Abstract
Background
Risk of arrhythmic death is considered highest in ischemic heart disease with severe left ventricular ejection fraction (LVEF) reduction. Non-invasive testing should improve decision-making of prophylactic defibrillator (ICD) implantation.
Methods and Results
We enrolled 120 patients with ischemic heart disease and LVEF <50% and 30 control subjects without ischemic heart disease and normal LVEF. An initial assessment, a second assessment after 3 years, and a final follow-up comprised of: pharmacological baroreflex testing (BRS), short-term spectral (low frequency (LF) to high frequency (HF) ratio) and long-term time domain analysis of heart rate variability (SDNN), exercise Microvolt T-wave alternans (MTWA) and others. The median follow-up was 7.5 years. Resuscitated cardiac arrest and arrhythmic death due to ventricular arrhythmias ≥ 240/min was observed in 18% and 15% of patients, respectively. Cardiac death was observed in 28% of patients. The incidence of arrhythmic death and resuscitated cardiac arrest was identical in patients with ischemic heart disease with LVEF <30% and ≥30%. No significant difference between subgroups with LVEF of <30%, 30% to 39% and ≥40% was found either. MTWA, BRS, SDNN and LF to HF ratio failed to identify patients at risk of arrhythmic death in a multiple regression model.
Conclusions
Ischemic heart disease patients with LVEF <30% and ≥30% face the same risk of arrhythmic death. Stratification techniques fail to identify high risk patients. Therefore, the current practice to constrain prophylactic ICDs to patients with severely reduced LVEF seems to be insufficient.
Keywords: Sudden cardiac death, non-invasive risk stratification, ischemic heart disease
INTRODUCTION
Randomized trials in patients with ischemic heart disease have shown that prophylactic implantation of a cardioverter-defibrillator (ICD) improves overall survival in selected patients with ischemic heart disease and markedly reduced left ventricular ejection fraction (LVEF).1–3 Prediction of sudden cardiac death and especially arrhythmic death (AD) based on arbitrary LVEF criteria (30% or less) has major limitations in accuracy. In patients with ischemic heart disease, noninvasive assessments of autonomic function4 or electrophysiological markers5 have been proposed to identify high risk patients. Combining both assessments might enhance risk stratification.6 There are limited prospective data available how combined assessment of autonomic and electrical substrate at single or multiple time points might improve ischemic heart disease management.7 The objective of this paper was to evaluate the clinical relevance of a combined assessment of autonomic function plus cardiac electrical substrate at multiple time points for the prediction of fatal arrhythmias in patients with ischemic heart disease.
METHODS
The local ethics committee approved this prospective observational, controlled, observer-blind study. All participants gave written consent. Screening, enrollment (2002–2003), clinical testing (first investigation (I1) in 2003/2004, second investigation (I2) in 2007/2008 and final follow-up in 2012/13) were conducted at the Medical University of Vienna, Austria. Test scoring, interpretation and statistical data processing (2009–2013) underwent a blinded assessment at the Vanderbilt Autonomic Dysfunction Center, Nashville, USA. After enrollment, optimal medical treatment was established. Test results were not disclosed to participants or their physicians. Antiarrhythmic drug therapy and ICD implantation were not guided by the study.
Population
A total of 120 consecutive patients with a history of myocardial infarction and ischemic heart disease and 30 control subjects without myocardial infarction and ischemic heart disease were studied. Patients were eligible to participate if they were at least 18 years of age, had an LVEF of 50% or less due to a myocardial infarction within the last 2 months or a normal LVEF (controls, no myocardial infarction), had recently undergone coronary angiography with ventriculography due to suspected myocardial infarction, had no history of sustained ventricular arrhythmia or permanent atrial fibrillation and were non-dependent on ventricular pacing. Coronary angiography with ventriculography as standard use of care in symptomatic patients with suspected coronary artery disease was independent from study participation and was not guided by the study.
Cardiac Pump Function
Left ventricular ejection fraction (LVEF) was initially calculated from echocardiography and ventriculography following coronary angiography (screening). LVEF was then reassessed by radionuclide angiography or by magnetic resonance imaging (gold standard) at enrollment which was close to I1, followed by a reassessment of LVEF close to I2 and thereafter every two years.
Cardiac Electrical Substrates
Corrected QT-times with Bazett´s formula (QTC) were obtained using automated analysis systems. Non-sustained ventricular tachycardia (nsVT) and signal-averaged ECG (SAECG) were obtained using 24-hour Holter monitoring devices. NsVT was defined as a run of ≥ 3 ventricular beats lasting no longer than 30 seconds without syncope. SAECG were obtained by time-domain analysis as previously described.8 Late potentials were present if two of the following criteria were fulfilled: QRSf >114 ms, LAS40 >38 ms, and RMS40 <20 AV. Microvolt T-wave alternans (MTWA) was assessed during submaximal bicycle exercise (CH 2000®, Cambridge Heart Inc., Bedford, MA, USA). Patients continued their regular cardiovascular medications except beta-blockers which were withheld on the day of the investigation. The MTWA test was automatically interpreted (Version D10) and verified. Standard invasive programmed ventricular stimulation (PVS) provided up to three extra stimuli delivered during sinus rhythm and after eight paced ventricular cycle lengths at 500, 430, 375, and 333 msec.9 The positive procedural endpoint of PVS was the induction of a sustained monomorphic VT. PVS was performed in all patients with coronary artery disease except those with a clear ICD indication (patients with severely reduced LVEF).
Autonomic Function
For baroreflex testing, continuous finger blood pressure by the vascular unloading technique, beat-to-beat stroke volume by impedance cardiography, and 4 lead ECG was recorded with the Task Force Monitor system (TFM®, CNSystems, Graz, Austria).10 After a resting period in supine position of at least 30 min, three bolus injections of Phenylephrine (2–4 µg/kg) were given intravenously at intervals of 10 min to raise systolic blood pressure by 15–40 mmHg.4 A regression line was fit between data pairs of blood pressure and R-R intervals. Only regression lines with a R2 coefficient either more than 0·80 or statistical significant linear regression (F-test on the regression model, p<0·05) were accepted. Baroreceptor reflex sensitivity (BRS, msec/mmHg) was obtained by calculating the mean from at least three slope determinations. Short-time spectral measurement of low frequency (LF) and high frequency (HF) of heart rate variability (HRV) was obtained during a 10-minutes resting period in supine position before pharmacological baroreflex testing.11 Normalized values as well as LF to HF ratio (LF/HF) were analyzed. Time domain measures and long-term spectral parameters of HRV were obtained from 24-hour Holter recordings. The standard deviation of all normal-to-normal R–R intervals (SDNN) was chosen as one of the favorite time domain parameter for the statistical model analysis.
End-points, classification of death
The primary end-point was time to arrhythmic death (AD) or resuscitated cardiac arrest (RCA). Cardiac death (CD) and all-cause mortality were secondary endpoints. Deaths were categorized utilizing an adapted form of the Hinkle classification.12 Appropriate ICD therapy without VT acceleration that failed to save the patient’s life at the time of arrhythmias was classified as AD. An RCA was ventricular fibrillation or VT >240 bpm leading to syncope before ICD therapy, and multiple slower VT episodes (electrical storm) leading to syncope and ICD discharge without ICD therapy related acceleration. All other ICD therapies due to VT <240 bpm were not taken as surrogate for AD. All ICD devices were programmed to allow maximum possible detection duration.
Sample size justification and statistical analysis
The accrual interval is one year with additional follow-up of 10 years. Based on our prior knowledge and literature review, the median survival time for ischemic heart disease patients was 15 years. If the true ratio (relative risk) of controls relative to ischemic heart disease patients is 0.4, we need to study 120 ischemic heart disease patients and 30 control patients to be able to reject the null hypothesis that the survival curves are equal with 89% power. The Type I error probability associated with this test of this null hypothesis is 0.05. Data were summarized as median with 1st and 3rd Quartiles or percentage (N) for continuous and categorical variables. Wilcoxon rank-sum test or Kruskal-Wallis test was used for continuous variables, chi-square test was used for categorical variables. Survival curves were estimated by cumulative incidence treating death as the competing event and the k-sample test was used. To assess whether MTWA, BRS, or SDNN adds prognostic value in addition to LVEF, we fit four separate Cox proportional-hazards models (all adjusted for age and gender). The added prognostic value was assessed by likelihood ratio test. All tests were two-tailed with 5% significance level. All analyses were performed at the Vanderbilt University School of Medicine, Department of Biostatistics, Nashville, TN, U.S.A. using an open source software R version 3.0.2. as previously described.13
RESULTS
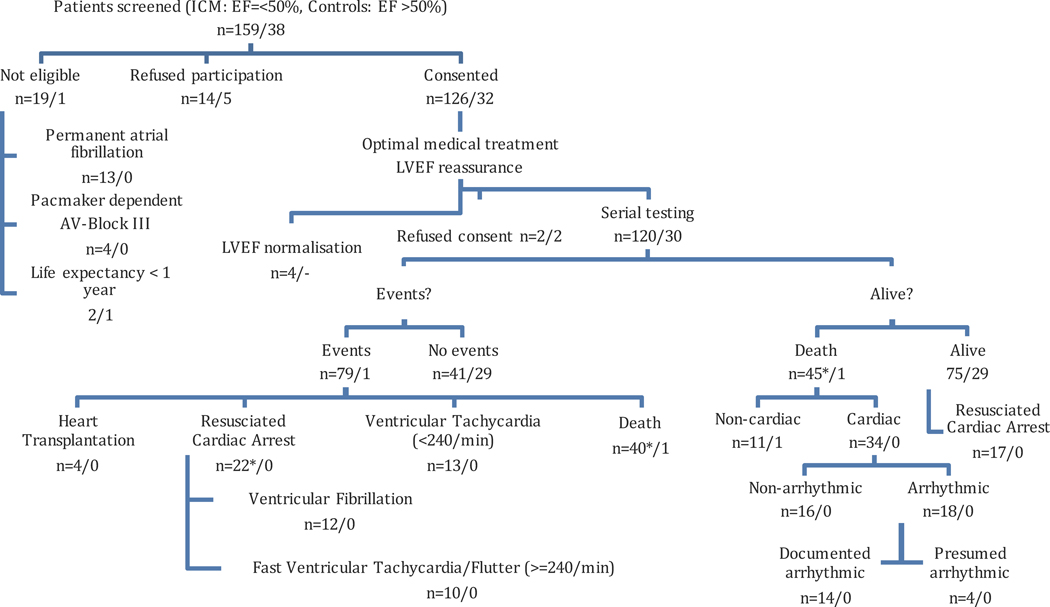
A total of 120 ischemic heart disease patients with LVEF of 50% or less and 30 control patients with a normal LVEF underwent serial clinical testing. (Figure 1, upper part). Distribution of LVEF and clinical characteristics of the two patient groups are listed in Table 1. The median LVEF was 34% in ischemic heart disease patients. During a median follow-up period of 7.5 years, 46 deaths (one in the control group) were observed. Of these, 34 were categorized as cardiac where 18 were considered as arrhythmic. In 22 patients a RCA was documented as their first event (Figure 1, left lower part). Five of these 22 patients died within the follow-up period. In 35 out of 70 ICD recipients (50%), ICD therapies (22 RCA events and 13 VT < 240/min events) were recorded. No RCA occurred in non-ICD recipients. Out of 18 patients died from AD, 9 (LVEF < 30%) had an ICD implanted but the device could not abort an electrical storm. Another 8 patients (LVEF≥30%) had no ICD implanted, and one patient had a CRT-Pacemaker only. Mortality rates are shown in Table 2.
Figure 1.
Patient Selection and Outcomes (2002–2013). A total of 150 patients (120 ischemic heart disease patients with LVEF=<50%, 30 controls with LVEF>50%) underwent serial testing. They were enrolled from a larger sample of consecutive patients. Ineligibility was mainly due to permanent atrial fibrillation. Other patients refused participation or LVEF normalized. During a median follow-up of 7 years, 46 (45 ICM patients and one Control patient) deaths were observed. Of these, 34 were categorized as cardiac and 18 as arrhythmic. In 22 patients a resuscitated cardiac arrest was documented as their first event. Five of these 22 patients died during further follow-up from a non-cardiac and non-arrhythmic reason. (* marked as 5 non-qualifying events)
Table 1.
Clinical characteristics
| Ischemic heart disease, n=120 |
Controls, n=30 |
P-value | |
|---|---|---|---|
| Age, years, Med (range) | 62 (30–79) | 59 (28–73) | 0.15 |
| Male | 108 (90) | 17 (57) | <0.001 |
| BMI, kg/m2, Med (IQR) | 27·4 (24·8–30·5) | 26·2 (24·1–29·1) | 0.16 |
| LVEF, %, Med (range) | 34 (15–50) | 67 (54–79) | <0.001 |
| LVEF = 40–50% | 29 (24) | 0 | |
| LVEF = 31–39% | 39 (39) | 0 | |
| LVEF = 25–30% | 29 (24) | 0 | |
| LVEF = < 25% | 23 (13) | 0 | |
| Hypertension | 105 (88) | 23 (77) | 0.13 |
| Hyperlipidemia | 91 (76) | 10 (33) | <0.001 |
| Diabetes mellitus | 47 (39) | 2 (7) | <0.001 |
| COPD | 25 (21) | 4 (13) | 0.35 |
| Renal dysfunction | 32 (27) | 0 | 0.001 |
| Major depression | 25 (21) | 8 (27) | 0.49 |
| Stroke | 19 (16) | 1 (3) | 0.072 |
| Syncope | 29 (24) | 1 (3) | 0.011 |
| Non-sustained VT | 27 (23) | 1 (3) | 0.78 |
| Beta-blocker | 107 (89) | 18 (60) | <0.001 |
| Class III (Sotalol, Amiodarone) | 27 (22) | 2 (7) | 0.05 |
| Statins | 88 (73) | 7 (23) | <0.001 |
| Diuretics | 83 (69) | 7 (23) | <0.001 |
| Antidepressants | 16 (13) | 15 (17) | 0.64 |
| Follow-up, years, Med (mean±SD) | 6.8 (5.9±2.5) | 8.7 (8.5±1) | <0.001 |
| ICD implanted | 70 (58) | 0 | <0.001 |
| ICD therapy | 35 (50) | 0 | <0.001 |
| History of myocardial infarction | 120 (100) | 0 | <0.001 |
| 1-vessel disease* | 23 (19) | 0 | <0.001 |
| 2-vessel disease* | 22 (18) | 0 | <0.001 |
| 3-vessel disease* | 75 (63) | 0 | <0.001 |
Data are n (%), unless otherwise indicated; Med=median (Q1–Q3); test used: Wilcoxon test or chi-square test. Renal dysfunction=persistent serum creatinine >1.2mg/dl.
from coronary angiography
Table 2.
Primary and secondary endpoints stratified according LVEF
| Ischemic heart dis- ease, LVEF <30% n=37 |
Ischemic heart disease, LVEF 30– 39% n=54 |
Ischemic heart dis- ease, LVEF 40–49% n=29 |
Control subjects n=30 |
|
|---|---|---|---|---|
| All-cause mortality | n=16 (43%) | n=18 (33%) | n=11 (38%) | n=1 (3%) |
| Cardiac death (CD) | n=14 (38%) | n=13 (24%) | n=7 (24%) | 0 |
|
Arrhythmic death (AD) /
Re- susciated cardiac arrest (RCA) |
n=11 (30%) | n=20 (37%) | n=9 (31%) | 0 |
Test results related to primary and secondary endpoints
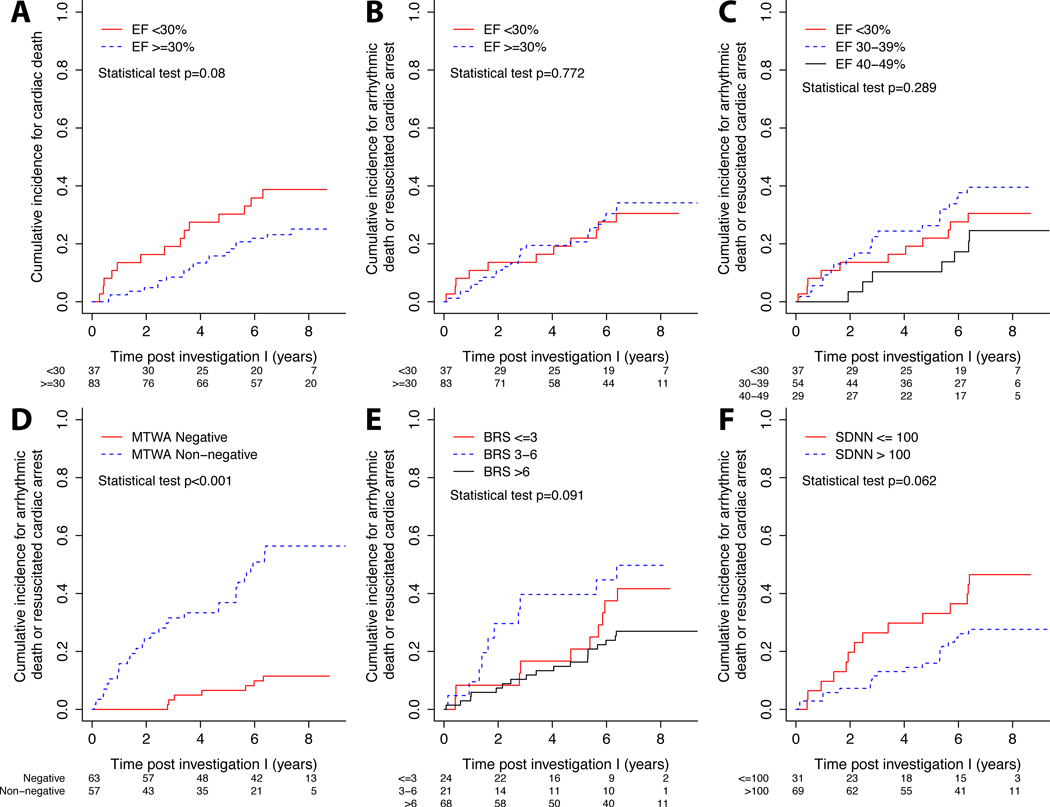
BRS and HRV testing at second investigation (I2), MTWA and LVEF at both investigations (I, I2) were significantly different associated with the primary endpoints of AD or RCA and other forms of death (Table 3a). All studied test results at both investigations (I1, I2) were significantly different associated according to the secondary endpoints of cardiac (n=21) and non-cardiac death (n=3) (Table 3b). Figure 2 demonstrates that MTWA only was associated with AD or RCA (p<0·001). LVEF was a non-significant predictor for cardiac death (Figure 2a) and arrhythmic events: the cumulative incidence for arrhythmic death or resuscitated cardiac arrest was not dependent on LVEF. This was true for LVEF <35% and ≥35% (p=0.093), <30% and ≥30% (p=0.772, Figure 2b) and <30% and 30–39% and 40–49% (p=0.289, Figure 2c). We fit a Cox proportional-hazards model for the association between LVEF and time to RCA and AD adjusted for age and gender. We added MTWA, BRS, and SDNN to the EF model one at a time to test for possible higher prognostic value. A comparison of likelihood ratios between the “LVEF+MTWA” model, “LVEF+BRS” model, “LVEF+SDNN” model, and the LVEF only model revealed that only MTWA but not BRS or SDNN added predictive information (Table 3c). Sensitivity, specificity, positive predictive value and negative predictive value regarding AD or RCA for “LVEF+MTWA” model for all patients were 83%, 78%, 64% and 91% regarding 1st assessment (I1) and 93%, 80%, 61% and 97% regarding 2nd assessment (I2), respectively.
Table 3.
| a Risk factors stratified by primary endpoints
defined as time to arrhythmic death (AD) or resusci- tated cardiac arrest (RCA) and all other deaths using first (I1) and second (I2) investigation. | |||||
|---|---|---|---|---|---|
|
| |||||
| N | Alive n=87 |
Arrhythmic death /resuscitated car-diac ar- rest, n=40 |
Death other n=23 |
P- value |
|
| Autonomic tone | |||||
| BRS_I1, ms/mmHG, Med | 92 | 9·6 (6·0–13·8) | 6.8 (4.8–9.9) | 6·5 (1·3–11·9) | 0.13 |
| BRS_I2, ms/mmHG, Med | 105 | 10·9
(6·5– 17·3) |
5.7 (3.2–11.6) | 6·4 (1·8–11·4) | 0.002 |
| LF/HF_I1, LF to HF ratio, Med |
109 | 2·4 (1·3–4·0) | 2.1 (0.9–3.7) | 2·0 (0·7–3·8) | 0.51 |
| LF/HF_I2, LF to HF ratio, Med |
112 | 2·8 (1·4–5·1) | 2.3 (0.8–4.6) | 1·9 (0·8–3·7) | 0.28 |
| HRV_I1, SDNN, ms, Med | 103 | 114 (97–134) | 105 (77–134) | 116 (84–131) | 0.51 |
| HRV_I2, SDNN, ms, Med | 92 | 115 (95–140) | 110 (93–127) | 85 (65–105) | 0.051 |
| Electrical substrate | |||||
| PVS_I1 | 85 | 0.061 | |||
| Negative | 8 (24) | 13 (11) | 6 (43) | ||
| Non-negative | 25 (76) | 25 (89) | 8 (57) | ||
| SAECG_I1 | 112 | 0.37 | |||
| Negative | 30 (51) | 22 (61) | 7 (41) | ||
| Non-negative | 29 (49) | 14 (39) | 10 (59) | ||
| SAECG_I2 | 98 | 0.36 | |||
| Negative | 49 (68) | 9 (50) | 5 (62) | ||
| Non-negative | 23 (32) | 9 (50) | 3 (38) | ||
| MTWA_I1 | 150 | <0.001 | |||
| Negative | 68 (78) | 7 (18) | 15 (65) | ||
| Non-negative | 19 (22) | 33 (82) | 8 (35) | ||
| MTWA_I2 | 132 | ||||
| Negative | 68 (80) | 2 (7) | 9 (50) | <0.001 | |
| Non-negative | 17 (20) | 27 (93) | 9 (50) | ||
| Clinical variables | 68 (80) | 2 (7) | 9 (50) | <0.001 | |
| History of syncope_I1 | 150 | 15 (17) | 11 (28) | 14 (17) | 0.38 |
| Non-sustained VT_I1 | 111 | 11 (19) | 12 (33) | 5 (29) | 0.27 |
| LVEF_I1, %, Med | 150 | 39 (30–65) | 33 (29–37) | 35 (28–40) | <0.001 |
| LVEF_I2, %, Med | 131 | 38 (29–65) | 30 (25–37) | 30 (23–35) | <0.001 |
| QTC_I1, ms, Med | 150 | 436 (424–453) | 454 (443–464) | 464 (428–474) | 0.084 |
| QTC_I2, ms, Med | 131 | 435 (419–458) | 472 (443–481) | 460 (421–468) | 0.13 |
| Crea_I1, mg/dl, Med | 150 | 1.2 (1.0–1.5) | 1.2 (1.0–1.3) | 1.5 (1.0–1.9) | 0.41 |
| Crea_I2, mg/dl, Med | 131 | 1.2 (1.0–1.4) | 1.2 (1.0–1.3) | 1.5 (1.1–1.7) | 0.37 |
| b Risk factors stratified by secondary
endpoints defined as time to cardiac death (CD) and non- cardiac death using first (I1) and second (I2) investigation. | |||||
|---|---|---|---|---|---|
|
| |||||
| N | Alive, n=104 |
Cardiac death, n=34 |
Non-cardiac death, n=12 |
P- value |
|
| Autonomic tone | |||||
| BRS_I1, ms/mmHG, Med | 92 | 9.6 (6.0–14.5) | 6.3 (2.6–8.3) | 7.3 (4.2–13.0) | 0.008 |
| BRS_I2, ms/mmHG, Med | 105 | 10.7 (6.1–17.2) | 4.7 (0.3–6.7) | 7.9 (2.2–13.7) | 0.001 |
| LF/HF_I1, LF to HF ratio, Med | 109 | 2.5 (1.2–4.4) | 1.9 (0.7–2.9) | 1.1 (0.6–3.3) | 0.06 |
| LF/HF_I2, LF to HF ratio, Med | 112 | 2.8 (1.3–5.3) | 1.5 (0.4–2.3) | 3.5 (0.9–3.7) | 0.047 |
| HRV_I1, SDNN, ms, Med | 103 | 117 (100–140) | 84 (67–116) | 123 (108–134) | 0.003 |
| HRV_I2, SDNN, ms, Med | 92 | 114 (96–140) | 94 (82–116) | 63 (60–74) | 0.003 |
| Electrical substrate | |||||
| MTWA_I1 | 150 | <0.001 | |||
| Negative | 74 (71) | 9 (26) | 7 (58) | ||
| Non-negative | 30 (29) | 25 (74) | 5 (42) | ||
| MTWA_I2 | 132 | ||||
| Negative | 69 (68) | 4 (18) | 6 (75) | <0.001 | |
| Non-negative | 33 (32) | 18 (82) | 2 (25) | ||
| Clinical variables | |||||
| LVEF_I1, %, Med | 150 | 38 (30–58) | 32 (27–37) | 38 (33–42) | 0.002 |
| LVEF_I2, %, Med | 131 | 37 (28–60) | 28 (20–34) | 35 (25–37) | <0.001 |
| c: Cox proportional-hazards model using
baseline clinical variables for arrhythmic death / resusci- tated death in ischemic heart disease patients alone. | ||||
|---|---|---|---|---|
|
| ||||
| Parameter | Haz- ard Ratio |
95%C I low- er |
95% CI upper |
p- value |
| (LV)EF (10% increase) | 0.97 | 0.92 | 1.02 | 0.212 |
| MTWA
(non- negative:negative) |
0.15 | 0.06 | 0.41 | 0.008 |
| BRS (1 unit increase) | 0.95 | 0.89 | 1.01 | 0.127 |
| SDNN (10 units increase) | 0.99 | 0.98 | 1.00 | 0.184 |
Data are n (%), unless otherwise indicated; Med=median (Q1–Q3); I1, I2 Investigation 1 and 2, N is the number of non-missing values; test used: Kruskal-Wallis test
Figure 2.
a–f. Kaplan-Meier curves show the association between non-invasive tests (MTWA, BRS, LVEF and SDNN) and secondary endpoint (2a, cardiac death) and primary endpoints (2b–f, arrhythmic death, resuscitated cardiac arrest) for ischemic heart disease patients.
Other parameters in addition to LVEF
We performed likelihood ratio test for time to primary endpoints (arrhythmic death, resuscitated cardiac arrest) comparing 3 models (“LVEF+MTWA”, “LVEF+BRS”, “LVEF+SDNN” model) to the LVEF only model with age and sex adjustments. The results indicate that MTWA adds more predictive values regarding to the primary endpoints in addition to LVEF. (age, sex, LVEF, MTWA: p<0.001; age, sex, LVEF, BRS: p=0.249; age, sex, SDNN: p=0.446). The area under the curve (AUC) keeps clearly below 0.85 (0.677 to 0.786).
Missed patients at risk for AD or RCA and possible benefits from ICD therapy
Considering that all 120 patients with ischemic heart disease would receive an ICD, the treatment ratio would be 3:1 as there were 40 AD/RCA events. In addition, if one considers nonfatal VT in the setting of ischemic heart disease as an ICD indication the percentage of patients benefitting from ICD will increase to 44% with a treatment ratio of 2.3:1. Considering that 57 out of 120 patients with ischemic heart disease and a non-negative TWA result would receive an ICD, the ICD treatment ratio would be 2.1:1. Considering that 33 out of these 57 patients had an AD/RCA event the combination of LVEF+TWA would result in an ICD treatment ratio of 1.2:1. The resulting percentage of ischemic heart disease patients missed at risk would be 35% for LVEF >30% and 11% for TWA, respectively.
DISCUSSION
To the best of our knowledge this is the first prospective, long-term (follow-up 10 years) observational study on multiple risk stratification techniques for primary prophylactic defibrillator implantation in patients with ischemic heart disease and both preserved and clearly reduced left ventricular function. The main finding is that none of the investigated techniques including the degree of left ventricular dysfunction reliably could identify patients at risk for sudden arrhythmic death.
The ACC/AHA/ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death dichotomize patients with ischemic heart disease according to the degree of left ventricular dysfunction: ICD therapy is recommended in patients with symptomatic heart failure (NYHA class II – III) and LV ejection fraction ≤35% after ≥3 months of optimal medical therapy.14 In patients with an ejection fraction of >35% the risk of sudden arrhythmic death is considered low not warranting primary prophylactic ICD implantation. Bigger et al. first described that ventricular arrhythmias and LV dysfunction are related, one at a time and jointly, to total mortality and to arrhythmic deaths.15 However, the mean follow-up period in this study has been 1.7 years only. These findings subsequently formed the rationale for the primary prevention studies MADIT II and SCD-Heft that used an ejection fraction of 30% and 35% as upper limit for patient inclusion.3, 16 In MADIT II and SCD-Heft primary preventive ICD implantation was associated with a significant absolute reduction in total mortality of 5.6 % and 5 %, respectively. The follow-up period was with maximum of 1.8 years in MADIT II and with median of 3.8 years in SCD-Heft considerably short.
The most important finding of our study is that over a projected follow up period of up to 10 years the cumulative incidence of arrhythmic death and resuscitated cardiac arrest is not significantly different in patients with (EF < 30 %) and without (> 30 %) left ventricular dysfunction. This finding is regardless of the degree of EF reduction (EF of < 30 %, EF between 30 and 39 % and patients with EF of > 40%). Similar to the patients in the Multicenter Post-Infarction Group Trial our patients with an ejection fraction of ≥ 30 % had an excellent prognosis and virtually no risk of sudden arrhythmic death during the first 2 years of follow up.15 However, our findings differ after the initial 2 years of follow-up. Thereafter, even in this favorable subpopulation the risk of arrhythmic events continuously increased to finally reach a virtually identical level of arrhythmic death or resuscitated cardiac arrest when compared to patients with severely depressed LV function
A highly significant difference in total cardiac mortality was found by dichotomizing the study population in patients with an EF <30% versus >30%. This excess in cardiac mortality in patients with left ventricular dysfunction may offset in part the gains made by the ICD and probably explains why in the primary prevention studies the absolute reduction in total mortality in the ICD groups was as low as 5 % and 5.6 %, respectively.3, 16 In the SCD-Heft Trial in ischemic heart disease patients the difference in total mortality reached borderline significance (p = 0.05) only.16
Would have all our study patients, irrespective of the degree of left ventricular dysfunction, undergone primary prophylactic ICD implantation, the treatment ratio would have been 3:1 as there were 40 arrhythmic deaths or resuscitated cardiac arrest events. In addition, if one considers nonfatal VT in the setting of ischemic heart disease an indication for ICD implantation the percentage of patients benefitting from ICD therapy would increase to 44 % with an treatment ratio of 2.3 : 1.
Among all other noninvasive tests applied in this study only an abnormal MTWA test was found to be able to identify patients at risk of arrhythmic death, although with false negative test results in 11% of cases which makes its applicability in clinical practice questionable. Furthermore, the possible significant advantage of MTWA becomes less evident in a Cox proportional-hazards model and the related likelihood ratio test. In addition, patients with known atrial fibrillation were not included and 8.2% of study patients could not be tested due to atrial fibrillation.
In the REFINE study neither MTWA or other non-invasive markers were found to be predictive for the primary outcome as stand-alone tests.7 The REFINE study found that only a combination of at least two tests predicted a significantly higher independent risk for the primary outcome 10 to 14 weeks after myocardial infarction. Main differences compared to our study were: tests at 2–4 and 10–14 weeks after myocardial infarction, study population size, no control group and the median follow-up of 1.9 years with an event rate of 9.4% compared to an event rate of 66% in the underlying study.
Neither SAECG nor the presence of nsVT on Holter recordings were found to be useful in fatal arrhythmic risk prediction. Also programmed ventricular stimulation with its high amount of false negative test results should therefore no longer be recommended for risk stratification. The markers of autonomic tone, HRV and BRS, were predictive for CD but not so for AD or RCA and therefore also should not be used for risk stratification.
Rationale for combined and multiple assessment
Our primary endpoint was AD or RCA. This is in contrast to many other studies using sudden death as endpoint that is not synonymous with a fatal ventricular tachyarrhythmia. The rational for multiple testing and a projected follow-up of 10 years was the generally low risk of fatal arrhythmias in post-MI patients,17 which significantly increases approximately 20 months after myocardial infarction.18 In addition, this study aimed to achieve a high rate of endpoints to sufficiently support statistical analysis. In fact, there was an event rate of 66% in ischemic heart disease patients (79/120 patients). This high event rate was driven by 1) the very long follow-up period, 2) the large percentage of implanted ICDs which allowed an increased event detection and 3) similar demographics to other landmark studies making data comparable. The reason to choose an upper LVEF limit of 50% was based on the ATRAMI study,4 where a relative risk of 2.5 for cardiac mortality in patients with an LVEF between 35% and 50% compared with patients with LVEF>50% was found.
Study limitations
This was a non-randomized, prospective, observational study performed to assess the risk of fatal arrhythmias during long-term follow-up. It is still possible that some of the events, defined as RCA, might not have led to death. Another limitation is the small sample size, which does not allow making firm conclusions. Based on our power calculation, our study has sufficient power of 89% to reject the null hypothesis that the survival curves are equal between ischemic heart disease patients and controls. Additionally, we introduced multivariable adjustment using Cox proportional-hazards model to study multiple parameters to gain statistical power. Although there is a potential beta error which is a limitation of the present study. This can be only investigated in a bigger population in the future. Therefore, any finding should be labeled as pilot study results and only randomized ICD trials can give definite answers about the true value of risk stratification.
Conclusions
Patients with ischemic heart disease and rather preserved left ventricular function carry the same risk of fatal arrhythmic events as those with markedly reduced left ventricular function. None of the available risk stratification techniques (single or combination rules) reliably can identify patients at risk for arrhythmic events. Therefore, the current practice to constrain prophylactic ICDs to patients with severely reduced LVEF seems to be insufficient.
Key messages.
Prophylactic defibrillator implantation is recommended in ischemic heart disease and left ventricular ejection fraction (LVEF) of 30–35% or less. Risk stratification is based on virtual borders of left ventricular ejection fraction. Noninvasive assessments of autonomic tone (heart rate variability, baroreflex testing) and cardiac electrical substrate (signal averaged ECG, microvolt T-wave alternans, MTWA) have been developed to identify high-risk patients.
Our findings are: One third of patients with ischemic heart disease and LVEF of 50% or less experience fatal arrhythmias over a median follow-up of 7 years.
The occurrence of fatal arrhythmias is not dependent on the degree of LVEF reduction.
None of the non-invasive risk stratification techniques appear useful in clinical decision-making.
Therefore, the current practice to constrain prophylactic ICDs to patients with severely reduced LVEF seems to be insufficient.
Acknowledgments
Research reported in this publication was partly supported by the National Center for Advancing Translational Science of the National Institute of Health (NIH) under Award Number UL1TR000445 and NIH 5P01 HL56693 and is solely the responsibility of the authors and does not necessarily represent official views of the NIH. All authors had access to all of the data in the study and take responsibility for the integrity and the accuracy of the data analysis.
Footnotes
For all authors: There is no conflict of interest.
Disclosures. None of the authors have any conflict of interest regarding this study.
All authors have seen and approved the paper. Authors takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
REFERENCES
- 1.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996 Dec 26;335(26):1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999 Dec 16;341(25):1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002 Mar 21;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 4.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998 Feb 14;351(9101):478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 5.Bloomfield DM, Steinman RC, Namerow PB, et al. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004 Oct 5;110(14):1885–1889. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 6.Gold MR, Bloomfield DM, Anderson KP, et al. A comparison of T-wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol. 2000 Dec;36(7):2247–2253. doi: 10.1016/s0735-1097(00)01017-2. [DOI] [PubMed] [Google Scholar]
- 7.Exner DV, Kavanagh KM, Slawnych MP, et al. Noninvasive risk assessment early after a myocardial infarction the REFINE study. J Am Coll Cardiol. 2007 Dec 11;50(24):2275–2284. doi: 10.1016/j.jacc.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 8.Pezawas T, Stix G, Kastner J, Schneider B, Wolzt M, Schmidinger H. Ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy: clinical presentation, risk stratification and results of long-term follow-up. Int J Cardiol. 2006 Mar 8;107(3):360–368. doi: 10.1016/j.ijcard.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 9.Pezawas T, Stix G, Kastner J, et al. Unexplained syncope in patients with structural heart disease and no documented ventricular arrhythmias: value of electrophysiologically guided implantable cardioverter defibrillator therapy. Europace. 2003 Jul;5(3):305–312. doi: 10.1016/s1099-5129(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 10.Gratze G, Fortin J, Holler A, et al. A software package for non-invasive, real-time beat-to-beat monitoring of stroke volume, blood pressure, total peripheral resistance and for assessment of autonomic function. Comput Biol Med. 1998 Mar;28(2):121–142. doi: 10.1016/s0010-4825(98)00005-5. [DOI] [PubMed] [Google Scholar]
- 11.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996 Mar;17(3):354–381. [PubMed] [Google Scholar]
- 12.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982 Mar;65(3):457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 13.Pezawas T, Diedrich A, Winker R, et al. Multiple autonomic and repolarization investigation of sudden cardiac death in dilated cardiomyopathy and controls. Circ Arrhythm Electrophysiol. 2014 Dec;7(6):1101–1108. doi: 10.1161/CIRCEP.114.001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2013 Mar 26;61(12):1318–1368. doi: 10.1016/j.jacc.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Bigger JT, Jr, Fleiss JL, Kleiger R, Miller JP, Rolnitzky LM. The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation. 1984 Feb;69(2):250–258. doi: 10.1161/01.cir.69.2.250. [DOI] [PubMed] [Google Scholar]
- 16.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005 Jan 20;352(3):225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 17.Huikuri HV, Raatikainen MJ, Moerch-Joergensen R, et al. Prediction of fatal or near-fatal cardiac arrhythmia events in patients with depressed left ventricular function after an acute myocardial infarction. Eur Heart J. 2009 Mar;30(6):689–698. doi: 10.1093/eurheartj/ehn537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huikuri HV, Tapanainen JM, Lindgren K, et al. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2003 Aug 20;42(4):652–658. doi: 10.1016/s0735-1097(03)00783-6. [DOI] [PubMed] [Google Scholar]