Abstract
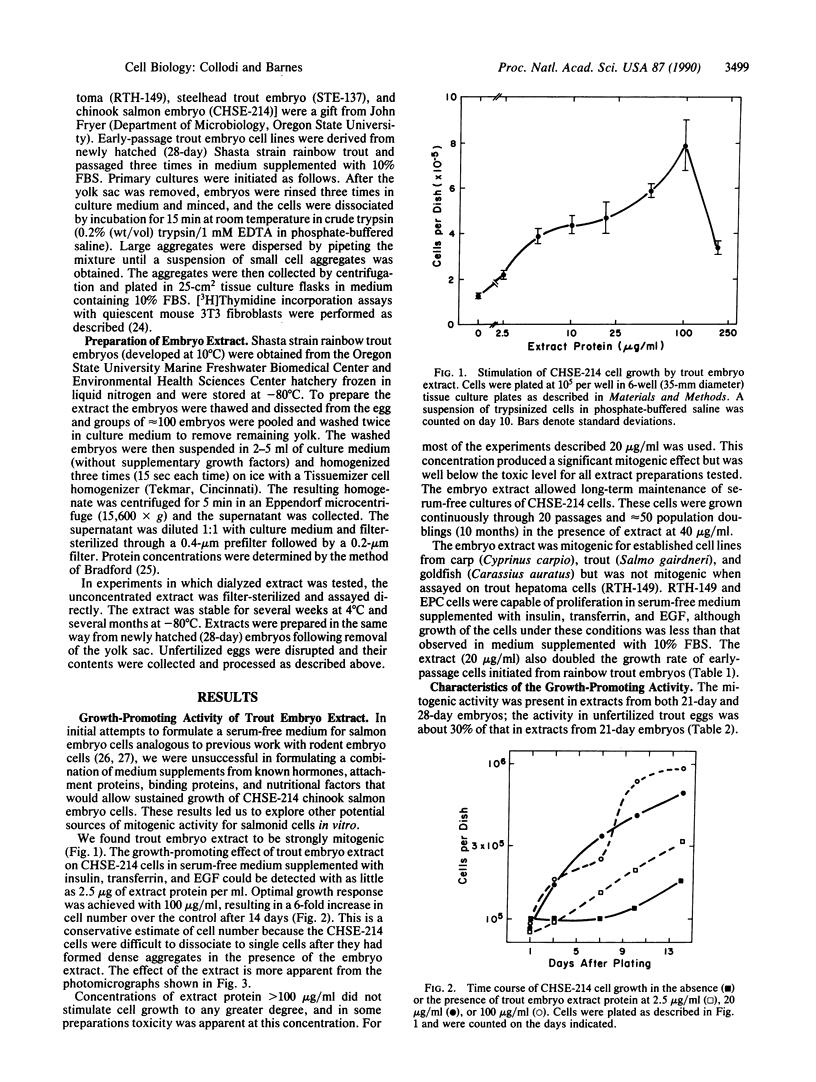
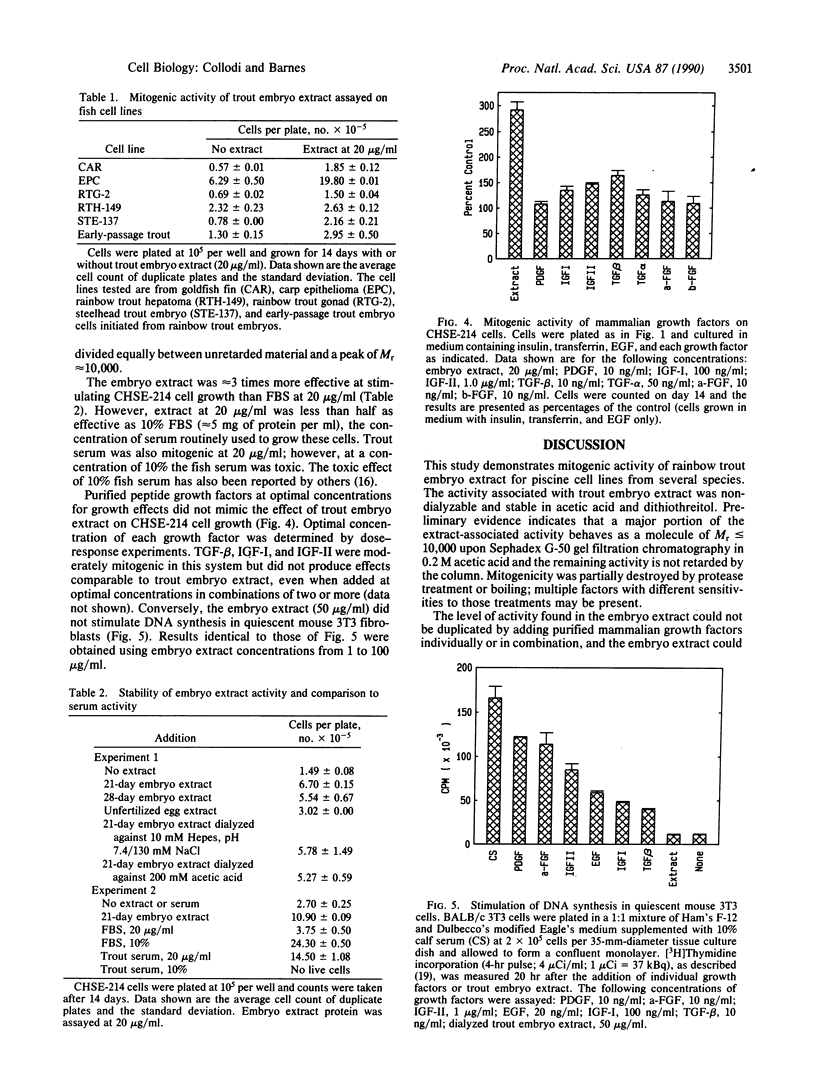
An extract of 21-day rainbow trout embryos stimulated growth of several piscine cell lines in the absence of added serum. Established lines from trout (RTG-2 and STE-137), salmon (CHSE-214), carp (EPC), and goldfish (CAR) and early-passage cells initiated from trout embryos grew in serum-free medium containing the embryo extract. In addition the extract was sufficient for maintaining long-term cultures of CHSE-214 cells for several months through a minimum of 20 passages (approximately 50 population doublings) in the absence of serum. Optimal response was achieved with 100 micrograms of extract protein per ml, but a significant growth-promoting effect was observed with as little as 2.5 micrograms/ml. The activity was nondialyzable, protease-sensitive, and stable in 200 mM acetic acid. The level of mitogenic response induced by the extract could not be duplicated with purified mammalian growth factors added individually or in combination, and the extract did not stimulate DNA synthesis in quiescent mouse fibroblasts. These results suggest that trout embryo extract may contain a novel growth-promoting activity for fish cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders F., Schartl M., Barnekow A. Xiphophorus as an in vivo model for studies on oncogenes. Natl Cancer Inst Monogr. 1984 May;65:97–109. [PubMed] [Google Scholar]
- Barnes D. W., Colowick S. P. Stimulation of sugar uptake and thymidine incorporation in mouse 3T3 cells by calcium phosphate and other extracellular particles. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5593–5597. doi: 10.1073/pnas.74.12.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chiang L. C., Silnutzer J., Pipas J. M., Barnes D. W. Selection of transformed cells in serum-free media. In Vitro Cell Dev Biol. 1985 Dec;21(12):707–712. doi: 10.1007/BF02620926. [DOI] [PubMed] [Google Scholar]
- Fryer J. L., Yusha A., Pilcher K. S. The in vitro cultivation of tissue and cells of Pacific salmon and steelhead trout. Ann N Y Acad Sci. 1965 Aug 10;126(1):566–586. doi: 10.1111/j.1749-6632.1965.tb14303.x. [DOI] [PubMed] [Google Scholar]
- Hendricks J. D., Meyers T. R., Casteel J. L., Nixon J. E., Loveland P. M., Bailey G. S. Rainbow trout embryos: advantages and limitations for carcinogenesis research. Natl Cancer Inst Monogr. 1984 May;65:129–137. [PubMed] [Google Scholar]
- Hendricks J. D., Scanlan R. A., Williams J. L., Sinnhuber R. O., Grieco M. P. Carcinogenicity of N-methyl-N'-nitro-N-nitrosoguanidine to the livers and kidneys of rainbow trout (Salmo gairdneri) exposed as embryos. J Natl Cancer Inst. 1980 Jun;64(6):1511–1519. doi: 10.1093/jnci/64.6.1511. [DOI] [PubMed] [Google Scholar]
- Hightower L. E., Renfro J. L. Recent applications of fish cell culture to biomedical research. J Exp Zool. 1988 Dec;248(3):290–302. doi: 10.1002/jez.1402480307. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Kuwada J. Y. Cell recognition by neuronal growth cones in a simple vertebrate embryo. Science. 1986 Aug 15;233(4765):740–746. doi: 10.1126/science.3738507. [DOI] [PubMed] [Google Scholar]
- Lee L. E., Martinez A., Bols N. C. Culture conditions for arresting and stimulating the proliferation of a rainbow trout fibroblast cell line, RTG-2. In Vitro Cell Dev Biol. 1988 Aug;24(8):795–802. doi: 10.1007/BF02623650. [DOI] [PubMed] [Google Scholar]
- Loo D. T., Fuquay J. I., Rawson C. L., Barnes D. W. Extended culture of mouse embryo cells without senescence: inhibition by serum. Science. 1987 Apr 10;236(4798):200–202. doi: 10.1126/science.3494308. [DOI] [PubMed] [Google Scholar]
- Parsons J. E., Thorgaard G. H. Production of androgenetic diploid rainbow trout. J Hered. 1985 May-Jun;76(3):177–181. doi: 10.1093/oxfordjournals.jhered.a110060. [DOI] [PubMed] [Google Scholar]
- Powers D. A. Fish as model systems. Science. 1989 Oct 20;246(4928):352–358. doi: 10.1126/science.2678474. [DOI] [PubMed] [Google Scholar]
- Rosa F., Roberts A. B., Danielpour D., Dart L. L., Sporn M. B., Dawid I. B. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988 Feb 12;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- Singh J. P., Chaikin M. A., Stiles C. D. Phylogenetic analysis of platelet-derived growth factor by radio-receptor assay. J Cell Biol. 1982 Nov;95(2 Pt 1):667–671. doi: 10.1083/jcb.95.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Streisinger G. Attainment of minimal biological variability and measurements of genotoxicity: production of homozygous diploid zebra fish. Natl Cancer Inst Monogr. 1984 May;65:53–58. [PubMed] [Google Scholar]
- Streisinger G., Coale F., Taggart C., Walker C., Grunwald D. J. Clonal origins of cells in the pigmented retina of the zebrafish eye. Dev Biol. 1989 Jan;131(1):60–69. doi: 10.1016/s0012-1606(89)80038-7. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Walker C., Dower N., Knauber D., Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature. 1981 May 28;291(5813):293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- Wales J. H., Sinnhuber R. O., Hendricks J. D., Nixon J. E., Eisele T. A. Aflatoxin B1 induction of hepatocellular carcinoma in the embryos of rainbow trout (Salmo gairdneri). J Natl Cancer Inst. 1978 May;60(5):1133–1139. doi: 10.1093/jnci/60.5.1133. [DOI] [PubMed] [Google Scholar]
- Wolf K., Mann J. A. Poikilotherm vertebrate cell lines and viruses: a current listing for fishes. In Vitro. 1980 Feb;16(2):168–179. doi: 10.1007/BF02831507. [DOI] [PubMed] [Google Scholar]
- de Pablo F., Chambers S. A., Ota A. Insulin-related molecules and insulin effects in the sea urchin embryo. Dev Biol. 1988 Nov;130(1):304–310. doi: 10.1016/0012-1606(88)90436-8. [DOI] [PubMed] [Google Scholar]




