660. 10.1124/dmd.32.6.647. [DOI] [PubMed] [Google Scholar]
- Chang J.; Lucas M. C.; Leonte L. E.; Garcia-Montolio M.; Singh L. B.; Findlay A. D.; Deodhar M.; Foot J. S.; Jarolimek W.; Timpson P.; Erler J. T.; Cox T. R. Pre-clinical evaluation of small molecule LOXL2 inhibitors in breast cancer. Oncotarget 2017, 10.18632/oncotarget.15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.