Abstract
BACKGROUND
Pancreatic ductal adenocarcinoma is the most common pancreatic cancer. Five-year overall survival is currently 3.3–6.0%. The aim of this review was to evaluate the prognostic value of lymph node ratio, number of positive nodes and total nodes examined on overall survival rate following pancreatic resection.
MATERIALS AND METHODS
A literature search was conducted of MEDLINE, EMBASE, the Cochrane Library and Central Register of Controlled Trials and the Cochrane Database of Systematic Review databases, from January 1996 to January 2016.
RESULTS
Overall, 19 studies including 4,883 patients examined the relationship between lymph node ratio and overall survival. A high lymph node ratio was associated with decreased overall survival in 17 studies. A total of 12 studies examined the relationship between the number of positive nodes and overall survival, and 11 studies revealed that an increase in the number of positive nodes was associated with decreased overall survival. In 15 studies examining the relationship between the total nodes examined and overall survival, there was no association with overall survival in 12 studies.
CONCLUSIONS
Lymph node ratio and number of positive nodes are factors associated with overall survival in pancreatic ductal adenocarcinoma, but not total nodes examined.
Keywords: Pancreatic Cancer, Lymph Node Status, Lymph Node Ratio, Survival
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common pancreatic cancer, with an incidence of 2.1% of all cancers.1 PDAC is considered to have the worst survival rates of all gastrointestinal cancers; the average life expectancy on diagnosis is 4–6 months and the 5-year overall survival rate is 3.3% in the UK. Surgical resection combined with adjuvant therapy is considered for only 10–20% of patients at the time of presentation and has the potential to increase the overall survival to approximately 18 months.2–5
One prognostic factor for PDAC is the lymph node ratio, which is the ratio of the number of positive nodes to the number of total nodes examined. Lymph node ratio has been reported to be associated with prognosis in a variety of cancers, including oesophageal, gastric, colonic and breast.6–9 In 2004, Berger et al. examined the association of lymph node ratio and total nodes examined on survival in patients with PDAC and reported that only lymph node ratio had an impact on overall and disease-free survival.10 Further studies were subsequently conducted on the prognostic role of lymph node ratio, lymph node ratio to delineate the most predictive marker of survival, but there was no consensus. Despite numerous studies since 1994, neither lymph node ratio nor number of positive nodes is included in the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control’s Tumour, Node, Metastasis staging system for pancreatic cancer, where the classic lymph node status (N0 vs. N1) is the only criterion available.
This systematic review evaluates the currently available evidence regarding the prognostic value of lymph node ratio, number of positive nodes and total nodes examined in patients with PDAC and the potential association with overall survival.
Materials and Methods
This systematic review was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11
Search strategy
A literature search was conducted for all published studies between January 1996 and January 2016. There was no restriction on language or country. A broad search approach was carried out owing to the expected scarcity of randomised controlled trials, so randomised, non-randomised, prospective and retrospective studies were included if they met the inclusion criteria. Studies were identified by searching the following databases: MEDLINE (via PubMed), EMBASE (via OvidSP), the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Review. Searches were adapted to each database and carried out using the specific controlled vocabulary of each database, if available (MeSH terms for MEDLINE and Emtree terms for EMBASE), In addition to free text words. Searches included the words ‘lymph node ratio’, ‘node status*’, ‘examined lymph nodes’, ‘metastatic lymph nodes’, ‘positive lymph nodes’ or ‘lymphadenectomy’ and ‘pancreatic cancer’, ‘pancreatic adenocarcinoma’, ‘pancreas*’, ‘tumour*’, ‘carcinoma*’, ‘pancreatectomy’, ‘Whipple’s procedure’, ‘pancreaticoduodenectomy’, ‘distal pancreatectomy’, ‘pylorus preserved pancreaticoduodenectomy’.
All titles and abstracts of papers identified by the search strategy were screened for relevance. At this stage, only clearly non-relevant articles were excluded. Full copies of all potentially relevant papers were obtained and texts were screened to assess eligibility for inclusion. Reference lists of included studies were also screened for additional articles. Case reports and reviews were also gathered to screen their reference lists for additional relevant articles but were excluded from the qualitative analysis.
Inclusion and exclusion criteria
Articles analysing the influence of lymph node ratio, number of positive nodes and total nodes examined on overall survival following pancreatic resections (Whipple’s procedure, distal pancreatectomy, pylorus-preserved pancreaticoduodenectomy, total pancreatectomy) for patients affected by PDAC within the past 20 years were included. Studies reporting data on ampullary cancer, duodenal carcinoma or cholangiocarcinoma and studies on PDAC without an analysis of the association between lymph node ratio, number of positive nodes, total nodes examined and overall survival were excluded. Reasons for exclusion were recorded.
Data collection and analysis of outcomes
Two authors (ME, GG) independently extracted information from included studies. Data regarding study design, number of patients, type of resections, nodal status, resection margin (R), lymph node ratio, number of positive nodes, total nodes examined and overall survival were collected if available and were summarised. Any discrepancies between the reviewers regarding the extraction of data were resolved by consensus. The primary outcome was the overall survival rate. The secondary outcome was lymph node ratio cut-off points associated with overall survival. Overall survival was calculated from the time of surgery. Kaplan–Meier survival curves were reviewed to confirm the impact on survival, especially if survival outcomes were not directly reported.
Assessment of risk of bias
The studies were assessed using the Cochrane Collaboration’s tool for assessing risk of bias in randomised and non-randomised trials.12
Statistical analysis
All data were entered into an Microsoft Excel® database. Patients were categorised either as ‘dead’ or ‘alive’ (categorical variables) and the descriptive statistics were elaborated upon in terms of occurrence rate and relative frequencies. The software package Review Manager (RevMan 5.3; Nordic Cochrane Centre, Cochrane Collaboration) was used for data analysis and synthesis. When articles with missing data relevant to the outcomes were found, these were excluded from the analysis. Results were considered significant if the probability of chance of occurrence was less than five percent (P < 0.05).
Results
A PRISMA flow diagram showing the selection process of articles for the systematic review is presented in Fig 1.
Figure 1.
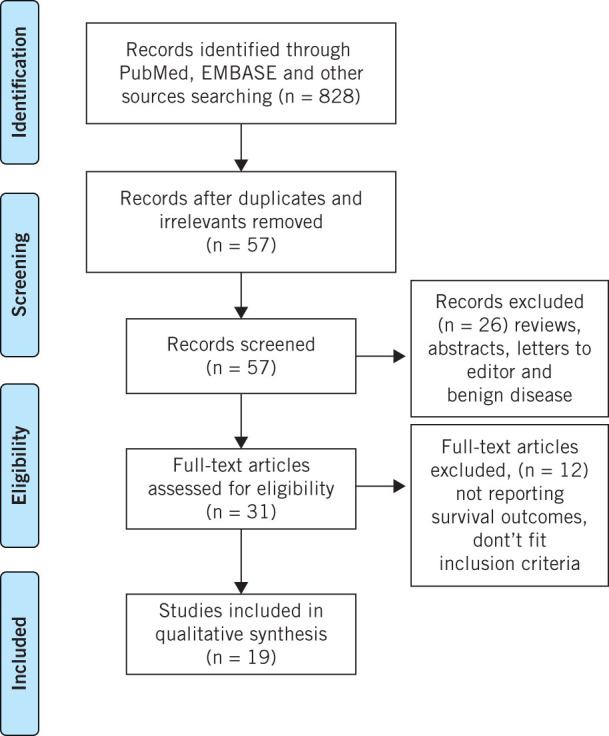
Preferred reporting Items for systematic reviews and meta-analyses flow diagram of study selection
Characteristics of the included studies
Nineteen studies comprising 4,883 patients who underwent pancreatic resections for PDAC were included.13–31 There were 5 prospective studies, 1 randomised controlled trial and 13 retrospective studies (Table 1). All included studies analysed survival using the Kaplan–Meier method, defining an event as the death of a patient and overall survival from the time elapsed from surgery to death. Univariate analysis of all variables related to overall survival was performed with the log rank test while multivariate analysis was calculated using Cox proportional hazard model. Whipple’s pancreaticoduodenectomy was the most common procedure (n = 2408; 49%), followed by pylorus-preserving pancreaticoduodenectomy (n = 1620; 33%), distal pancreatectomy (n = 278; 6%) and total pancreatectomy (n = 48; 1%). Node-negative disease (N0) was observed in 1,527 patients (31%) and node-positive disease was observed in 3,094 patients (63%). The surgical resection margin was microscopically negative (R0) in 2,653 patients (54%) and was microscopically positive (R1) in 1,535 patients (31%). Only four studies did not report the nodal and R status.28,30–32 Adjuvant therapy (chemoradiotherapy) data were available in 10 studies that included 1,739 patients (36%),13,14,19,21–23,26,28–30 and data on neoadjuvant therapy were available in only two studies.15,29
Table 1.
Characteristics of the included studies
| Study | Year | Design | Patients (n) | LNR cut-off points(%) | NPN n (range) | TNE n (range) |
|---|---|---|---|---|---|---|
| Pawlik et al.24 | 2007 | Prospective | 905 | 0, 20, 40 | – | 17 (6–28) |
| House et al.16 | 2007 | Prospective | 696 | 0, 20, 40 | 4 ± 3 | 17 ± 9.5 |
| Riediger et al.25 | 2009 | Retrospective | 182 | 0, 20, 30 | 1 (0–22) | 16 (2–47) |
| Massucco et al.22 | 2009 | Prospective | 77 | 10 | 4 (1–29) | 29 (10–54) |
| Showalter et al.28 | 2010 | RCT | 445 | 15, 33 | 1 (0–18) | 9–11 (1–56) |
| Bhatti et al.14 | 2010 | Retrospective | 84 | 20, 30 | – | 9 (1–26) |
| Murakami et al.23 | 2010 | Retrospective | 119 | 0, 10, 20 | 2 (0–40) | 28 (2–75) |
| La Torre et al.18 | 2011 | Retrospective | 101 | 0, 20, 40 | – | 19 (3–26) |
| Sanjay et al.27 | 2012 | Prospective | 51 | 20 | – | – |
| Robinson et al.26 | 2012 | Retrospective | 134 | 15 | – | – |
| Ausborn et al.13 | 2013 | Retrospective | 106 | 0.095 (0.0–0.26) median | – | – |
| John et al.17 | 2013 | Retrospective | 70 | 14 | 2 | 14 |
| Yamamoto et al.30 | 2014 | Retrospective | 56 | 20 | 2 | 25 |
| Strobel et al.29 | 2014 | Prospective | 811 | 0, 20, 40 | 3 (1–7) | 24 (18–32) |
| La Torre et al.19 | 2014 | Retrospective | 143 | 0, 20, 30 | ≥ 1 | 15 |
| Liu et al.20 | 2014 | Retrospective | 167 | 20, 40 | 2 (1–27) | 10 (0–44) |
| Malleo et al.21 | 2015 | Retrospective | 255 | 0, 20, 40 | 4.9 ± 4.7 | 30.8 ± 14 |
| Zhan et al.31 | 2015 | Retrospective | 83 | 20 | – | 8.2 ± 6.1 |
| Fischer et al.15 | 2016 | Retrospective | 398 | 19 | 2 (1–25) | – |
LNR, lymph node ratio; NPN, number of positive nodes; RCT, randomised controlled trial; TNE, total nodes examined
Risk of bias in the included studies
The risk of bias is presented in Fig 2. Studies were classified as low risk, unclear risk or high risk of bias.
Figure 2.
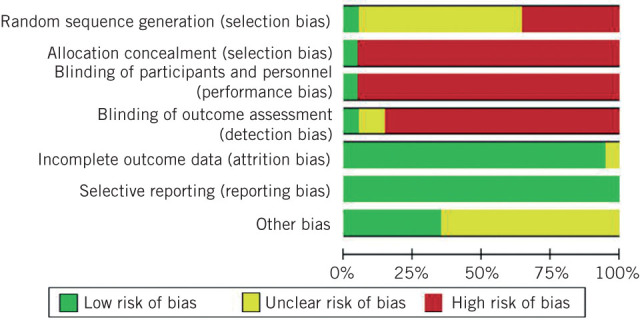
Review authors’ judgements about each risk of bias item presented as percentages across all included studies
Lymph node ratio association with overall survival
A total of 19 studies stratified the lymph node ratio into subgroups based on cut-off values identified through sensitivity analyses (Table 2).13–31 Association between these subgroups and overall survival was investigated and presented using Kaplan–Meier curves. A high lymph node ratio was associated with decreased overall survival in 17 studies,13–22,24–28,30,31 and no association was found in two studies.23,29 Lymph node ratio was significantly associated with overall survival in most studies.
Table 2.
Studies comparing associations with overall survival
| Study | LNR | NPN | TNE |
|---|---|---|---|
| Pawlik et al.24 | Yes | – | No |
| House et al.16 | Yes | Yes | No |
| Riediger et al.25 | Yes | Yes | No |
| Massucco et al.22 | Yes | Yes | No |
| Showalter et al.28 | Yes | Yes | Yes |
| Bhatti et al.14 | Yes | – | No |
| Murakami et al.23 | No | Yes | No |
| La Torre et al.18 | Yes | – | No |
| Sanjay et al.27 | Yes | – | – |
| Robinson et al.26 | Yes | – | – |
| Ausborn et al.13 | Yes | – | – |
| John et al.17 | Yes | Yes | No |
| Yamamoto et al.30 | Yes | Yes | No |
| Strobel et al.29 | No | Yes | Yes |
| La Torre et al.19 | Yes | No | Yes |
| Liu et al.20 | Yes | Yes | No |
| Malleo et al.21 | Yes | Yes | No |
| Zhan et al.31 | Yes | – | No |
| Fischer et al.15 | Yes | Yes | – |
LNR, lymph node ratio; NPN, number of positive nodes; TNE, total nodes examined; Yes = associated with overall survival; No = not associated with overall survival
Number of positive nodes association with overall survival
Twelve studies examined the relationship between the number of positive nodes and overall survival (Table 2).15–17,19–23,25,28–30 Eleven studies revealed that an increase in the number of positive nodes was associated with decreased overall survival.15–17,20–23,25,28–30Only one study showed no association with overall survival.19 Number of positive nodes was significantly associated with overall survival in most studies.
The association of total nodes examined with overall survival
Fifteen studies examined the relationship between total nodes examined and overall survival (Table 2).14,16–25,28–31 Total nodes examined was not linked with overall survival in 12 studies.14,16–18,20–25,30,31 Only three studies showed that an increase in total nodes examined was associated with improved overall survival.19,28,29 Total nodes examined was not associated with overall survival in most studies.
Discussion
This systematic review evaluated the association between three lymph node parameters (ratio, number of positive nodes and total nodes examined) with overall survival through the analysis of 18 observational studies and one randomised controlled trial involving 4,883 patients affected by PDAC. Only studies that reported data over the past 20 years were included, to minimise confounding due to changes in the surgical and non-surgical management of PDAC. Evidence demonstrated that there is a negative association between overall survival and lymph node ratio in N1 patients, and where different cut-off values were investigated, a ratio greater than 20% was frequently associated with poor overall survival in numerous studies. Similar results for lymph node ratio were also noted in other malignancies, such as gastric, oesophageal and colorectal cancers,33–35 where it is a well-established independent prognostic factor for overall survival.33 A systematic review by Ceelen et al. on lymph node ratio in stage III colorectal cancer confirmed a hazard ratio for overall survival of 2.36 (95% confidence interval 2.14–2.61). In this review, lymph node ratio appeared to be superior to total nodes examined and number of positive nodes as a predictor of survival.36
The number of positive nodes has been shown to be an independent prognostic factor for overall survival in PDAC and it was superior to lymph node ratio as a prognostic marker for survival in two studies.23,29 One particular study by Murakami et al. (2010)23 found that the number of positive nodes is a stronger prognostic element than lymph node ratio following pancreaticoduodenectomy. The study was retrospective and included 119 patients.23 The other study was by Strobel et al. (2014),29 who reported on 811 patients and found that the number of positive nodes and lymph node ratio were significantly correlated with overall survival on univariate analysis in N1 patients. However, on multivariate analysis, only number of positive nodes remained an independent prognostic factor of survival.29 In this retrospective single-centre analysis, the authors noted that an increase in the total number of nodes examined was associated with an increase in the number of positive nodes. Nevertheless, the number of patients with higher lymph node ratio decreased, even though more positive lymph nodes were identified. They concluded that the inverse tendency of number of positive nodes and lymph node ratio reflects an underestimation of the extent of lymph node involvement by ratio when the total number of nodes examined increases. This might explain why number of positive nodes was shown to be a superior prognostic factor.29
Number of positive nodes is a factor dependent on the total number of nodes examined, as the minimum number of nodes examined for an adequate and reliable staging is 15, according to the Royal College of Pathologists, and 12 according to the AJCC TNM classification 7th edition.37,38 The total nodes examined is affected by the extent of the lymph node dissection, the surgeon’s experience, the location of the tumour and the quality of the pathological examination. All these variables have the potential to impact the identification of nodes (total examined) and consequently the finding of nodes involved by PDAC (number of positive nodes). They all therefore influence any eventual association of number of positive nodes and total number examined with overall survival. The median number examined varied among the included studies, but in most studies no association was found with overall survival in N1 patients (Table 2).
To the best of the authors’ knowledge, this review is the first to analyse these lymph node parameters and their associations with overall survival in PDAC. A formal meta-analysis was not possible because of the heterogeneity between studies, due to the various clinical, surgical and pathological factors. The current systematic review has also limitations, as it was based predominantly on retrospective studies with variable risk of bias. Furthermore, examining the influence of neoadjuvant therapy on lymph node ratio, number of positive nodes and total number examined was not possible, as only two studies reported these data.
The findings of this review may help to improve the TNM staging system for pancreatic cancer, which currently depends on lymph node status, either positive or negative, to stratify the N stage. Considering the poor outcome following pancreatic resections, this will identify a subgroup of patients with reduced survival. In addition, it will help to inform the decision to employ adjuvant treatments.
Conclusion
The currently available data suggest that lymph node ration and number of positive nodes but not total number examined are factors associated with overall survival in PDAC. Further large randomised controlled trials are required to confirm these findings.
References
- 1.Ghadirian P, Lynch HT, Krewski D. Epidemiology of pancreatic cancer: an overview. Cancer Detect Prev 2003; : 87–93. [DOI] [PubMed] [Google Scholar]
- 2.Cress RD, Yin D, Clarke L et al. . Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes Control 2006; : 403–409. [DOI] [PubMed] [Google Scholar]
- 3.Cleary SP, Gryfe R, Guindi M et al. . Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg 2004; : 722–731. [DOI] [PubMed] [Google Scholar]
- 4.Mitry E, Rachet B, Quinn MJ et al. . Survival from cancer of the pancreas in England and Wales up to 2001. Br J Cancer 2008; 99(Suppl 1): S21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcea G, Dennison AR, Pattenden CJ et al. . Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP 2008; : 99–132. [PubMed] [Google Scholar]
- 6.Liu D, Chen Y, Deng M et al. . Lymph node ratio and breast cancer prognosis: a meta-analysis. Breast Cancer 2014; : 1–9. [DOI] [PubMed] [Google Scholar]
- 7.Feng JF, Huang Y, Chen L et al. . Prognostic analysis of esophageal cancer in elderly patients: metastatic lymph node ratio versus 2010 AJCC classification by lymph nodes. World J Surg Oncol 2013; : 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang BY, Yuan J, Cui ZS et al. . Evaluation of the prognostic value of the metastatic lymph node ratio for gastric cancer. Am J Surg 2014; : 555–565. [DOI] [PubMed] [Google Scholar]
- 9.Attaallah W, Gunal O, Manukyan M et al. . Prognostic impact of the metastatic lymph node ratio on survival in rectal cancer. Ann Coloproctol 2013; : 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger AC, Watson JC, Ross EA et al. . The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg 2004; : 235–240. [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; : b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011) http://handbook.cochrane.org (cited October 2016). [Google Scholar]
- 13.Ausborn NL, Wang T, Wentz SC et al. . 53BP1 expression is a modifier of the prognostic value of lymph node ratio and CA 19-9 in pancreatic adenocarcinoma. BMC Cancer 2013; 13: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatti I, Peacock O, Awan AK et al. . Lymph node ratio versus number of affected lymph nodes as predictors of survival for resected pancreatic adenocarcinoma. World J Surg 2010; : 768–775. [DOI] [PubMed] [Google Scholar]
- 15.Fischer LK, Katz MH, Lee SM et al. . The number and ratio of positive lymph nodes affect pancreatic cancer patient survival after neoadjuvant therapy and pancreaticoduodenectomy. Histopathology 2016; (2): 210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.House MG, Gonen M, Jarnagin WR et al. . Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg 2007; : 1,549–1,555. [DOI] [PubMed] [Google Scholar]
- 17.John BJ, Naik P, Ironside A et al. . Redefining the R1 resection for pancreatic ductal adenocarcinoma: tumour lymph nodal burden and lymph node ratio are the only prognostic factors associated with survival. HPB (Oxford) 2013; : 674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La Torre M, Cavallini M, Ramacciato G et al. . Role of the lymph node ratio in pancreatic ductal adenocarcinoma. Impact on patient stratification and prognosis. J Surg Oncol 2011; : 629–633. [DOI] [PubMed] [Google Scholar]
- 19.La Torre M, Nigri G, Petrucciani N et al. . Prognostic assessment of different lymph node staging methods for pancreatic cancer with R0 resection: pN staging, lymph node ratio, log odds of positive lymph nodes. Pancreatology 2014; : 289–294. [DOI] [PubMed] [Google Scholar]
- 20.Liu ZQ, Xiao ZW, Luo GP et al. . Effect of the number of positive lymph nodes and lymph node ratio on prognosis of patients after resection of pancreatic adenocarcinoma. Hepatobiliary Pancreat Dis Int 2014; : 634–641. [DOI] [PubMed] [Google Scholar]
- 21.Malleo G, Maggino L, Capelli P et al. . Reappraisal of nodal staging and study of lymph node station involvement in pancreaticoduodenectomy with the Standard International Study Group of Pancreatic Surgery definition of lymphadenectomy for cancer. J Am Coll Surg 2015; : 367–379. [DOI] [PubMed] [Google Scholar]
- 22.Massucco P, Ribero D, Sgotto E et al. . Prognostic significance of lymph node metastases in pancreatic head cancer treated with extended lymphadenectomy: not just a matter of numbers. Ann Surg Oncol 2009; : 3,323–3,332. [DOI] [PubMed] [Google Scholar]
- 23.Murakami Y, Uemura K, Sudo T et al. . Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J Am Coll Surg 2010; : 196–204. [DOI] [PubMed] [Google Scholar]
- 24.Pawlik TM, Gleisner AL, Cameron JL et al. . Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007; : 610–618. [DOI] [PubMed] [Google Scholar]
- 25.Riediger H, Keck T, Wellner U et al. . The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg 2009; : 1,337–1,344. [DOI] [PubMed] [Google Scholar]
- 26.Robinson SM, Rahman A, Haugk B et al. . Metastatic lymph node ratio as an important prognostic factor in pancreatic ductal adenocarcinoma. Eur J Surg Oncol 2012; : 333–339. [DOI] [PubMed] [Google Scholar]
- 27.Sanjay P, de Figueiredo RS, Leaver H et al. . Preoperative serum C-reactive protein levels and post-operative lymph node ratio are important predictors of survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. JOP 2012; : 199–204. [PubMed] [Google Scholar]
- 28.Showalter TN, Winter KA, Berger AC et al. . The influence of total nodes examined, number of positive nodes, and lymph node ratio on survival after surgical resection and adjuvant chemoradiation for pancreatic cancer: a secondary analysis of RTOG 9704. Int J Radiat Oncol Biol Phys 2010; : 1,328–1,335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strobel O, Hinz U, Gluth A et al. . Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg 2014; : 961–969. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto Y, Ikoma H, Morimura R et al. . The clinical impact of the lymph node ratio as a prognostic factor after resection of pancreatic cancer. Anticancer Res 2014; : 2,389–2,394. [PubMed] [Google Scholar]
- 31.Zhan HX, Xu JW, Wang L et al. . Lymph node ratio is an independent prognostic factor for patients after resection of pancreatic cancer. World J Surg Oncol 2015; : 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Luo G, Guo M et al. . Lymph node status predicts the benefit of adjuvant chemoradiotherapy for patients with resected pancreatic cancer. Pancreatology 2015; : 253–258. [DOI] [PubMed] [Google Scholar]
- 33.Petrelli F, Borgonovo K, Barni S. The emerging issue of ratio of metastatic to resected lymph nodes in gastrointestinal cancers: an overview of literature. Eur J Surg Oncol 2011; : 836–847. [DOI] [PubMed] [Google Scholar]
- 34.Siewert JR, Bottcher K, Stein HJ et al. . Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998; : 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smit JK, Pultrum BB, van Dullemen HM et al. . Prognostic factors and patterns of recurrence in esophageal cancer assert arguments for extended two-field transthoracic esophagectomy. Am J Surg 2010; : 446–453. [DOI] [PubMed] [Google Scholar]
- 36.Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol 2010; : 2,847–2,855. [DOI] [PubMed] [Google Scholar]
- 37.Campbell F, Foulis AK, Verbeke CS. Dataset for the histopathological reporting of carcinomas of the pancreas, ampulla of Vater and common bile duct Royal College of Pathologists https://www.rcpath.org/resourceLibrary/dataset-for-the-histopathological-reporting-of-carcinomas-of-the-pancreas--ampulla-of-vater-and-common-bile-duct.html (cited October 2016).
- 38.Edge S, Byrd DR, Compton CC et al. , AJCC Cancer Staging Manual (7th ed.) American Joint Committee on Cancer New York: Springer; 2010. [Google Scholar]


