Abstract
Plastic bronchitis is used to designate endobronchial plugs of rubber-like consistency that form into bronchial trees. It has been described in several diseases like asthma, cystic fibrosis, pulmonary infection, cyanotic congenital heart disease and in few young children with homozygous sickle cell disease. We report the first sickle cell adult case of plastic bronchitis during acute chest syndrome. He developed severe acute respiratory distress syndrome. This unusual presentation related to obstruction by voluminous casts may alert physicians to focus more on the bronchi in sickle cell patients. Realization of fiberoptic bronchoscopy to diagnose endobronchial injury and preventive measures such as fluidification of sputum at the early stage of thoracic vaso-occlusive crisis are essential.
1. Case report
A 19-year old non smoking young man with autistic disorder and homozygous SCD was referred to the Emergency Department of our hospital for excruciating pain in back and right knee. He had already experienced two stays in the intensive care unit (ICU) for acute chest syndrome (ACS). The symptoms had begun six days before with fever treated with amoxicillin and ibuprofen. Initial physical examination and Chest-X-ray (CXR) were normal. The patient was admitted to the Department of Internal Medicine with a diagnosis of vaso-occlusive crisis, and multimodal analgesia was started.
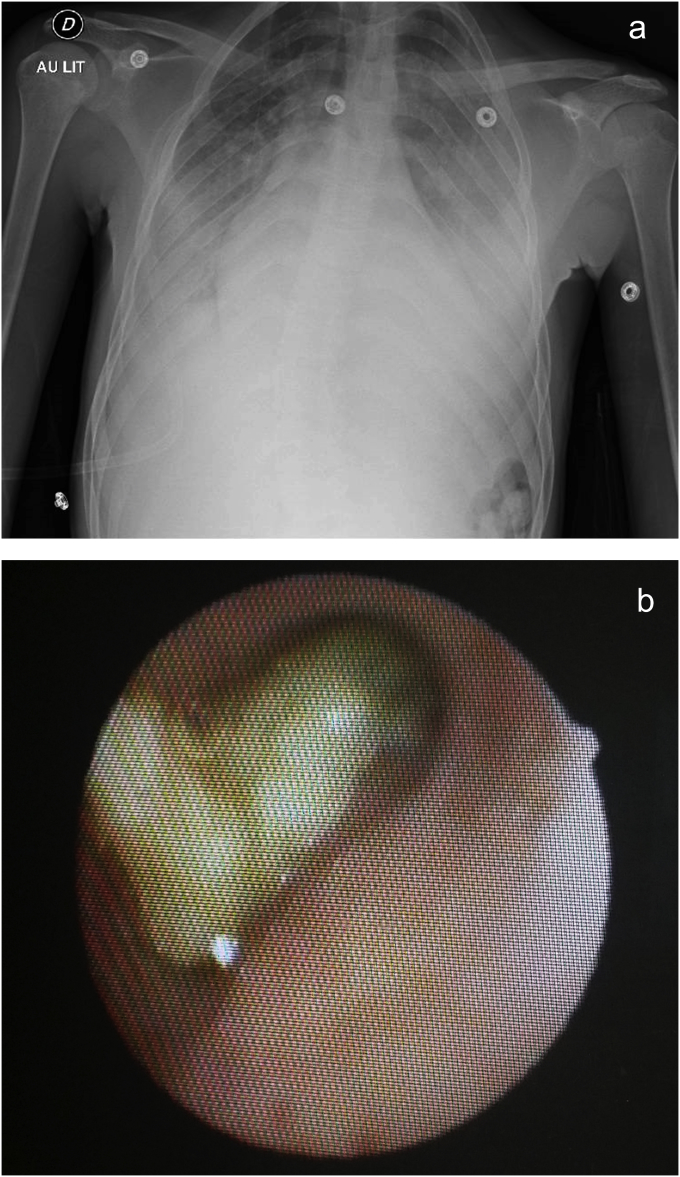
Three days later, the patient became dyspneic and febrile. Physical exam revealed pulmonary bilateral basal crackles with tubular sounds. Oxygen saturation dropped down to 93% on 6 L/min oxygen, with a corresponding PaO2 of 84 mm Hg. CXR revealed bilateral alveolar consolidation with left lung 'white out' (Fig. 1-A). The patient was admitted to the ICU and received empirical antimicrobial therapy combining cefotaxime and spiramycin. As the condition of the patient allowed it, a fiberoptic flexible bronchoscopy (FOB) was performed under high-flow nasal cannula oxygen therapy (FiO2 = 1) with a Remifentanil target-controlled infusion. Voluminous golden casts occluded the bronchial tree at many levels, predominantly at the left main bronchus (Fig. 1-B). The casts were firmly wedged, and could not be removed by the suction channel. N-acetylcystein nebulization was started, in association with repeated flushing using 0.9% NaCl to fragment the casts. As profound hypoxemia developed, tracheal intubation for mechanical ventilation was started. A severe acute respiratory distress syndrome (ARDS) rapidly developed with a PaO2/FiO2 ratio of 110. A lung-protective ventilatory strategy was administered using a low tidal volume (6 mL/kg of predicted body weight) and a PEEP of 10 cm H2O, and curarisation. Trans-thoracic echocardiography showed normal cardiac function and normal pulmonary artery pressure assessed by tricuspid regurgitant jet velocity. Prone-positioning session was attempted on D1 hypothesizing it might help draining the bronchial tree. Unfortunately, the PaO2/FiO2 ratio continued to decrease, and prone ventilation was stopped. Daily aspirations using FOB were performed during the first week and permitted to gradually remove the bronchial casts. Aerosolized mucolytic agents were administered every hour the first day, and then 4 to 6 times a day during the first week, using sodium 2-mercapto-ethane sulphonate and N-acetylcystein. Gas exchanges improved, as the secretions became more fluid and less obstructive. Cloxacillin was administered for 10 days to treat a methicillin-sensitive Staphylococcus aureus (MSSA) identified in the respiratory tract samplings. Red blood cell exchange transfusion decreased hemoglobin S level to 45%. Extubation was performed at D10, and incentive spirometry was administered. Bilateral lung involvement improved on a repeated CT scan at D12, with a persistent left lower lobe consolidation. The patient was discharged from ICU at D13 and hospital at D15.
Fig. 1.
Chest-X-ray showing bilateral alveolar condensation (A) and fiberoptic view of bronchial casts occluding the left main bronchus (B).
2. Discussion
We report the first case of plastic bronchitis, an unusual complication of ACS, in a young adult with SCD. This case highlights the importance of diagnosis and treatment of plastic bronchitis in the non pediatric SCD population.
‘Bronchial casts’ or ‘plastic bronchitis’ designate endobronchial plugs of rubber-like consistency that form into bronchial trees. It has been described in asthma, cystic fibrosis, pulmonary infection, cyanotic congenital heart disease and infrequently during ACS in young children (Table 1). Moser et al. [1] found a prevalence of 72% of plastic bronchitis in a series of 29 children (median age 8 years) suffering ACS, and suggested that plastic bronchitis may be under diagnosed, due to the unusual realization of FOB during ACS.
Table 1.
Reported cases of sickle cell acute chest syndrom with bronchial casts.
| Authors | Patients, No | Age | Sex | PMH | Cast histology | Microbiology |
|---|---|---|---|---|---|---|
| Manna et al. Arch Dis Child 2003 [7] |
1 | 7 years | M | Homozygous SCD No ACS |
Bronchial epithelial cells, mature squamous cells, polymorphs, and fat laden macrophages | ND |
| Moser et al. Chest 2001 [1] |
21 | Median 8 years (3–18) |
11 F 10 M |
NA | Bronchial epithelial cells and fibrinous material 37% of BAL with lipid laden macrophages 7% of BAL were positive for iron-laden alveolar macrophage |
S. pneumoniae Rhinovirus S. aureus E. aerogenes Mycoplasma Legionella |
| Raghuram et al. Pediatrics 1997 [5] |
3 | 23 months | F | ACS × 1, no intubation | Fibrinous material with inflammatory cells | sterile |
| 3.5 years | M | Asthma ACS × 9, no intubation |
Acellular with fibrinous material | sterile | ||
| 6.5 years | M | Asthma ACS × 5, no intubation |
Acellular with fibrinous material | sterile | ||
| Mariana M et al. Int J of Surgical Path 2008 |
1 | 2 years | M | NA | Mucofibrinous with numerous eosinophils and Charcot-Leyden crystals | ND |
| Feray et al. 2016 |
1 | 19 years | M | ACS × 2, no intubation Homozygous SCD |
ND | S. aureus |
Several classifications of plastic bronchitis have been proposed, either based on cast histology [2], separating inflammatory and 'non inflammatory' casts, or on the associated disease [3] defining allergic/asthmatic, cardiac, or idiopathic plastic bronchitis. Madsen et al. [4] recommended a classification based on the associated disease completed with cast histology, when the etiology of plastic bronchitis is unclear. There was no available histology of the bronchial cast in our patient.
The pathogenesis of bronchial casts formation remains poorly known during ACS, and may be explained by multiple factors, including airways inflammation. Both lower respiratory tract infection and asthma may cause excessive production of bronchial secretions that could lead to cast formation in case of clearance impairment. Our patient had a MSSA pneumonia, but no history of asthma or allergy, and the eosinophils differential count was in normal ranges. During vaso-occlusive crisis, patients may likely splint their chest wall by limiting cough or deep breaths. In addition, ischemia of the bronchial tree related to vaso-occlusion may alter airway clearance [5]. Pulmonary vascular hypertension may develop during ACS, and increase lymphatic load by augmentation of lung blood volume. It has been suggested that these conditions may contribute to the lymph leakage into the bronchi, and participate to the development of bronchial casts [2]. In our patient, there were no echocardiographic signs of pulmonary hypertension. However, we can not exclude its existence, and Madsen et al. [4] underlined the fact that bronchial cast formation is not described in other diseases with pulmonary vascular hypertension. Altogether, both excessive bronchial secretions due to MSSA pneumonia and clearance impairment due to thoracic pain and bronchial tree ischemia could have participate to the development of plastic bronchitis.
The mainstay of therapy of bronchial cast is symptomatic, i.e. improving alveolar oxygenation, and etiologic. The role of FOB realization in the occurrence of acute respiratory failure may be questioned in our patient. However, the patient respiratory status was already critical and FOB allowed us to diagnose plastic bronchitis and start invasive treatment.
The topical management of bronchial cast is not well defined, and there is no recommendation for using one mucolytic agent or another. Bronchial cast may be difficult to remove with single bronchial instillation of 0.9% saline or bronchoscopic suction. Bronchodilators and mucolytics may disintegrate the secretions. The use of tissue plasminogen activator (t-PA) has been described in plastic bronchitis occurring after extracardiac Fontan operation in children [6]. Recombinant human DNase (rhDNAse) has been used to decrease sputum viscoelasticity in cystic fibrosis patients. Intra-tracheal rhDNase instillation under direct bronchoscopic vision has been described to treat plastic bronchitis in ACS [7]. In our case, we administered local mucolytics instillations with sodium 2-mercapto-ethane sulphonate and N-acetylcystein.
To sum, we described an unusual presentation of ACS, with endobronchial injury related to the obstruction of proximal bronchial tree by voluminous casts in association with lung parenchymal involvement, resulting in severe ARDS. This observation may alert physicians to focus on the bronchi in SCD patients with severe or rapidly aggravating ACS. Risk factors such as dehydration, airway inflammation or infection, hypereosinophilia and aspergillar disease should be searched for and treated. Preventive measures with incentive spirometry, multimodal analgesia and sputum fluidification at the early stage of thoracic vaso-occlusive crisis or ACS are mandatory.
References
- 1.Moser C., Nussbaum E., Cooper D.M. Plastic bronchitis and the role of bronchoscopy in the acute chest syndrome of sickle cell disease. Chest. 2001;120:608–613. doi: 10.1378/chest.120.2.608. [DOI] [PubMed] [Google Scholar]
- 2.Seear M., Hui H., Magee F. Bronchial casts in children: a proposed classification based on nine cases and a review of the literature. Am. J. Respir. Crit. Care Med. 1997;155:364–370. doi: 10.1164/ajrccm.155.1.9001337. [DOI] [PubMed] [Google Scholar]
- 3.Brogan T.V., Finn L.S., Pyskaty D.J. Plastic bronchitis in children: a case series and review of the medical literature. Pediatr. Pulmonol. 2002;34:482–487. doi: 10.1002/ppul.10179. [DOI] [PubMed] [Google Scholar]
- 4.Madsen P., Shah S.A., Rubin B.K. Plastic bronchitis: new insights and a classification scheme. Paediatr. Respir. Rev. 2005;6:292–300. doi: 10.1016/j.prrv.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Raghuram N., Pettignano R., Gal A.A. Plastic bronchitis: an unusual complication associated with sickle cell disease and the acute chest syndrome. Pediatrics. 1997;100:139–142. doi: 10.1542/peds.100.1.139. [DOI] [PubMed] [Google Scholar]
- 6.Heath Lauren, Ling Shelley, Racz Jennifer. Prospective, longitudinal study of plastic bronchitis cast pathology and responsiveness to tissue plasminogen activator (tPA) Pediatr. Cardiol. 2011;32(8):1182–1189. doi: 10.1007/s00246-011-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manna S.S., Shaw J., Tibby S.M., Durward A. Treatment of plastic bronchitis in acute chest syndrome of sickle cell disease with intra-tracheal rhDNase. Arch. Dis. Child. 2003;88:626–627. doi: 10.1136/adc.88.7.626. [DOI] [PMC free article] [PubMed] [Google Scholar]