Abstract
Introduction
To evaluate the impact of intermittent portal clamping (IPC) on long-term postoperative outcomes in patients with hepatocellular carcinoma (HCC).
Methods
Clinical records of 355 patients underwent curative liver resection for HCC in January 2007 to December 2010 were retrospectively reviewed. According to how portal clamping was performed, patients were grouped as: IPC, n=113; other portal clamping (OPC), n=190; and no portal clamping (NPC), n=52.
Results
Median recurrence-free survival (RFS) was statistically significantly shorter in the IPC (39.4 months) than OPC (47.3 months, p=0.010) and NPC groups (51.4 months, p=0.008). Median overall survival (OS) was also significantly shorter with IPC (46.3 months), versus 52.9 months with OPC (p=0.022) and 56.2 months with NPC (p=0.015). Kaplan–Meier survival analysis revealed that 5-year cumulative RFS was much lower in the IPC (42.5%) than OPC (50.9%, p=0.014) and NPC groups (49.6%, p=0.013). Five-year cumulative OS was also much lower in the IPC (44.9%) than OPC (58.0%, p=0.020) and NPC groups (57.7%, p=0.025). On univariate analysis, tumour grade, size and number, TNM stage, blood transfusion, vascular invasion and IPC were significantly inversely correlated with RFS and OS. On multivariate analysis, tumour size and number, blood transfusion, vascular invasion and IPC remained significant.
Conclusions
Our study suggests that IPC is an independent risk factor for poor long-term postoperative outcomes in patients with HCC.
Keywords: Hepatocellular carcinoma, Hepatectomy, Survival analysis
Liver resection remains the curative therapy of choice for hepatocellular carcinoma (HCC).1 However, the effective control of intraoperative bleeding is crucial to completing the operation and reducing postoperative morbidity and mortality.2 Portal inflow occlusion (the Pringle manoeuvre) is the primary approach for reducing operative haemorrhage,3 and intermittent portal clamping (IPC), a modification of the Pringle manoeuvre, is frequently applied in liver surgery.4 However, it is unclear whether or not patients undergoing IPC have favorable long-term outcomes. It is has been reported that, in comparison with other clamping techniques, IPC maneuver may come with some pitfalls,5 such as more intraoperative blood loss, prolonged duration of operation and excessive surgical manipulation.
To address this issue, we reviewed the clinical records of a large cohort of patients who underwent curative liver resection, with the aim of evaluating the impact of IPC on long-term postoperative outcomes in patients with HCC.
Methods
Three hundred and fifty five patients who underwent curative hepatectomy for HCC at the Union Hospital, Tongji Medical College, Huazhong Science and Technology University (Wuhan, China) or at the Third Xiangya Hospital of Central South University (Changsha, China) between January 2007 and December 2010 were included. Two hundred and thirteen patients were male and 142 female. Their mean age was 48.5±10.3 years (range 18–79 years). Written consent was obtained for all patients and the study was approved by the relevant institutional review boards.
Liver resection was carried out using Pean fracture or finger fracture approach, or the Cavitron Ultrasonic Surgical Aspirator (CUSA) technique. A minor hepatectomy was defined as resection of less than three segments of liver, while a major hepatectomy required resection of at least three segments. IPC was performed in 113 patients, which consisted of occlusion of the portal flow via clamping for 15 minutes followed by unclamping for 5 minutes, with the cycle repeated 2–3 times whenever necessary. A further 190 patients received other portal clamping (OPC), consisting of the continuous Pringle manoeuvre, preconditioning portal clamping or selective portal clamping, and 52 patients had the CUSA technique and thus no portal clamping (NPC). The choice over clamping was left to surgeon preference.
Following surgery, patients were followed-up with serum alpha-fetoprotein (AFP) measurements and imaging, comprising ultrasound, computed tomography and magnetic resonance imaging, every 2 months in the first year and every 3–4 months thereafter. Recurrence was defined as rebound AFP levels and the appearance of a new lesion on imaging. Recurrence-free survival (RFS) was defined as the interval between surgery and the date of diagnosis of first recurrence. Overall survival (OS) was defined as the interval between surgery and the date of death or last follow-up.
Statistical analysis
Data are presented as mean (standard deviation), median (range) and percentage. Demographic and clinical factors were assessed on univariate analysis, with between-group differences analyzed using Pearson's chi-squared test. For factors on univariate analysis with a p value of <0.05, multivariate analysis was performed to identify those predicting postoperative survival. Survival was compared between the groups using Kaplan–Meier analysis. Statistical analyses were performed using SPSS Statistics, version 19.0 (IBM, Armonk, New York, USA). p<0.05 was considered statistically significant.
Results
As shown in Table 1, demographic and clinicopathological features were comparable between the groups. Mean follow-up was 49.7 months (range 6–66 months), and 328 patients had a minimum follow-up of 6 months. Seventeen patients were lost to follow-up within 1–5 months (six in the IPC group, nine in the OPC group and two in the NPC group), and 10 patients died within 3 months of surgery (three IPC patients, six OPC patients and one NPC patient).
Table 1.
Demographic and clinicopathologic factors
| Variables |
IPC (N=113) |
OPC (N=190) |
NPC (N=52) |
P Value |
| Age (mean, year) | 51.7 | 53.6 | 55.0 | 0.665 |
| Sex: Male (male/female) | 67.3 (76/37) | 68.4 (130/60) | 71.1 (37/15) | 0.750 |
| Child-Pugh score: A (A/B) | 65.5 (74/39) | 64.7 (123/67) | 67.3 (35/17) | 0.700 |
| TNM Stage: I-II (I–II/III) | 61.9 (70/43) | 67.9 (129/61) | 69.2 (36/16) | 0.613 |
| Differentiation: H-M (H-M/L-U) | 69.0 (78/35) | 63.2 (120/70) | 61.5 (32/20) | 0.486 |
| Tumour size ≤5cm (≤5cm/>5cm) | 35.4 (40/73) | 40.0 (76/114) | 42.3 (22/30) | 0.575 |
| Tumour number <2 (<2/≥2) | 60.2 (67/46) | 61.6 (117/73) | 65.4 (34/18) | 0.379 |
| Hepatectomy Minor (Minor/Major) | 37.2 (42/71) | 42.1 (80/110) | 48.1 (25/27) | 0.323 |
| Virolgy HBV/HCV(+) [HBV-HCV(+)/(-)] | 81.4 (92/21) | 78.9 (150/40) | 82.7 (43/9) | 0.478 |
All values % (n/n), unless otherwise stated
HBV = hepatitis B virus; HCV = hepatitis C virus; H-M = high-moderate; IPC = intermittent portal clamping; L-U = low-undifferentiated; NPC = no portal clamping; OPC = other portal clamping
The overall perioperative mortality rate was 2.8% (10/355). Among the 328 patients follow-up for a minimum of 6 months, 146 had recurrence (53/104 IPC patients, 75/174 OPC patients and 12/49 NPC patients), and 120 died of cancer-related causes (48 IPC, 65 OPC and 14 NPC patients). Median RFS and OS in the IPC group was 39.4 months and 46.3 months, respectively, which was significantly shorter than that seen in the OPC group (47.3 months, p=0.003, and 52.9 months, p=0.001, respectively) in the OPC group (51.4 months, p=0.009, and 56.2 months, p=0.002, respectively).
Five-year cumulative RFS was 42.5% in the IPC group, which was significantly lower than that among OPC patients (50.9%, p=0.025) and NPC patients (49.6%, p=0.020) (Figure 1). Five-year cumulative OS was also significantly shorter in the IPC than in the OPC and NPC groups, at 44.9% versus 58.0% (p=0.014) and 57.7% (p=0.012), respectively (Figure 2).
Figure 1.
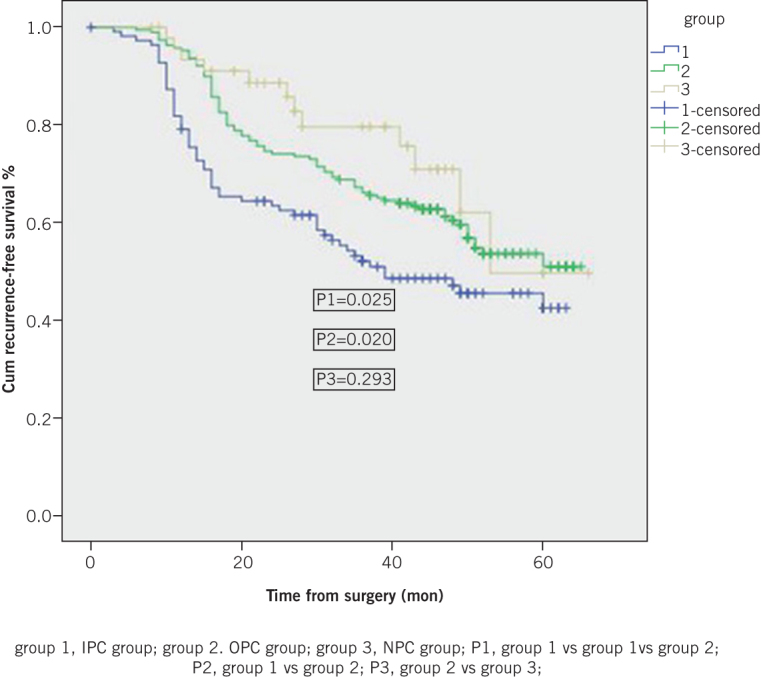
Five-year cumulative recurrence-free survival
Figure 2.
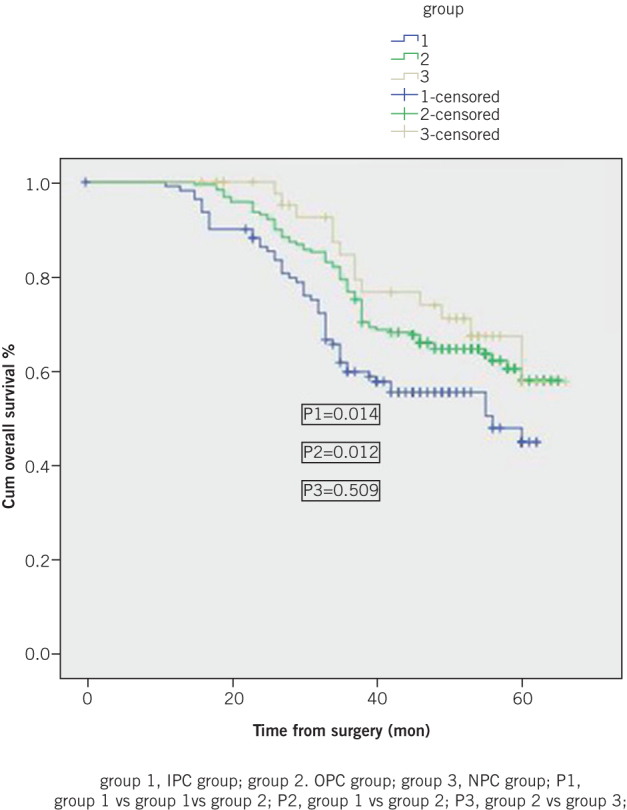
Five-year cumulative overall survival
Thirteen clinicopathological factors were evaluated on univariate and multivariate analysis to assess their correlations with RFS and OS. On univariate analysis, tumour grade (low/undifferentiated), tumour size (>5 cm), tumour number (≥2), TNM stage (III), blood transfusion, vascular invasion, liver cirrhosis, hepatitis B virus (HBV) replication and IPC were significantly negatively correlated with RFS. Tumour size (>5 cm), tumour number (≥2), liver cirrhosis, HBV replication, blood transfusion, vascular invasion and IPC remained significant on multivariate analysis (Table 2).
Table 2.
Factors associated with recurrence-free survival
| Factor | Univariate | Multivariate | ||||
| 95% CI | RR | P | 95% CI | RR | P | |
| Sex (M vs F) | 0.46, 1.45 | 0.81 | 0.55 | |||
| Age (≤60 vs >60) | 0.92,1.93 | 0.97 | 0.232 | |||
| Child-Pugh score (A vs B) | 0.68, 1.35 | 0.79 | 0.361 | |||
| TNM stage (I, II vs III) | 0.98,1.12 | 1 | 0.023 | |||
| Differentiation (H-M vs L-U) | 1.77, 4.48 | 2.6 | 0.017 | |||
| Tumour number (1 vs ≥2) | 1.86, 5.15 | 2.83 | 0.001 | 2.99, 6.33 | 4.35 | 0 |
| Tumour diameter (≤5 vs >5 cm) | 1.50, 3.90 | 2.55 | 0.01 | 2.21,4.88 | 3.66 | 0.001 |
| Vascular invasion (Yes vs No) | 1.28, 3.87 | 2.63 | 0.014 | 2.75, 5.56 | 3.86 | 0 |
| Duration of occlusion (≤45 vs >45 minutes) | 0.95, 1.66 | 1.05 | 0.26 | |||
| Liver cirrhosis (Yes vs No) | 1.90, 3.92 | 2.85 | 0.001 | 3.02,5.85 | 3.88 | 0.001 |
| HBV replication (Yes vs No) | 1.88, 4.02 | 3 | 0.001 | 2.95, 6.15 | 3.5 | 0.001 |
| Blood transfusion (Yes vs No) | 1.10, 2.48 | 1.76 | 0.022 | 2.34, 3.89 | 3.351 | 0.001 |
| Portal clamp modality (IP vs OP) | 1.47, 2.94 | 2.05 | 0.013 | 2.25, 3.69 | 2.936 | 0.001 |
CI = confidence interval; H-M = high-moderate; HBV = hepatitis B virus; IP = intermittent portal; L-U = low-undifferentiated; OP = other portal; RFS = recurrence-free survival rate; RR = risk ratio.
There were similar results on univariate analysis for factors associated with OS, with tumour grade (low/undifferentiated), size (>5 cm) and number (≥2), and TNM stage (III), blood transfusion, vascular invasion and IPC all significantly negatively correlated with OS. However, multivariate analysis demonstrated that liver cirrhosis, HBV replication, TNM stage and tumour grade were no longer associated with OS, while tumour size/number, vascular invasion, blood transfusion and IPC remained significant (Table 3).
Table 3.
Factors for overall survival
| Factor | Univariable | Multivariable | ||||
| 95%CI | RR | P | 95%CI | RR | P | |
| Sex (M vs F) | 0.46, 1.45 | 0.81 | 0.55 | |||
| Age (≤60 vs >60) | 0.92,1.93 | 0.97 | 0.232 | |||
| Child-Pugh score (A vs B) | 0.68, 1.35 | 0.79 | 0.361 | |||
| TNM stage (I, II vs III) | 0.98,1.12 | 1 | 0.023 | |||
| Differentiation (H-M vs L-M) | 1.77, 4.48 | 2.6 | 0.017 | |||
| Tumour number (1 vs ≥2) | 1.86, 5.15 | 2.83 | 0.001 | 2.99, 6.33 | 4.35 | 0 |
| Tumour diameter (≤5 vs >5 cm) | 1.50, 3.90 | 2.55 | 0.01 | 2.21,4.88 | 3.66 | 0.001 |
| Vascular invasion (Yes vs No) | 1.28, 3.87 | 2.63 | 0.014 | 2.75, 5.56 | 3.862 | 0 |
| Duration of occlusion (≤45 vs >45 minutes) | 0.95, 1.66 | 1.05 | 0.26 | |||
| Liver cirrhosis (Yes vs No) | 1.90, 3.92 | 2.85 | 0.001 | |||
| HBV replication (Yes vs No) | 1.88, 4.02 | 3 | 0.001 | |||
| Blood transfusion (Yes vs No) | 1.10, 2.48 | 1.76 | 0.022 | 2.34, 3.89 | 3.351 | 0.001 |
| Portal clamp modality (IP vs OP) | 1.47, 2.94 | 2.05 | 0.013 | 2.25, 3.69 | 2.936 | 0.001 |
CI = confidence interval; H-M = high-moderate; HBV = hepatitis B virus; IP = intermittent portal; L-U = low-undifferentiated; OP = other portal; OS = overall survival rate; RR = risk ratio.
Discussion
HCC is the sixth most common malignancy and the third most common cause of cancer-related death worldwide.6 Overall 5-year survival is typically less than 40–60%.7,8 A number of factors affect the survival of HCC patients after surgery, and these can be classified as:9 tumour-related, including the size and number of tumours, HCC grade, macroscopic or microscopic vascular invasion and TNM stage; patient-related, such as liver cirrhosis, HBV/hepatitis infection and impaired liver reserve; and surgery-related, including intraoperative blood loss, blood transfusion and frequent manipulations.
In this cohort of patients, we found that the median RFS and OS was significantly shorter in IPC patients than in the OPC group following liver resection. Moreover, Kaplan–Meier analysis revealed that 5-year cumulative RFS and OS were much lower in IPC than in OPC patients. Our results indicated that, when compared with OPC, IPC patients experience earlier recurrence and more tumour-related deaths, suggesting that IPC may have promoted tumour recurrence and progression.
Tumour characteristics are highly associated with HCC prognosis,10–13 with tumour size, number and differentiation and vascular invasion reported as crucial risk factors for a poor long-term postoperative outcome. In the current study, we found that tumour size, tumour number, and vascular invasion were associated high relative risks in terms of both RFS and OS (all p<0.001).
The evidence for the impact of surgery-related factors on postoperative survival among HCC patients is not quite as definitive. Some authors found that surgery-associated factors such as intraoperative blood loss, operative complications, operative time and the duration of portal occlusion were associated with poor postoperative survival,14,15 whereas others did not.16,17 Nonetheless, blood transfusion is usually considered as a predictor of poor prognosis in HCC patients,18,19 and our study supported the notion that blood transfusion can have an adverse impact on outcomes.
More importantly, the present study revealed that IPC was an independent risk factor associated with poor RFS and OS among HCC patients. The IPC manoeuvre is virtually an ischemia reperfusion injury process.20 Evidence from the literature suggests that liver ischemic reperfusion-injury induced by portal clamping plays a critical role in tumour recurrence and progression in damaged remnant liver, both in animal models,21–23 and in clinical practice.24,25 This process involves a number of signal pathways and pathological cascades that facilitate tumour cell metastasis and growth.26–28
Taken together, we believe that surgery-related factors can play an important role in postoperative survival in HCC patients. Previous studies have found that hepatectomy manipulations can significant influence the dislodging of cancer cells from the main tumour.29,30 Moreover, pressure gradients between tumour vessels and portal vein affect the spread of tumour cells into portal veins.31 Indeed, excessive manipulation, prolonged operative times, dramatic pressure fluctuations in the tumourinterstitial space with IPC all can facilitate the shedding of cancer cells from main tumour and their spread along portal system to form metastases. Therefore, in addition to the ‘favourable soil’ produced by ischaemic reperfusion injury with portal inflow occlusion, IPC can enhance the spread of ‘viable seeds’, which ultimately increases the probability of HCC recurrence and progression.
Conclusions
To our knowledge, the current study is the first report with a large cohort of patients to demonstrate a significant, adverse impact of IPC on the HCC recurrence and progression. It suggests that IPC is an independent risk factor for poor long-term outcomes in HCC patients undergoing curative hepatectomy. However, a larger scale, randomised, prospective study is required to elucidate the underlying mechanism by which IPC induces poor postoperative survival among HCC patients.
Acknowledgements
We declare no conflicts of interest and no funding.
References
- 1.van Gulik TM, de Graaf W, Dinant S et al. . Vascular occlusion techniques during liver resection. Dig Surg 2007; : 274–281. [DOI] [PubMed] [Google Scholar]
- 2.Jarnagin WR, Gonen M, Fong Y et al. . Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002; : 397–406; discussion 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg 1908; : 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belghiti J, Noun R, Malafosse R et al. . Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg 1999; : 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nuzzo G, Giuliante F, Giovannini I et al. . Hepatic resections in normothermic ischemia. Surgery 1996; : 852–858. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray F, Ferlay J et al. . Global cancer statistics, 2002. CA Cancer J Clin 2005; : 74–108. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Sun RL, Jarnagin W et al. . An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999; : 790–9; discussion 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CH, Chau GY, Lui WY et al. . Long-term results of hepatic resection for hepatocellular carcinoma originating from the noncirrhotic liver. Arch Surg 2004; : 320–5; discussion 326. [DOI] [PubMed] [Google Scholar]
- 9.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000; : 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah SA, Greig PD, Gallinger S et al. . Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg 2006; : 275–283. [DOI] [PubMed] [Google Scholar]
- 11.Regimbeau JM, Abdalla EK, Vauthey JN et al. . Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. J Surg Oncol 2004; : 36–41. [DOI] [PubMed] [Google Scholar]
- 12.Imamura H, Matsuyama Y, Tanaka E et al. . Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003; : 200–207. [DOI] [PubMed] [Google Scholar]
- 13.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int 2009; : 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusano T, Sasaki A, Kai S et al. . Predictors and prognostic significance of operative complications in patients with hepatocellular carcinoma who underwent hepatic resection. Eur J Surg Oncol 2009; : 1,179–1,185. [DOI] [PubMed] [Google Scholar]
- 15.Hanazaki K, Kajikawa S, Shimozawa N et al. . Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg 2000; : 381–388. [DOI] [PubMed] [Google Scholar]
- 16.Bruns H, Krätschmer K, Hinz U et al. . Quality of life after curative liver resection: a single center analysis. World J Gastroenterol 2010; : 2,388–2,395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei Z, Chang L, Fan-Di M et al. . Exploration on surgical-related factors influencing HCC patients prognosis. Hepatogastroenterology 2012; : 1,541–1,543. [DOI] [PubMed] [Google Scholar]
- 18.Katz SC, Shia J, Liau KH et al. . Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 2009; : 617–623. [DOI] [PubMed] [Google Scholar]
- 19.de Boer MT, Molenaar IQ, Porte RJ. Impact of blood loss on outcome after liver resection. Dig Surg 2007; : 259–264. [DOI] [PubMed] [Google Scholar]
- 20.Petrowsky H, McCormack L, Trujillo M et al. . A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg 2006; : 921–8; discussion 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Liu Y, Zhang A et al. . Inhibitory CpG sequences reduced ischemia/reperfusion-induced hepatic metastases of liver tumor in a murine model. J Surg Res 2012; : 248–254. [DOI] [PubMed] [Google Scholar]
- 22.Doi K, Horiuchi T, Uchinami M et al. . Hepatic ischemia-reperfusion promotes liver metastasis of colon cancer. J Surg Res 2002; : 243–247. [DOI] [PubMed] [Google Scholar]
- 23.Nicoud IB, Jones CM, Pierce JM et al. . Warm hepatic ischemia-reperfusion promotes growth of colorectal carcinoma micrometastases in mouse liver via matrix metalloproteinase-9 induction. Cancer Res 2007; : 2,720–2,728. [DOI] [PubMed] [Google Scholar]
- 24.Ishizuka M, Kubota K, Kita J et al. . Duration of hepatic vascular inflow clamping and survival after liver resection for hepatocellular carcinoma. Br J Surg 2011; : 1,284–1,290. [DOI] [PubMed] [Google Scholar]
- 25.Nijkamp MW, van der Bilt JD, Snoeren N et al. . Prolonged portal triad clamping during liver surgery for colorectal liver metastases is associated with decreased time to hepatic tumour recurrence. Eur J Surg Oncol 2010; : 182–188. [DOI] [PubMed] [Google Scholar]
- 26.Man K, Ng KT, Lo CM et al. . Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases--activation of cell invasion and migration pathways. Liver Transpl 2007; : 1,669–1,677. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Fu SY, Lau WY et al. . Selective main portal vein clamping to minimize the risk of recurrence after curative liver resection for hepatocellular carcinoma. Hepatogastroenterology 2012; : 1,560–1,565. [DOI] [PubMed] [Google Scholar]
- 28.Knudsen AR, Kannerup AS, Dich R et al. . Expression of genes involved in rat liver angiogenesis after ischaemia and reperfusion: effects of ischaemic pre- and post-conditioning. HPB (Oxford) 2010; : 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamanaka N, Okamoto E, Fujihara S et al. . Do the tumor cells of hepatocellular carcinomas dislodge into the portal venous stream during hepatic resection. Cancer 1992; : 2,263–2,267. [DOI] [PubMed] [Google Scholar]
- 30.Matsumata T, Kanematsu T, Takenaka K et al. . Lack of intrahepatic recurrence of hepatocellular carcinoma by temporary portal venous embolization with starch microspheres. Surgery 1989; : 188–191. [PubMed] [Google Scholar]
- 31.Tanaka T, Yamanaka N, Oriyama T et al. . Factors regulating tumor pressure in hepatocellular carcinoma and implications for tumor spread. Hepatology 1997; : 283–287. [DOI] [PubMed] [Google Scholar]


