Abstract
INTRODUCTION
Benign and malignant primary retroperitoneal tumours (PRT) have different clinical manifestations and pathological characteristics. We explore the characteristics and evaluate the value of computed tomography (CT) for PRT.
METHODS
Fifty-six benign and fifty-seven malignant PRT with clinical, radiographic and surgical data were retrospectively investigated. Statistical analysis was carried out using SPSS software.
RESULTS
We found that mean size of benign tumours was 12.4 ± 7.7cm but 20.7 ± 11.6cm in malignant cases (P < 0.05). The accuracy of CT localisation was approximately 70%. The sensitivity and specificity of CT diagnosis for benign tumours were 96% and 85%, respectively; 51 (91%) underwent complete resection among benign cases but 42 (74%) in malignant cases (P < 0.05). Sensitivity of CT evaluation for invaded organs and vessels was 50% and 65%, respectively, and specificity was 88% and 81%, respectively.
CONCLUSIONS
Large size, no capsule, irregular margin, heterogeneous density and specific enhancement pattern on CT are highly suggestive of malignancy. CT is the most useful tool in assessing the size and characteristics of PRT but it does not have the sensitivity to assess the involvement of organs and vessels.
Keywords: Primary retroperitoneal tumour, Characteristics, Computed tomography
Introduction
Primary retroperitoneal tumours (PRT) are divided into benign and malignant neoplasms that arise within the retroperitoneal space. They account for 0.2–0.6% of all neoplasms.1 PRT originate from mesenchymal, neurogenous or embryonic tissue in the retroperitoneal space, excluding tumours in retroperitoneal organs such as kidney, adrenal, pancreas and metastatic.2 They often do not come to clinical attention until they have reached a considerable size with involvement of adjacent vital structures. Consequently, surgical resection of these tumours is often challenging, which affects local recurrence, postoperative outcomes and long-term survival rates.3,4
Computed tomography (CT) is the most useful tool in the evaluation of retroperitoneal tumours. It cannot only assess the tumour’s location and its relationship to adjacent organs, but also identify metastatic lesions in peritoneal cavity.5 We relied primarily on CT scan in the evaluation of PRT from 113 patients in our department. The aim of this retrospective study was to identify the characteristics of benign and malignant PRT through clinical manifestations, CT findings and postoperative pathologic specimens. By understanding the characteristics of PRT we can conduct better preoperative evaluation to obtain the most appropriate therapy plans for different PRT.
Materials and Methods
Patients
From January 2005 to December 2014, 113 patients with PRT (56 benign and 57 malignant) were treated in Tongji Hospital. We retrospectively investigated their data: sex, age, clinical manifestations, histopathological diagnosis, CT findings (image of localisation, diagnosis, density, enhancement and relation to adjacent structures) and surgical data (surgical approach, resected organs, adjacent structures and tumour profile). All patients underwent CT diagnosis and received surgical therapy and pathological examination. We compared the data of benign and malignant tumours.
Statistical analysis
Statistical analysis was carried out by SPSS software (SPSS, Chicago, IL, USA). Descriptive statistics were represented as the mean ± standard deviation (SD). Chi-square or Fisher’s exact test was adopted to analyse categorical variables. The t-test was used to analyse continuous variables with a normal distribution and a P value of less than 0.05 was considered significant.
Results
There were 46 males and 67 females patients with PRT. Mean age was 47 years (range 13–92 years). Among benign cases, the mean size (maximal diameter) was 12.4 ± 7.7cm (range 3–45cm) but 20.7 ± 11.6cm (range 5–55cm) in malignant cases. Thus, the size of malignant tumours is significantly larger than that of the benign (P < 0.05).
Clinical symptoms of most patients frequently appeared with the expansion of the tumour and its invasion into the adjacent organs in a restricted space. Some asymptomatic patients did not learn of the abdominal mass until taking physical examinations. The clinical presentation of 56 benign and 57 malignant tumours is shown in Figure 1. The main clinical manifestation of benign and malignant PRT was abdominal mass and abdominal distension, respectively.
Figure 1.

Distribution of clinical manifestations of benign and malignant cases; A: the main clinical manifestation of 56 benign primary retroperitoneal tumours (PRT) was abdominal mass (49%); B: the main clinical manifestation of 57 malignant PRT was abdominal distension (31%)
Most primary retroperitoneal tumours originate from mesenchymal tissue, neurogenous tissue and embryonic tissue. Postsurgical pathological findings are summarised in Table 1. Pathology confirmed that 50% of tumours were benign. A majority of benign tumours derived from mesenchymal tissue and neurogenous tissue, while malignant tumours mostly originated from mesenchymal tissue.
Table 1.
Pathological findings of primary retroperitoneal tumours
| Finding | Patients (n) |
|---|---|
| Benign: | 56 |
| Lymphangioma | 11 |
| Leiomyoma | 6 |
| Lipoma | 3 |
| Paraganglioma | 9 |
| Benign teratoma | 6 |
| Ganglioneuroma | 6 |
| Neurilemmoma | 11 |
| Neurofibroma | 3 |
| Telangiectatic fibroma | 1 |
| Malignant: | 57 |
| Liposarcoma | 34 |
| Leiomyosarcoma | 6 |
| Rhabdomyosarcoma | 1 |
| Mucinous cystadenoma | 1 |
| Malignant mesothelioma | 1 |
| Malignant mesenchyma | 1 |
| Melanoma | 1 |
| Synovial sarcoma | 1 |
| Aggressive angiomyxoma | 1 |
| Neurblastoma | 2 |
| Spindle cell sarcoma | 2 |
| Clear cell sarcoma | 1 |
| Undifferentiated carcinoma | 2 |
| Fibrosarcoma | 2 |
| Primitive neurotodermal tumour | 1 |
Although CT can demonstrate important characteristics of PRT, diagnosis is often challenging for radiologists. Diagnostic challenges include the precise localisation of the lesion, the extent of invasion and the characteristics of these tumours.6 The first step is to determine whether the tumour is located within the retroperitoneal space, then whether it is benign or malignant. Finally, the extent of the tumours and their characteristics such as enhancement and density are described. CT findings are listed in Table 2. The accuracy of CT localisation (approximately 70%) was not significantly different between benign and malignant tumours (P > 0.05). However, malignant tumours were significantly more enhanced and heterogeneous on CT than benign tumours (P < 0.05). The sensitivity and specificity of CT diagnosis for benign tumours were 96% and 85%, respectively (Table 3).
Table 2.
Computed tomography findings of benign and malignant primary retroperitoneal tumours
| CT Findings | Benign | Malignant | χ2 | P value |
|---|---|---|---|---|
| Right localisation | 36 | 31 | 0.177 | 0.674 |
| False localisation | 14 | 15 | ||
| Homogeneous density | 21 | 3 | 16.827 | 0.000 |
| Heterogeneous density | 17 | 30 | ||
| Enhancement | 17 | 25 | 8.538 | 0.003 |
| No enhancement | 18 | 5 |
Table 3.
Correlation between computed tomography and pathological diagnosis (sensitivity 96%; specificity 85%)
| Computed tomography | Pathology | Total | |
|---|---|---|---|
| Benign | Malignant | ||
| Benign | 45 | 7 | 52 |
| Malignant | 2 | 39 | 41 |
| Total | 47 | 46 | 93 |
Surgical resection is the most effective method for the treatment of PRT. In our study, 51 patients (91%) with benign tumours underwent complete resection and 42 (74%) in malignant cases (Table 4). The complete resection rate for benign tumours was significantly higher than malignant tumours (P < 0.05). There were 14 cases with combined organ resection among PRT. The resected organs included the colon (the majority), followed by kidney, spleen, pancreas, uterus and psoas major. The rate of organ resection was significantly higher in malignant cases (P < 0.05). In addition, we found that benign tumours were more inclined to have capsule and regular surface relative to the malignant (P < 0.05).
Table 4.
Surgical data for benign and malignant cases
| Surgical data | Benign | Malignant | χ2 | P value |
|---|---|---|---|---|
| Resection: | ||||
| Complete | 51 | 42 | 5.441 | 0.020 |
| Incomplete | 5 | 15 | ||
| Combined organ | 2 | 12 | 8.207 | 0.004 |
| No combined organ | 52 | 40 | ||
| Capsule: | ||||
| (positive) | 50 | 26 | 13.647 | 0.000 |
| (negative) | 4 | 17 | ||
| Surface: | ||||
| Regular | 40 | 11 | 22.457 | 0.000 |
| Irregular | 13 | 32 |
Finally, we compared the tumour’s relation to adjacent structures between surgical observation and presurgical CT findings (Table 5). The sensitivity and specificity of CT diagnosis for invaded organs was 50% and 88%, respectively, relatively low as compared with magnetic resonance imaging (MRI) diagnosis. In addition, the sensitivity and specificity of CT diagnosis for invaded vessels was 65% and 81%, respectively, relatively low in comparison with CT angiography (CTA) diagnosis.
Table 5.
Comparison of the relation of adjacent structures between surgical observation and computed tomography findings
| Surgical observation (n) | Total (n) | ||
|---|---|---|---|
| Benign | Malignant | ||
| CT (organ):a | |||
| Benign | 15 | 6 | 21 |
| Malignant | 15 | 45 | 60 |
| Total | 30 | 51 | 81 |
| MRI (organ):b | |||
| Benign | 6 | 1 | 7 |
| Malignant | 2 | 13 | 15 |
| Total | 8 | 14 | 22 |
| CT (vessel):c | |||
| Benign | 13 | 11 | 24 |
| Malignant | 7 | 46 | 53 |
| Total | 20 | 57 | 77 |
| CT angiogram (vessel):d | |||
| Benign | 14 | 1 | 15 |
| Malignant | 0 | 5 | 5 |
| Total | 14 | 6 | 20 |
a Sensitivity 50%, specificity 88%
b Sensitivity 75%, specificity 93%
c Sensitivity 65%, specificity 81%
d Sensitivity 100%, specificity 83%
Discussion
Primary retroperitoneal tumours comprise a rare and diverse group of neoplasms. Characterising this diverse group of PRT is difficult, owing to the relative rarity of each subtype.7 PRT are malignant in 85% of cases; of these, nearly 50% are sarcomas.8 In our study, a majority of benign PRT originated from neurogenous tissue, while liposarcomas comprised the majority of malignant PRT. The main clinical manifestation of benign and malignant PRT was abdominal mass and abdominal distension, respectively. When symptoms are present, they relate to the extension of tumour or local invasion. Sepideh Gholami et al reported that the duration of symptoms was inversely proportional to the prognosis.9 This meant the patients who had symptoms for longer periods did worse and had a shorter recurrence-free survival time.
Complete surgical resection is the most effective therapy for initial and recurrent retroperitoneal tumours, even if adjacent normal organs need to be removed.10 Combined organ resection is required in the majority of cases, most frequently involving the kidney, colon, small bowel and bladder.11 Sufficient preoperative preparation is therefore the key to successful surgery. CT can clearly demonstrate the characteristics of the mass, the localisation of tumours and the relation to adjacent organs and vessels. It is also used to detect pulmonary, bone or hepatic metastases and local recurrence in patients who have already been resected,12 which is helpful in making precise surgical plans.
In our experience, the accuracy of CT localisation is approximately 70%. The sensitivity and specificity of CT diagnosis for benign tumours are more than 85%. It is not satisfactory that the sensitivity of CT findings for invaded organs or vessels is less than 65%. When the tumour is near a vital structure but routine CT does not show their relationship, MRI or CT angiography presents a greater advantage. Nakashima et al reported some promising clinical and radiological criteria, including the tumour size, symptom, margin and calcification for the differential diagnosis between benign and malignant retroperitoneal tumours.13 One study demonstrates that the irregularity of the margins and the specific enhancement pattern on dynamic MRI may be important predictive factors of malignant retroperitoneal tumours.14 Another study reported that benign retroperitoneal tumours are usually regular, smaller, with clear margins and a less solid area, they are slower growing, rarely with necrosis and haemorrhage, no metastasis and are rarely recurrent after resection.15 In our study, the large size, no capsule, irregular margin, heterogeneous density and specific enhancement pattern on CT are highly suggestive of malignancy. Figures 2 and 3 show the characteristics of the benign and malignant tumours, respectively.
Figure 2.
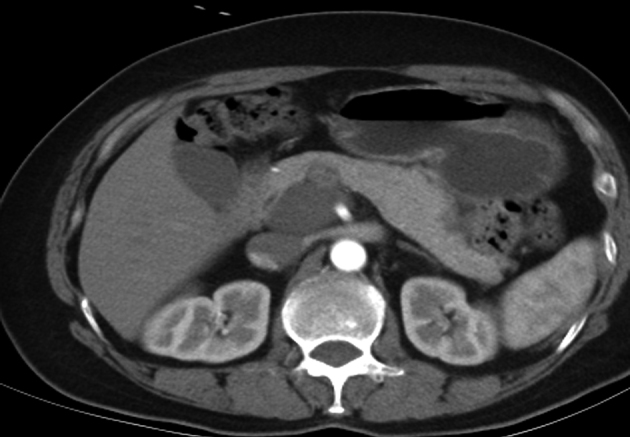
Characteristics of the benign retroperitoneal tumour; computed tomography shows ganglioneuroma in postcaval region, with regular margin, homogeneous density and no enhancement
Figure 3.
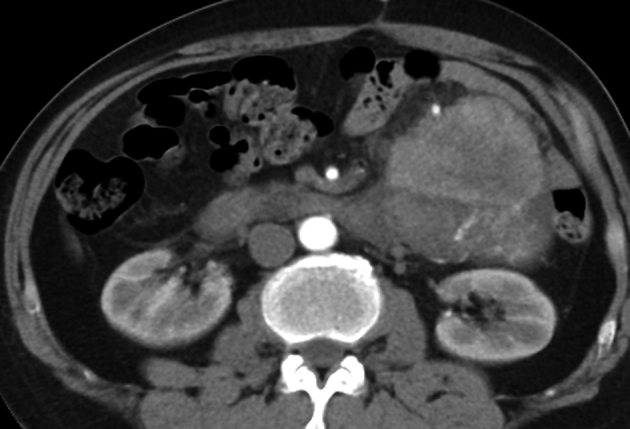
Characteristics of the malignant retroperitoneal tumour; computed tomography shows liposarcoma in left lower abdomen, with irregular margin, heterogeneous density and minor enhancement
Although CT findings of PRT are not specific for the histological diagnosis, a number of CT features may suggest specific diagnosis: (i) the presence of calcification in malignant fibrous histiocytoma; (ii) the presence of fat in a mass lesion of heterogeneous density in liposarcoma; (iii) large regions of necrosis in leiomyosarcoma; (iv) catecholamine excess and para-aortic location in paraganglioma; (v) homogeneous, low density of neurofibroma; (vi) homogeneous, fat density of lipoma; and (vii) characteristic mixed components of teratoma.16
Malignant retroperitoneal tumours usually have a poor prognosis. The 5-year survival rate after surgical resection remains low, ranging from 36% to 63%.17 Prognostic factors for overall survival for retroperitoneal sarcoma include grade, stage, histology, size and margin status.18 In summary, the steps to improve curative effects include: (i) early finding, early diagnosis and early operation; (ii) complete resection; (iii) regular re-examination after operation. Regular follow-up with CT examination to detect recurrence is necessary every 6 months for 3 years.19,20
Conclusions
It is beneficial to discriminate characteristics between benign and malignant PRT for preoperative preparation. Large size, no capsule, irregular margin, heterogeneous density and specific enhancement pattern on CT are highly suggestive of malignancy. CT is the most useful tool in assessing the size and characteristics of PRT but it is not sufficiently sensitive to assess the involvement of organs and vessels.
References
- 1.Kutta A, Engelmann U, Schmidt U et al. Primary retroperitoneal tumors. Urol Int 1992. : 353–357. [PubMed] [Google Scholar]
- 2.Melicow M. Primary tumors of the retroperitoneum: clinicopathologic analysis of 162 cases: review of literature and tables of classification. J Int Coll Surg 1953. : 401–449. [PubMed] [Google Scholar]
- 3.Alvarenga JC, Ball AB, Fisher C, et al. Limitations of surgery in the treatment of retroperitoneal sarcoma. Br J Surg 1991. : 912–916. [DOI] [PubMed] [Google Scholar]
- 4.Scibe R, Massa M, Verdolini R et al. Retroperitoneal tumors. Ann Ital Chir 1999. : 731–736. [PubMed] [Google Scholar]
- 5.Lahat G, Madewell JE, Anaya DA et al. Computed tomography scan-driven selection of treatment for retroperitoneal liposarcoma histologic subtypes. Cancer 2009. :1,081–1,090. [DOI] [PubMed] [Google Scholar]
- 6.Nishino M, Hayakawa K, Minami M et al. Primary retroperitoneal neoplasms: CT and MR imaging findings with anatomic and pathologic diagnostic. Radiographics 2003. : 45–57. [DOI] [PubMed] [Google Scholar]
- 7.Xu YH, Guo KJ, Guo RX et al. Surgical management of 143 patients with adult primary retroperitoneal tumor. World J Gastroenterol 2007. : 2,619–2,621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An JY, Heo JS, Noh JH et al. Primary malignant retroperitoneal tumors: analysis of a single institutional experience. Eur J Surg Oncol 2007. : 376–382. [DOI] [PubMed] [Google Scholar]
- 9.Gholami S, Jacobs CD, Kapp DS et al. The value of surgery for retroperitoneal sarcoma. Sarcoma 2009. : 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonvalot S, Rivoire M, Castaing M et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol 2009. : 31–37. [DOI] [PubMed] [Google Scholar]
- 11.Hassan I, Park SZ, Donohue JH et al. Operative management of primary retroperitoneal sarcomas: a reappraisal of an institutional experience. Ann Surg 2004. : 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrero DR, Moreno PF, Huertas VE et al. Liposarcoma retroperitoneal calcificado. Actas Urol Esp 2004. : 234–237. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima J, Ueno M, Nakamura K et al. Differential diagnosis of primary benign and malignant retroperitoneal tumors. Int J Urol 1997. : 441–446. [DOI] [PubMed] [Google Scholar]
- 14.Tambo M, Fujimoto K, Miyake M et al. Clinicopathological review of 46 primary retroperitoneal tumors. Int J Urol 2007. : 785–788. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Z, Zhao XM, Zhao YF et al. Evaluation of CT findings for the differentiation of benign from malignant primary retroperitoneal tumors. Chin Med J 2014. : 114–119. [PubMed] [Google Scholar]
- 16.Lane RH, Stephens DH, Reiman HM. Primary retroperitoneal neoplasms: CT findings of 90 cases with clinical and pathologic correlation. AJR 1989. : 83–89. [DOI] [PubMed] [Google Scholar]
- 17.Abbott AM, Habermann EB, Parsons HM et al. Prognosis for primary retroperitoneal sarcoma survivors: a conditional survival analysis. Cancer 2012. : 3,321–3,329. [DOI] [PubMed] [Google Scholar]
- 18.Lewis JJ, Leung D, Woodruff JM et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998. : 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez PJA, Martĺn MA, Bonilla PR et al. Linfangioma quistico retroperitoneal. Una patologia silente en adultos. Actas Urol Esp 2002. : 356–360. [DOI] [PubMed] [Google Scholar]
- 20.Arguelles SE, Congregado RCB, Medina LRA et al. Histiocitoma maligno fibroso retroperitoneal. Actas Urol Esp 2004. : 624–626. [DOI] [PubMed] [Google Scholar]


