Abstract
A 43-year-old female patient suffered from persistent anastomotic leakage after Roux-en-Y gastric bypass for morbid obesity. Endoscopic stenting of the anastomotic leakage was performed. The patient presented with haematemesis 3 weeks later. An aorto-oesophageal fistula was diagnosed, most likely due to ulceration of the oesophageal stent. The fistula was closed with an endovascular covered aortic stent and a new gastrojejunostomy was created. One year after surgery, the patient is in good condition.
Endoscopic stents are increasingly being used to treat anastomotic leakage in bariatric patients. An aorto-oesophageal fistula is a life-threatening complication of stent placement, early clinical recognition is essential. More data are needed on the complications of anastomotic stenting in bariatric patients.
Keywords: Gastric bypass, Bariatric surgery, Anastomotic leakage, Stents, Oesophageal fistula
Background
Bariatric surgery is the most effective treatment for morbid obesity in the long term. Roux-en-Y gastric bypass (RYGB) is the most performed bariatric procedure. Staple-line leakage is a severe complication of RYGB, with a prevalence of 1–5%.1 Revisional surgery is often needed, although conservative management is sometimes sufficient. In cases of persistent leakage after revisional surgery or in selected cases when there is minor leakage, endoscopic placement of a self-expandable metal stent can be an alternative to surgery.2 The success rate of stent placement for anastomotic leakage after bariatric surgery varies, but is generally above 50%. Frequent complications of stent placement include ulceration and migration.
We present a case of a 43-year-old female patient who underwent endoscopic stenting of an anastomotic leakage after RYGB. Three weeks after stent placement she presented with haematemesis from an aorto-oesophageal fistula, most likely caused by ulceration of the stent.
Case history
A 43-year-old morbidly obese female was referred to our bariatric centre after previous bariatric surgery. In 2004, an open adjustable gastric banding procedure was performed in another hospital at a body mass index (BMI) of 42.4kg/m2 (weight 130kg). After an initial weight loss to 83kg (BMI 27.1), she had slowly regained weight and weighed 118kg (BMI 38.5) at initial presentation at our centre. Adjustment of the band was no longer possible, owing to recurrent problems with the infusion port. She had no further relevant medical history. There were no obesity-related comorbidities and preoperative screening by an internist, psychologist and dietician revealed no contraindications for surgery.
A laparoscopic revision from gastric banding to RYGB was performed in one step. The procedure consisted of adhesiolysis in the midline (post-laparotomy) and at the place where the gastric band had been. The band had eroded through the stomach wall and was completely located inside the stomach. There were no defects in the stomach wall nor signs of infection. The band was removed via a gastrotomy and, subsequently, an RYGB was performed in an antegastric, antecolic fashion. The gastrojejunal and jejuno-jejunal anastomoses were achieved by linear stapling. There were no perioperative complications and the postoperative situation was stable. The patient was discharged on the first postoperative day in a good condition. On the sixth postoperative day, she was readmitted with signs of peritonitis. A laparoscopy was performed the same day. A leakage of the vertical staple line of the gastric pouch was seen and the defect was closed with sutures. The abdomen was irrigated and drains were placed. The patient was fed through a nasojejunal feeding tube and received intravenous antibiotics. Because of a persistent leak a second revisional surgery was performed on postoperative day 10, but leakage persisted, proven with methylene blue test. On postoperative day 15, a fully covered double-bump oesophageal stent (Niti-S BetaTM, TaeWoong Medical, Goyang-si, South Korea) was placed endoscopically by the gastroenterologist. Only after replacement with a new stent of the same type and later reposition of this stent did the leakage stop. The stents did cause persistent inconvenience, however, such as nausea and retrosternal pain, which was only partially alleviated by metoclopramide and opioids. On postoperative day 50, the patient was discharged.
Three days later (day 53) she presented to the emergency department with acute haematemesis, nausea and retrosternal pain. Blood pressure was 110/61mmHg, heart rate 104 beats/minute and respiratory rate 30 breaths/minute. Haemoglobin level at admission was 6.3 mmol/l (comparable to discharge 1 week before) and there was no evidence of coagulopathy. A computed tomography (CT) scan of the abdomen demonstrated air along the full length of the oesophagus but no fluid or free air in the abdominal cavity. The hypothesis was that the patient had suffered from a gastric or oesophageal bleed. She was admitted to the intensive care unit for haemodynamic surveillance and received intravenous proton pump inhibitors. After elective intubation, oesophagogastroduodenoscopy was performed. A blood clot was seen on the proximal edge of the stent, but no active bleeding. Following removal of the stent, there was active bleeding located proximally to where the stent was positioned. Because the patient became haemodynamically unstable and there were no endoscopic treatment options at this moment, a Sengstaken balloon was placed. Erythrocytes and fresh frozen plasma were transfused. After stabilisation of the patient, CT-angiography was performed and the patient underwent immediate laparotomy, during which an active bleeding from the distal oesophagus was seen. Complete haemostasis was not possible.
Perioperative review of the CT-angiography revealed an aorto-oesophageal fistula with active bleeding (Figs 1 and 2). The vascular surgeon was consulted and the fistula was successfully closed with an endovascular covered aortic stent. The gastric pouch and proximal jejunum were resected and a new gastrojejunostomy was created. Postoperatively, antibiotics and total parenteral nutrition were administered and her recovery was good. The patient was discharged 1 week after the aortic stent placement, 66 days after the initial gastric bypass procedure. She continued on prophylactic antibiotics for 6 weeks; 12 months after the initial RYGB, she was in good clinical condition, on oral nutrition only, and her weight was 89kg (BMI 29.1). CT-angiography demonstrated a good position of the aortic stent.
Figure 1.
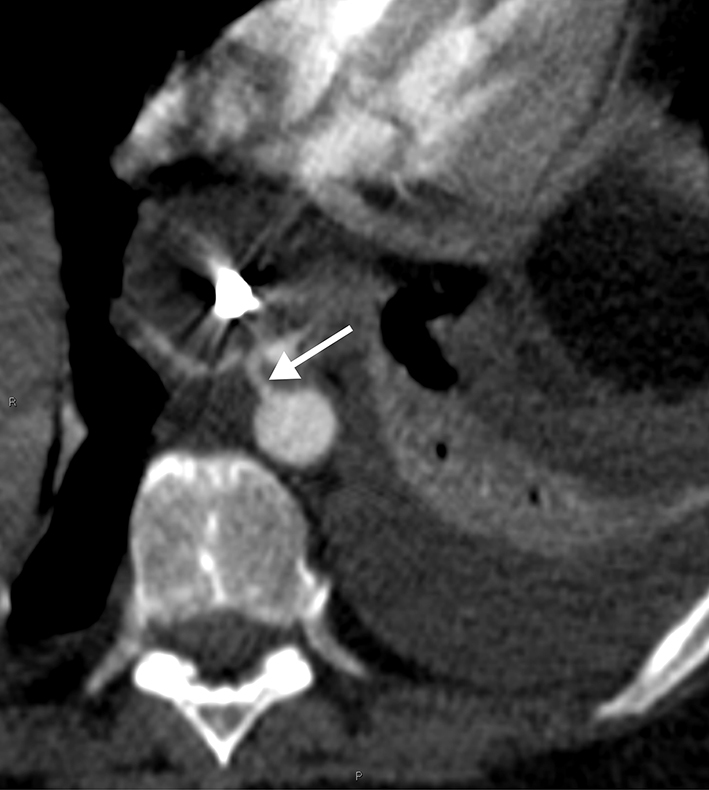
Axial computed tomography angiograph of the descending thoracic aorta at the level of thoracic vertebrae 7–8 showing the fistula (arrow) at the position of 11 o’clock between the aorta and oesophagus
Figure 2.
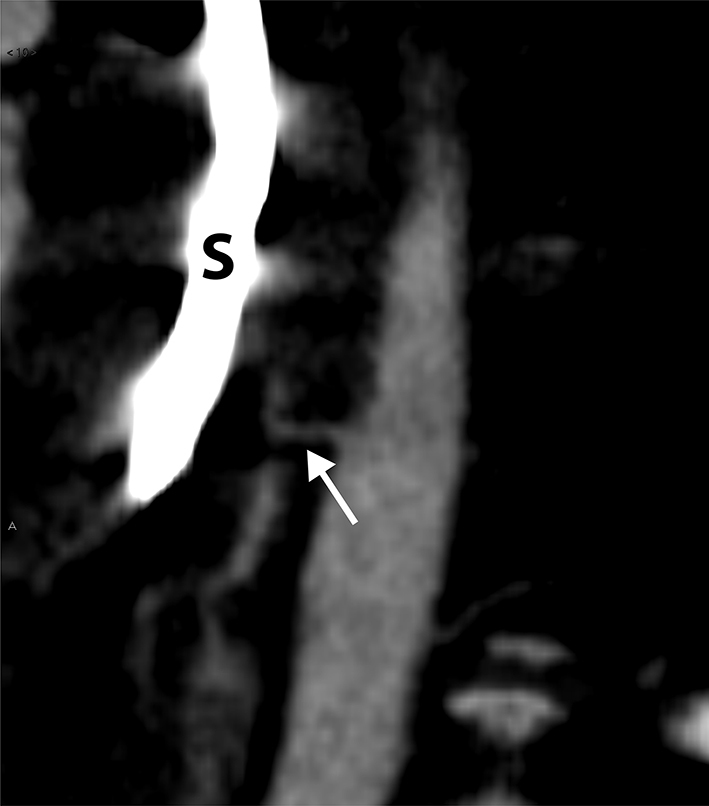
Sagittal reformatted image of the descending thoracic aorta shows the fistula (arrow) transversing anteriorly to the oesophagus; a Sengstaken–Blakemore tube (S) has been positioned in the oesophagus
Discussion
To our knowledge, this is the first case of an aorto-oesophageal fistula following stent placement for anastomotic leakage after RYGB.
The formation of an aorto-oesophageal fistula is mainly described as a complication of thoracic aortic aneurysm, aortic or oesophageal surgery and malignancy.3 There are several cases of an aorto-oesophageal fistula after implantation of an oesophageal stent. However, none of these patients underwent RYGB. To our knowledge, there is one similar case where the patient underwent gastric sleeve4 and one case where a patient developed an aortogastric fistula after implantation of the same type of stent as we used in this case.5
There are several factors that might have contributed to the formation of the aorto-oesophageal fistula; most likely the combined effect of infection and local pressure on the oesophagus caused by the oesophageal stent. The Niti-S BetaTM stent is specifically designed for anastomotic leakage after bariatric surgery. It is hypothesised that the double-bump prevents migration, but no clinical trials have been performed. It is therefore uncertain whether the double-bump might cause specific complications and whether this contributed to oesophageal ulceration and development of an aorto-oesophageal fistula in this case.
It is debatable whether the one-step approach for the revisional gastric bypass is also a causative factor. In our centre, it is considered safe to perform the gastric bypass at the same time as removing a band for erosion, if the tissue quality permits safe approximation and closure.6
In retrospect, awareness of the possibility of an aorto-oesophageal fistula in this case would have facilitated immediate surgical treatment. Although it is rare, bariatric surgeons and endoscopists should be aware of this life-threatening complication.
References
- 1.Almahmeed T, Gonzalez R, Nelson LG et al. Morbidity of anastomotic leaks in patients undergoing Roux-en-Y gastric bypass. Arch Surg 2007; : 954–957. [DOI] [PubMed] [Google Scholar]
- 2.Puli SR, Spofford IS, Thompson CC. Use of self-expandable stents in the treatment of bariatric surgery leaks: a systematic review and meta-analysis. Gastrointest Endosc 2012; : 287–293. [DOI] [PubMed] [Google Scholar]
- 3.Canaud L, Ozdemir BA, Bee WW et al. Thoracic endovascular aortic repair in management of aortoesophageal fistulas. J Vasc Surg 2014; : 248–254. [DOI] [PubMed] [Google Scholar]
- 4.Almadi MA, Bamihriz F, Aljebreen AM. Fatal aortoesophageal fistula bleeding after stenting for a leak post sleeve gastrectomy. World J Gastrointest Surg 2013; : 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deflandre J, Henroteaux D, Dandrifosse AC. Aorto-gastric fistula caused by an esophageal stent used in the treatment of bariatric surgery’s complications. Acta Gastroenterol Belg 2014; : 77–78. [PubMed] [Google Scholar]
- 6.Dang JT, Switzer NJ, Wu J et al. Gastric band removal in revisional bariatric surgery, one-step versus two-step: a systematic review and meta-analysis. Obes Surg 2016; : 866873. [DOI] [PubMed] [Google Scholar]


