Abstract
INTRODUCTION
Unplanned conversion to thoracotomy remains a major concern in video assisted thoracoscopic surgery (VATS) lobectomy. This study aimed to investigate the development of a VATS lobectomy programme over a five-year period, with a focus on the causes and consequences of unplanned conversions.
METHODS
A single centre retrospective review was performed of patients who underwent complete anatomical lung resection initiated by VATS between January 2010 and April 2015.
RESULTS
In total, 1,270 patients underwent a lobectomy in the study period and 684 (53.9%) of these were commenced thoracoscopically. There were 75 cases (10.9%) with unplanned conversion. The proportion of lobectomies started as VATS was significantly higher in the second half of the study period (2010–2012: 277/713 [38.8%], 2013–2015: 407/557 [73.1%], p<0.001). The conversion rate dropped initially from 20.4% (11/54) in 2010 to 9.9% (15/151) in 2013 and then remained consistently under 10% until 2015. Conversions were most commonly secondary to vascular injury (26/75, 34.7%). Patients undergoing unplanned conversion had a longer length of stay than VATS completed patients (9 vs 6 days, p<0.001). There was a higher incidence of respiratory failure (10/75 [14.1%] vs 23/607 [3.8%], p<0.001) and 30-day mortality (7/75 [9.3%] vs 6/607 [1.0%], p=0.003) in patients with unplanned conversion than in those with completed VATS.
CONCLUSIONS
As our VATS lobectomy programme developed, the unplanned conversion rate dropped initially and then remained constant at approximately 10%. With increasing unit experience, it is both safe and technically possible to complete the majority of lobectomy procedures thoracoscopically.
Keywords: Video assisted thoracic surgery, Thoracotomy, Pulmonary surgical procedures, Lung neoplasms
Video assisted thoracoscopic surgery (VATS) is associated with equivalent survival and recurrence rates, a shorter length of stay (LOS) and fewer postoperative complications than conventional thoracotomy for anatomical lung resection.1–4 It is advocated as the gold standard resection approach for early stage non-small cell lung cancer (NSCLC).5 With VATS lobectomy gaining popularity, structured training programmes have allowed high volume centres to develop extended indications for the approach. These include larger and centrally located tumours, cases with mediastinal lymphadenopathy and patients who have received neoadjuvant treatment.6 Despite this, the use of thoracoscopic lobectomy remains variable and it comprised less than 25% of all lobectomy procedures performed for NSCLC in Europe in 2013.7–9
Concerns regarding the impact of intraoperative complications and subsequent conversion have hindered the widespread implementation of VATS lobectomy. The reported incidence of unplanned conversion to thoracotomy ranges from less than 5% to over 20%.10–12 The reasons behind intraoperative conversion, the postoperative impact and trends in conversion rates over time have not been widely analysed. The aim of this study was to investigate the development of a thoracoscopic lobectomy programme over a five-year period with a focus on the causes and consequences of unplanned intraoperative conversion.
Methods
A retrospective review of a single unit’s lobectomy database was performed. Patients who underwent a lobectomy between January 2010 and April 2015 with individual division of hilar structures were included in the study. Only patients in whom the procedure was commenced thoracoscopically with no rib spreading were deemed eligible. Those undergoing pneumonectomy, segmentectomy or wedge resection were excluded. Patients who underwent thoracoscopic inspection (with or without a frozen section sample) and went on to receive a thoracotomy were also not included in the study. Postoperative complications were defined as per Society of Thoracic Surgeons data collection guidelines.9
Operative technique
All patients had general anaesthesia and single lung ventilation with a double lumen endotracheal tube. The majority underwent an anterior multiport (2 or 3 ports) VATS approach with either lymph node dissection or (in patients over 80 years of age) lymph node sampling. The aim was generally to dissect or sample lymph nodes from at least their separate mediastinal stations (paratracheal, subcarinal and paraoesophageal on the right; subaortic, subcarinal and paraoesophageal on the left). The thoracotomy conversion was performed by extending the utility incision or by connecting two port incisions.
Classifying reason for conversion
In the instances where an unplanned intraoperative conversion from VATS to thoracotomy occurred, the reasons were studied in detail and classified according to the VALT ‘Open’ system.13 Developed as a quality assurance tool, this classification system subdivides reason for conversion into ‘vascular’ (pulmonary artery injury, pulmonary vein injury or other vascular injury (eg intercostal vessel), ‘anatomical’ (adhesions or tumour size/location), ‘lymph nodes’ (bulky, sticky, calcified) or ‘technical’ (stapler misfire or equipment failure). The subsequent conversion is then classified as one of ‘elected to open with anticipation of difficulties’, ‘controlled and opened following difficulty’ or ‘uncontrolled open surgery with cardiorespiratory instability’. Data regarding clinicopathological characteristics and postoperative outcomes were collected for all patients.
Statistical analysis
All statistical analyses were performed using SPSS® version 11.0 (SPSS, Chicago, IL, US). For normally distributed data, Student’s t-test was used for comparison of values. Categorical variables were analysed with the chi-squared test or Fisher’s exact test as appropriate. A p-value of <0.05 was considered statistically significant.
Results
During the study period, 1,270 patients underwent a lobectomy in our department. Of these, 684 (53.9%) were commenced thoracoscopically and 75 patients (10.9%) underwent unplanned conversion. The proportion of lobectomies started as VATS was significantly higher in second half of the study period (2010–2012: 277/713 [38.8%], 2013–2015: 407/557 [73.1%], p<0.001) (Fig 1). The conversion rate dropped initially from 20.4% (11/54) in 2010 to 9.9% (15/51) in 2013 and then remained consistently under 10% until 2015.
Figure 1.
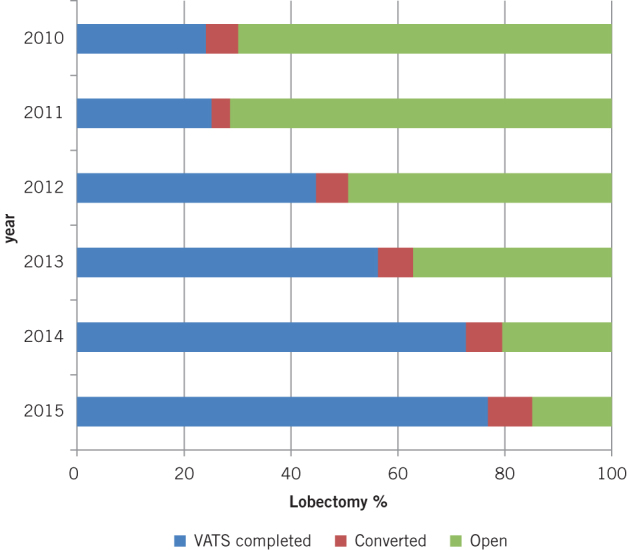
Change in approach to lobectomy between January 2010 and April 2015
The clinicopathological characteristics for patients with a completed thoracoscopic procedure and those with unplanned conversion are shown in Table 1. There were no significant differences in pathological tumour, nodal or metastasis stage. The proportions of procedures with positive resection margins were not significantly different between the groups.
Table 1.
Clincopathological characteristics of patients undergoing VATS completed lobectomy and unplanned conversion
| VATS (n=607) | Conversion (n=75) | p-value | |
|---|---|---|---|
| Mean age in years | 67.8 (SD: 10.0) | 67.2 (SD: 9.9) | 0.74* |
| Male sex | 253 (41.7%) | 36 (48.0%) | 0.22** |
| Pathological tumour stage | 0.60** | ||
| T1 | 210 (42.0%) | 27 (45.0%) | |
| T2 | 258 (51.6%) | 27 (45.0%) | |
| T3 | 31 (6.2%) | 6 (8.0%) | |
| T4 | 1 (0.2%) | 0 (0%) | |
| Pathological nodal stage | 0.92** | ||
| N0 | 400 (80.0%) | 46 (76.7%) | |
| N1 | 67 (13.4%) | 9 (15.0%) | |
| N2 | 22 (4.4%) | 3 (5.0%) | |
| Nx | 11 (2.2%) | 2 (3.3%) | |
| Unknown staging | 42 (6.9%) | 6 (8.0%) | 0.74** |
| Benign disease | 40 (6.6%) | 5 (6.7%) | 0.98** |
| Lobectomy for metastatic disease | 25 (4.1%) | 4 (5.3%) | 0.63** |
| R1 resection | 14 (2.3%) | 3 (4.0%) | 0.21** |
VATS = video assisted thoracoscopic surgery; SD = standard deviation
*Student’s t-test; **chi-squared test
The main reason for conversion was vascular (26/75, 34.7%), followed by anatomical (23/75, 30.7%), technical (14/75, 18.7%) and lymph nodes (12/75, 16.0%) (Tables 2 and 3). Of the 26 patients converted secondary to vascular causes, the primary source of bleeding was the pulmonary artery or a segmental branch (n=18). Three patients underwent conversion owing to pulmonary artery rupture or tear with associated blood loss of more than 2,000cc. Two of these subsequently died in the 30-day postoperative period. Half of the conversions secondary to technical reasons (7/14) occurred in the first year of the study period (2010), when five of these technical conversions were due to equipment failure (stapler misfire, thoracoscope and inadequate lighting). All subsequent conversions for technical reasons were due to failure of lung isolation.
Table 2.
Cause of conversion according to the VALT ‘Open’ classification system13
| n | |
|---|---|
| Total VATS cases | 684 (100%) |
| Vascular | 26 (3.8%) |
| Pulmonary artery injury | 18 |
| Pulmonary vein injury | 2 |
| Other | 6 |
| Anatomical | 23 (3.4%) |
| Adhesions | 15 |
| Tumour | 8 |
| Lymph nodes | 12 (1.8%) |
| Technical | 14 (2.0%) |
| Stapler | 2 |
| Equipment | 12 |
| ‘Open’ | |
| Elected to open with anticipation of difficulties | 44 (6.4%) |
| Controlled and opened following difficulty | 26 (3.8%) |
| Uncontrolled open surgery with cardiorespiratory instability | 5 (0.7%) |
Table 3.
Reasons for conversion over study period
| 2010 (n=178) | 2011 (n=230) | 2012 (n=305) | 2013 (n=240) | 2014 (n=244) | 2015 (Jan – April) (n=73) | |
|---|---|---|---|---|---|---|
| Started VATS | 54 (30.3%) | 66 (28.7%) | 157 (51.5%) | 151 (62.9%) | 194 (79.5%) | 62 (84.9%) |
| VATS converted | 11 (20.4%) | 8 (12.1%) | 19 (12.1%) | 15 (9.9%) | 16 (8.2%) | 6 (9.7%) |
| Vascular | 2 (3.7%) | 1 (1.5%) | 5 (3.2%) | 9 (6.0%) | 6 (3.1%) | 3 (4.8%) |
| Anatomical | 2 (3.7%) | 3 (4.6%) | 8 (5.1%) | 3 (2.0%) | 5 (2.6%) | 2 (3.2%) |
| Lymph nodes | 0 (0%) | 4 (6.1%) | 4 (2.6%) | 1 (0.7%) | 3 (1.6%) | 0 (0%) |
| Technical | 7 (13.0%) | 0 (0%) | 2 (1.3%) | 2 (1.3%) | 2 (1.0%) | 1 (1.6%) |
VATS = video assisted thoracoscopic surgery
When analysing urgency of conversion, the majority of conversions (44/75, 58.7%) were performed in anticipation of difficulties. There were five (6.7%) uncontrolled conversions with cardiorespiratory instability.
Patients undergoing unplanned conversion had a mean LOS of 9 days compared with 6 in those with a VATS procedure (p<0.001) (Table 4). There was a higher incidence of respiratory failure and empyema in those with unplanned conversion (p<0.001 and p=0.023 respectively). The frequency of all other postoperative complications (including total complications, reoperations and readmissions) was comparable in both groups. The mortality rate in the 30-day postoperative period was higher in patients with unplanned conversion (9.3% [7/75] vs 1.0% [6/607], p<0.001). Death in patients with unplanned conversion was due to acute respiratory distress syndrome (n=3), cardiac causes (n=1) and stroke (n=1). There were two deaths from an unknown cause.
Table 4.
Length of stay and postoperative complications of patients undergoing VATS completed lobectomy and unplanned conversion
| VATS (n=607) | Conversion (n=75) | p-value | |
|---|---|---|---|
| Mean length of stay in days | 6.4 (SD: 7.3) | 9.3 (SD: 2.4) | <0.001* |
| 30-day mortality | 6 (1.0%) | 7 (9.3%) | 0.003** |
| Any postoperative complication | 224 (36.9%) | 36 (52.9%) | 0.14** |
| Return to theatre | 43 (7.1%) | 6 (8.8%) | 0.78** |
| Reoperation for bleeding | 6 (1.0%) | 2 (2.9%) | 0.21*** |
| Readmission within 30 days | 41 (6.8%) | 5 (7.4%) | 0.33** |
| Respiratory failure | 23 (3.8%) | 10 (14.1%) | <0.001** |
| Empyema | 13 (2.1%) | 5 (7.4%) | 0.023** |
| Pneumonia | 57 (9.4%) | 12 (17.7%) | 0.09** |
| Arrhythmia | 34 (5.6)% | 8 (11.7%) | 0.10** |
| Pulmonary embolus | 8 (1.3%) | 2 (2.9%) | 0.36*** |
| Myocardial infarction | 3 (0.5%) | 0 (0%) | 0.54*** |
| Cerebrovascular accident | 1 (0.2%) | 0 (0%) | 0.73*** |
| Air leak >5 days | 123 (20.3%) | 15 (22.1%) | 0.96** |
VATS = video assisted thoracoscopic surgery; SD = standard deviation
*Student’s t-test; **chi-squared test; ***Fisher’s exact test
Discussion
This study analyses a large number of consecutive VATS lobectomy patients over a period of rapid expansion of a VATS lobectomy programme in a single unit. The causes and consequences of unplanned conversion over a six-year period are reported. In our unit, the proportion of lobectomy procedures that were safely completed thoracoscopically increased to over 75% without a subsequent increase in unplanned conversions. A drop in conversion rate in line with growing unit experience was demonstrated by Puri et al in 2015, where conversion rates fell from 28% to 11% over a nine-year period while rates for procedures commenced as VATS rose from 16% to 76%.10
With greater emphasis on healthcare cost containment, there is a desire to reduce the collateral impact of surgery on the patient. Despite this, the application of VATS lobectomy has lagged behind the use of minimally invasive techniques in other surgical specialties where laparoscopy is now the accepted standard. The thoracic surgery community may use the lessons learnt from well established laparoscopic surgical procedures (such as fundoplication) to progress the adoption of VATS lobectomy. In all applications of minimally invasive techniques, some circumstances will mandate conversion. In such cases, conversion may represent good judgement as opposed to a perceived ‘failure’. Furthermore, the presence of a dedicated theatre team trained in laparoscopic techniques can positively affect surgical outcomes.14,15 Finally, examining the ‘root cause’ of conversion, as done previously for laparoscopic cholecystectomy, is an important step in assessing the safety of a technique and determining patient suitability.16
Our results show that as experience in VATS increases over time, conversion rates secondary to technical reasons decrease. This reflects the increasing experience of the surgeons and theatre teams with the thoracoscopic equipment and techniques. The proportions of patients converted owing to vascular (bleeding), anatomical or nodal considerations remained consistent over the study period. This suggests that while surgeons can quickly learn to foresee a variety of technical challenges, conversion rates should not be expected to decrease below a certain proportion.
Concerns regarding major intraoperative complications may be a significant factor hindering the widespread adoption of VATS lobectomy, relating in particular to the ability to thoracoscopically control and (if necessary) repair vascular structures, predominately the pulmonary artery.10,17 The incidence of conversion due to intraoperative bleeding is reported as ranging from less than 1% to 5%,3,11,12 which is in line with the results of our series. Although uncommon, catastrophic bleeding encountered during VATS is associated with poor postoperative outcomes8 and is thought to be underreported in the literature.17 Despite the expanding patient cohort undergoing VATS lobectomy in our unit, our results demonstrate that the incidence of intraoperative major bleeding necessitating conversion has remained low. A timely decision to convert when experiencing difficult vascular dissection may reduce the risk of subsequent emergency conversion due to major bleeding.
Significant nodal disease on preoperative imaging has been described as a key factor associated with increased risk of conversion.11,18–20 The low incidence of conversion secondary to nodal disease in our series suggests accurate interpretation of preoperative imaging and subsequent appropriate assessment of suitability for a thoracoscopic procedure. Emergency conversion associated with cardiovascular instability was uncommon, comprising fewer than 1% of all procedures commenced thoracoscopically. Similar complication rates have been demonstrated between emergency and non-emergency conversions although emergency conversion is associated with a higher transfusion rate.10
Few variables have been demonstrated consistently to predict conversion in VATS lobectomy. This could relate to the difficulty in establishing a uniformly accepted definition of conversion. A case controlled study from 2015 showed age >65 years, forced expiratory volume in 1 second of <1.8 litres and fibrocalcified lymph nodes on preoperative chest computed tomography to be independently associated with conversion.18 Despite this, it is acknowledged that owing to the nature of these risk factors, they would already be assumed to infer greater operative risk even in those undergoing planned thoracotomy. As patients at high risk of conversion cannot be predicted reliably at present, a balance must be struck between striving to increase the use of VATS lobectomy and the consequences of unplanned and potential emergency conversion to thoracotomy.
Our study demonstrates that conversion can be associated with an increased incidence of respiratory failure and 30-day mortality as well as longer LOS. Evidence reporting the impact of conversion on postoperative outcomes is conflicting. The median LOS demonstrated in the present study for both VATS completed and converted patients is consistent with published analyses from other units.21,22 Previous studies have shown unplanned conversion to lengthen operative time as well as increasing blood loss, LOS and respiratory complications.10,18,21–23 Nevertheless, several studies have found no increase in associated morbidity or mortality following conversion.21,22 It is suggested that the comparison group for morbidity following unplanned converted thoracoscopic procedures should comprise those undergoing a planned thoracotomy.10 Previous literature has not demonstrated a difference in postoperative outcomes between these patient groups, indicating the risk of conversion should not be an insurmountable barrier to increasing the proportion of VATS lobectomies.10,11
Study limitations
This study is limited by its retrospective design, with the reason for conversion being assigned by the authors, potentially resulting in classification bias. Furthermore, the meaning of the term ‘conversion’ may vary between centres and individual surgeons. The results of unplanned conversions have not been compared with those for patients with planned open lobectomy, an area identified previously as being underreported.10 Although 26 patients had unplanned conversion due to vascular injury, the incidence of vascular injuries managed thoracoscopically without necessitating conversion in this patient cohort has not been explored. Data regarding cancer recurrence and overall survival are also not presented but have been demonstrated to be comparable in previous series.19,22 In addition, operative duration has not been reported. The exact time of conversion could provide an insight into intraoperative decision making and factors affecting the prompt anticipation of potential difficulties.
Conclusions
Our results suggest that as a VATS lobectomy programme develops, the conversion rates in lobectomies drop initially and then remain constant at approximately 10%. With increasing experience and technical expertise, surgeons become more skilled in both the preoperative identification of patients suitable for VATS lobectomy and completing lobectomy procedures thoracoscopically even in more complex cases.
Acknowledgement
The material in this paper was presented at the 29th Annual Meeting of the European Association for Cardio-Thoracic Surgery held in Amsterdam, October 2015.
References
- 1.Nwogu CE, D’Cunha J, Pang H et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015; : 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flores RM, Park BJ, Dycoco J et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009; : 11–18. [DOI] [PubMed] [Google Scholar]
- 3.Villamizar NR, Darrabie MD, Burfeind WR et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009; : 419–425. [DOI] [PubMed] [Google Scholar]
- 4.Walker WS, Codispoti M, Soon SY et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003; : 397–402. [DOI] [PubMed] [Google Scholar]
- 5.Detterbeck FC, Lewis SZ, Diekemper R et al. Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; : 7S–37S. [DOI] [PubMed] [Google Scholar]
- 6.Pischik VG. Technical difficulties and extending the indications for VATS lobectomy. J Thorac Dis 2014; : S623–S630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcoz PE, Puyraveau M, Thomas PA et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016; : 602–609. [DOI] [PubMed] [Google Scholar]
- 8.Decaluwe H, Petersen RH, Hansen H et al. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015; : 588–598. [DOI] [PubMed] [Google Scholar]
- 9.STS National Database Society of Thoracic Surgeons. http://www.sts.org/national-database (cited July 2016).
- 10.Puri V, Patel A, Majumder K et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg 2015; : 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samson P, Guitron J, Reed MF et al. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: a retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg 2013; : 1,512–1,518. [DOI] [PubMed] [Google Scholar]
- 12.McKenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006; : 421–425. [DOI] [PubMed] [Google Scholar]
- 13.Gazala S, Hunt I, Valji A et al. A method of assessing reasons for conversion during video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011; : 962–964. [DOI] [PubMed] [Google Scholar]
- 14.Chan SW, Hensman C, Waxman BP et al. Technical developments and a team approach leads to an improved outcome: lessons learnt implementing laparoscopic splenectomy. ANZ J Surg 2002; : 523–527. [DOI] [PubMed] [Google Scholar]
- 15.Kenyon TA, Lenker MP, Bax TW, Swanstrom LL. Cost and benefit of the trained laparoscopic team. A comparative study of a designated nursing team vs a nontrained team. Surg Endosc 1997; : 812–814. [DOI] [PubMed] [Google Scholar]
- 16.Scott TR, Zucker KA, Bailey RW. Laparoscopic cholecystectomy: a review of 12,397 patients. Surg Laparosc Endosc 1992; : 191–198. [PubMed] [Google Scholar]
- 17.Berry MF. Pulmonary artery bleeding during video-assisted thoracoscopic surgery: intraoperative bleeding and control. Thorac Surg Clin 2015; : 239–247. [DOI] [PubMed] [Google Scholar]
- 18.Byun CS, Lee S, Kim DJ et al. Analysis of unexpected conversion to thoracotomy during thoracoscopic lobectomy in lung cancer. Ann Thorac Surg 2015; : 968–973. [DOI] [PubMed] [Google Scholar]
- 19.Sawada S, Komori E, Yamashita M. Evaluation of video-assisted thoracoscopic surgery lobectomy requiring emergency conversion to thoracotomy. Eur J Cardiothorac Surg 2009; : 487–490. [DOI] [PubMed] [Google Scholar]
- 20.Mason AC, Krasna MJ, White CS. The role of radiologic imaging in diagnosing complications of video-assisted thoracoscopic surgery. Chest 1998; : 820–825. [DOI] [PubMed] [Google Scholar]
- 21.Augustin F, Maier HT, Weissenbacher A et al. Causes, predictors and consequences of conversion from VATS to open lung lobectomy. Surg Endosc 2016; : 2,415–2,421. [DOI] [PubMed] [Google Scholar]
- 22.Park JS, Kim HK, Choi YS et al. Unplanned conversion to thoracotomy during video-assisted thoracic surgery lobectomy does not compromise the surgical outcome. World J Surg 2011; : 590–595. [DOI] [PubMed] [Google Scholar]
- 23.Paul S, Altorki NK, Sheng S et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010; : 366–378. [DOI] [PubMed] [Google Scholar]


