Abstract
INTRODUCTION
In the 75–80% of urothelial bladder cancers (UBC) presenting as non-muscle invasive bladder cancer (NMIBC), transurethral resection of bladder tumour (TURBT) is the key treatment and staging procedure. In the 20–25% of patients with muscle invasive bladder cancer (MIBC), further cross-sectional imaging is required to complete the staging process before considering radical treatment. Given the adverse effects of ionising radiation, clinicians identify patients believed to have MIBC, and so requiring further imaging pre-TURBT, at the tumour histology/stage based on the tumour’s visual characteristics. There is minimal evidence describing the accuracy of such predictions in newly-diagnosed patients.
METHODS
Over a 6-year period, a database of patients undergoing resection of newly-diagnosed bladder lesions in a single UK centre was prospectively established. Predictions based on histology were simultaneously recorded, and the accuracy of these predictions of histology/stage subsequently assessed.
RESULTS
One hundred and twenty two (73.1%) patients with histologically confirmed NMIBC had predictions recorded versus 45 (26.9%) patients with MIBC. Visual assessment predictions of MIBC had a sensitivity of 88.9% (95% confidence interval [CI] 76.5%–95.2%) and a specificity of 91.0% (95% CI 84.6%–94.9%), giving a positive predictive value of 78.4% (95% CI 65.4%–87.5%) and a negative predictive value of 95.7% (95% CI 90.3%–98.1%).
CONCLUSIONS
We find that visual assessment is accurate in predicting the presence of MIBC. This supports the practice of stratifying patients at the time of initial cystoscopy for those requiring further radiological staging pre-TURBT.
Keywords: Transitional cell carcinoma, Cystoscopy, Urinary bladder neoplasms
Urothelial bladder cancer (UBC) is the fifth most common malignancy in Western nations. The typical diagnostic pathway relies on cystoscopy followed by transurethral resection to obtain histological confirmation of the tumour type, grade and stage. Transurethral resection of bladder tumour (TURBT) is therefore both a diagnostic and staging procedure, as the specimen can be analysed for depth of invasion. In the 75%–80% of tumours that present as non-muscle-invasive bladder cancer (NMIBC),1,2 TURBT, with or without re-transurethral resection (TUR),3 is also the key treatment modality and, in most cases, is the only staging procedure required. In the remaining 20%–25% of patients with muscle-invasive bladder cancer (MIBC), further cross-sectional imaging, usually with computed tomography (CT), is required for complete staging to identify lymph node involvement and distant metastases. Moreover, TURBT is inadequate for local disease control and further radical treatment, usually with chemotherapy and cystectomy or radiotherapy, is required.1,4
Post-TURBT, cross-sectional pelvic imaging is hard to interpret due to post-procedure artefacts such as perivesical inflammation, or reactive lymph nodes interpreted as nodal spread.5–7 It is therefore advantageous to carry out imaging prior to TURBT. Due to the adverse effects of ionising radiation, clinicians stratify, at the time of cystoscopic diagnosis, patients depending on the need to expose them to these risks prior to further surgical intervention. Such decisions should be based upon the visual characteristics of suspicious lesions, and thus rely on the individual urologist’s experience of how tumours of different stages tend to appear. This can be relatively subjective; for example, non-papillary tumours, those with a sessile configuration and those 1cm or larger have a significantly higher risk of muscle-invasion.8
Although the correlation of visual impression and final histology has previously been investigated in recurrent disease,9 the impact of bias on these results is unknown, as knowledge of a recurrent tumour’s previous grade and stage may influence the assessment. Little is known about how accurately urologists are able to predict visually the stage of a tumour based on cystoscopic findings in newly-diagnosed patients.
The aim of this study was to assess the clinical prediction of UBC stage based solely upon cystoscopic appearances at the first TURBT, and correlate these predictions with the final histology in a group of patients newly-diagnosed with bladder cancer.
Methods
Between 1996 and 2002, a record of all patients undergoing resection of newly-diagnosed bladder lesions in a single, district general hospital in the United Kingdom (The Wye Valley NHS Trust, UK) was prospectively established to facilitate discussions between surgeons and pathologists. Predictions of final histology were recorded in a database prior to histological confirmation. Predictions and resections were undertaken by two consultant urologists with a general urological practice (GMS, AKJ).
A retrospective analysis was undertaken some years later to assess the ability of surgeons to predict final histological stage. Both predictions and histopathology were reported using the World Health Organization 1973 classification.10 Where re-resection took place (ie in high-risk tumours following initial resection), the final histology after re-resection is presented.
Statistical analysis
The sensitivity and specificity of visual prediction for the presence of muscle-invasive disease was calculated using a standard 2×2 table comparing the relative ratios of true/false positives and negatives. Confidence intervals (CIs) for sensitivity and specificity were generated using the Wilson score.11
Results
Of 289 consecutive newly-diagnosed bladder cancer patients identified during the study period, 75 patients were subsequently excluded due to the presence of pathologies other than UBC, such as lymphoma in one specimen, or because the original stage and/or grade prediction at cystoscopy could not be confidently reconciled with the histopathology record. Of the remaining 214 patients, 161 (75.2%) were diagnosed with NMIBC and 53 (24.8%) with MIBC on final pathological analysis.
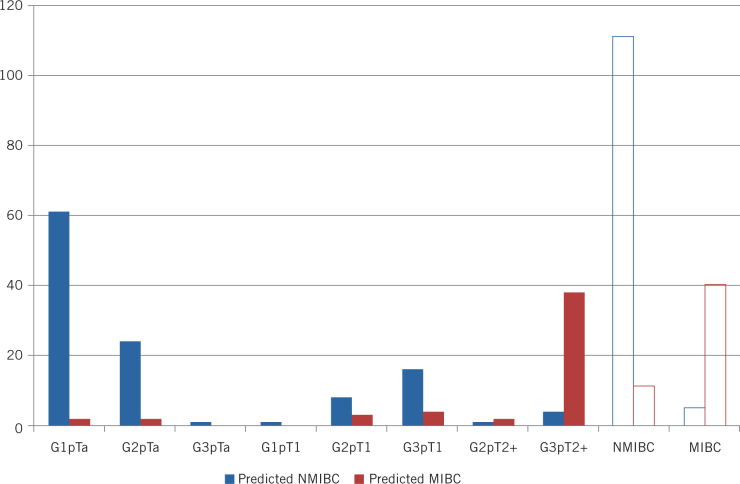
A stage prediction was recorded at the time of first TURBT for 167 (78%) patients. This was based purely on the visual appearances of the tumour at the time of resection. Of these, 122 (73.1%) patients were later confirmed on subsequent histology to have NMIBC, and 45 (26.9%) MIBC. The tumour characteristics are detailed in Table 1 and Figure 1.
Table 1.
Prediction of NMIBC and MIBC by grade and stage of tumour
| Final Histology | N (%) | Correctly Predicted N (%) |
|---|---|---|
| NMIBC or MIBC | ||
| NMIBC | 122 (73.1) | 111 (91.0) |
| MIBC | 45 (26.9) | 40 (88.9) |
| G1 pTa | 63 (37.7) | 61 (96.8) |
| G2 pTa | 26 (15.6) | 24 (92.3) |
| G3 pTa | 1 (0.6) | 1 (100.0) |
| G1 pT1 | 1 (0.6) | 1 (100.0) |
| G2 pT1 | 11 (6.6) | 8 (72.7) |
| G3 pT1 | 20 (12.0) | 16 (80.0) |
| G2 pT2+ | 3 (1.8) | 2 (66.7) |
| G3 pT2+ | 42 (25.1) | 38 (90.5) |
| Both Grade and Stage | ||
| G1 pTa | 16 | 10 (62.5) |
| G2 pTa | 2 | 0 (0.0) |
| G3 pTa | 0 | - |
| G1 pT1 | 0 | - |
| G2 pT1 | 2 | 1 (50.0) |
| G3 pT1 | 6 | 6 (100.0) |
| G2 pT2+ | 1 | 0 (0.0) |
| G3 pT2+ | 10 | 9 (90.0) |
MIBC = muscle-invasive bladder cancer; NMIBC = non-muscle-invasive bladder cancer.
Figure 1.
Prediction of NMIBC or MIBC by grade and stage of tumour
Of the 122 patients later confirmed as having NMIBC, 111 (91.0%) were correctly predicted based on visual appearance, while the remainder (11, 9.0%) were considered, incorrectly, to be MIBC. In the 45 patients later confirmed as having MIBC, 40 (88.9%) cases were correctly predicted as MIBC, while five (11.1%) were incorrectly considered to be NMIBC.
Cystoscopy as a diagnostic test for muscle-invasive tumours based solely on visual inspection therefore had a sensitivity of 88.9% (95% CI 76.5%–95.2%) and a specificity of 91.0% (95% CI 84.6%–94.9%). This yielded a positive predictive value of 78.4% (95% CI 65.4%–87.5%) and a negative predictive value of 95.7% (95% CI 90.3%–98.1%). The accuracy of these predictions by grade and T stage are shown in Table 1 and Figure 1.
Grade was also predicted in this cohort of patients, albeit less frequently than stage. Of the 167 patients in whom a stage prediction was made, only 37 also had a grade prediction recorded. Where both tumour grade and stage were predicted, there was complete concordance with the final histopathological assessment in 26 cases (70.3%) (Table 1).
Discussion
Prediction of UBC stage at the time of diagnosis is commonplace in clinical practice. However, there are little data in the literature as to the accuracy of such predictions.8,9,12 The majority that exist are focussed on the distinction of low-risk, non-invasive tumours to identify those that are amenable for office fulguration.9,13 This study, however, concentrated on the clinical ability to accurately predict muscle-invasion at the time of diagnosis, as this may allow the appropriate use of radiological staging prior to diagnostic TURBT. This is also the largest group of patients assessed to date in the literature. The findings suggest that the impression gained from initial cystoscopy is not only accurate at predicting the presence of muscle-invasion but also much more accurate at excluding its presence. A positive predictive value of 78.4% is not sufficient evidence to proceed to more aggressive, radical treatment based on the cystoscopic visual appearance of the tumour alone, but it lends a strong argument to staging the patient early (pre-TURBT) by way of cross-sectional imaging. A negative predictive value of 95.7% would support the omission of cross-sectional imaging for staging purposes in patients whose tumours do not have the characteristic appearances of MIBC and, therefore, avoids subjecting these patients to unnecessary risks from ionising radiation.
Others have studied the efficacy of cystoscopic impression as a tool for predicting pathological diagnosis in UBC. Satoh et al provided evidence, via a multivariate analysis, that visual characteristics of a tumour commonly used by urologists to gain an impression of tumour stage (size, configuration, previous histology, etc) are useful in predicting muscle invasion.8 Satoh et al, however, did not include any predictions of stage or grade prior to histological confirmation, unlike the data we present here.
Herr et al attempted prospective prediction of both stage and grade of bladder tumours using cystoscopy, and found that cystoscopic impression was highly accurate, with 94% of non-invasive (pTa) and 93% of low-grade neoplasias (G1) correctly identified using cystoscopy alone.9 This cohort of patients had recurrent disease, however, and previous histology from prior tumours was not blinded from the investigators. The authors concluded that the results that they presented may have been influenced by the knowledge that low-grade, low-stage tumours rarely progress on either parameter on recurrence.9 It is also noteworthy that the predictive accuracy in patients with G3pTa or pT1 disease of any grade were less impressive, at 59% and 60%, respectively, and that only one patient in the entire cohort had MIBC proven pathologically.9 The data that we present are in a group of newly-diagnosed patients with no prior histology available to influence the prediction.
Mitropoulos et al also investigated the accuracy of cystoscopic impression of both stage and grade of bladder tumours.12 They concluded that cystoscopy was not an accurate way of predicting stage, as they found complete agreement with pathological stage in only 64.5% of cases. The authors did not, however, consider grouping patients as NMIBC or MIBC, as in our study, which we believe to be of greater clinical significance. The patients selected by Mitropoulos et al were also a mixture of newly-diagnosed and recurrent patients, which may have influenced their results, as described earlier. Similarly, Cina et al concluded in a cohort of both newly-diagnosed and recurrent tumours that cystoscopic impression was not accurate in predicting invasion into the lamina propria or beyond (pT1+), as the sensitivity of cystoscopic impression alone was 88%, with a specificity of 57%.13 The sensitivity and specificity for predicting low-grade tumours was 91% and 46%, respectively.
In our patients, grade was much less commonly predicted than stage and, as the numbers are small, conclusions must be drawn carefully from these data. In the small group in whom predictions were made for both stage and grade, it is worth noting that this was completely accurate for both stage and grade in only 70.3% of cases. The choice of treatment of tumours in the urological office under local anaesthetic often relies solely on the visual features of the tumour, without histopathological assessment. The benefits of this approach in terms of convenience and reduced patient burden should be weighed carefully against the risks of not undertaking an histopathological examination of the lesion and potentially missing grade or stage progression.14 The balance of risks depends upon the experience of the clinician and their ability to correctly identify low-risk Ta tumours visually.14 Published data has shown that clinicians with a specialised bladder cancer practice can correctly predict G1 pTa recurrences in 93%–99% of patients,9 but, outside of such settings, predictions are less accurate,12 as we have demonstrated here. Clinically, such treatments are nevertheless usually reserved for the surveillance/recurrent tumour setting rather than newly-diagnosed tumours; the authors acknowledge that this was not the purpose of this study and suggest that further research into this area may be required.
Our study has a number of limitations. The source data originated between 1996 and 2002 and, at the time, they were collected prospectively to inform meetings and discussions between pathologists and urologists. More recently, the issue of stage prediction at cystoscopy and the potential for undertaking cross-sectional imaging prior to TURBT has become pertinent. As a consequence, the relevance of these data increased and they were analysed for the first time in 2015. Due to the passage of time, stage predictions for a number of the original consecutive series of patients could not be confidently reconciled with the histopathology data and, given the original purpose of the source data, no predictions were made for some patients. This latter factor may have introduced bias, especially if predictions were not made in those patients whose tumours had an ambiguous appearance. However, since predictions were made in over 78% patients where the necessary data were available, we feel that our findings remain valid. Furthermore, it should be noted that our data are derived from observations made during rigid cystoscopy immediately before TURBT, and not during flexible cystoscopy in the outpatient setting prior to TURBT. Overall, we feel that our findings are entirely transferrable to the flexible cystoscopy setting, as many workers consider the image quality of the latest colour charge-coupled device flexible cystoscopes to be as good if not better than that of rigid cystoscopes.
For MIBC, TURBT is frequently sub-optimal for staging,15 may delay definitive MIBC-appropriate therapy, and may contribute to dissemination of the tumour either extravesically due to bladder perforation or systemically by increasing the number of circulating tumour cells.16,17 It is conceivable that, in the future, the current pathways for the diagnosis, staging and treatment of suspected MIBC will be altered and, with improvements in imaging modalities, TURBT may be unnecessary and undesirable in MIBC patients. The key to commencing on any such pathway is the accurate prediction at the time of cystoscopy in combination with small pinch biopsies to confirm a cancer diagnosis and cross-sectional imaging for staging,18 approaches utilised in most other solid-tumour settings. Although such an approach would be considered a sea change in the management of bladder cancer, and that CT scanning has its staging limitations and is associated with radiation exposure, multiparametric magnetic resonance imaging (mpMRI) offers improvements over both.19–24 Such a pathway re-design is the subject of the National Institute for Health Research-funded randomised-controlled trial BladderPath: Image Directed Redesign of Bladder Cancer Treatment Pathways, which is currently being established. For this, patients who appear to have MIBC as assessed at flexible cystoscopy in a haematuria clinic and who are confirmed to have a high-grade bladder cancer on biopsy will be randomised to the existing pathway of TURBT, confirmation of MIBC, cross-sectional imaging, definitive therapy or to a new pathway of mpMRI to demonstrate MIBC and definitive therapy. Our evidence offers some credence to this approach.
Conclusions
We have demonstrated that cystoscopy is an accurate screening test for diagnosing MIBC based upon tumour appearance alone. This may permit appropriate radiological staging prior to TURBT in patients whose tumours appear to be muscle-invasive. A prospective, multicentre study should be conducted to validate these findings in contemporary clinical practice. Further data on the ability of urologists to visually predict grade and differentiate between NMIBC risk categories is also required.
Acknowledgement
We thank Sonia Warner for her assistance in managing the study database.
Conflicts of interest
RT Bryan has previously contributed to an advisory board for Olympus Medical Systems with regard to narrow band imaging cystoscopy.
References
- 1.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet 2009; : 239–249. [DOI] [PubMed] [Google Scholar]
- 2.Bryan RT, Zeegers MP, van Roekel EH et al. A comparison of patient and tumour characteristics in two UK bladder cancer cohorts separated by 20 years. BJU Int 2013; : 169–175. [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Zigeuner R et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013; : 639–653. [DOI] [PubMed] [Google Scholar]
- 4.Witjes JA, Compérat E, Cowan NC et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014; : 778–792. [DOI] [PubMed] [Google Scholar]
- 5.Swinnen G, Maes A, Pottel H et al. FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur Urol 2010; : 641–647. [DOI] [PubMed] [Google Scholar]
- 6.Maurer T, Souvatzoglou M, Kübler H et al. Diagnostic efficacy of [11C]choline positron emission tomography/computed tomography compared with conventional computed tomography in lymph node staging of patients with bladder cancer prior to radical cystectomy. Eur Urol 2012; : 1,031–1,038. [DOI] [PubMed] [Google Scholar]
- 7.Vargas HA, Akin O, Schüder H et al. Prospective evaluation of MRI, 11C-acetate PET/CT and contrast-enhanced CT for staging of bladder cancer. Eur J Radiol 2012; : 4,131–4,137. [DOI] [PubMed] [Google Scholar]
- 8.Satoh E, Miyao N, Tachiki H et al. Prediction of muscle invasion of bladder cancer by cystoscopy. Eur Urol 2002; : 178–181. [DOI] [PubMed] [Google Scholar]
- 9.Herr HW, Donat SM, Dalbagni G. Correlation of cystoscopy with histology of recurrent papillary tumors of the bladder. J Urol 2002; : 978–980. [DOI] [PubMed] [Google Scholar]
- 10.Mostofi FK, Sobin LH, Torloni H. Histological typing of urinary bladder tumours. International Histological Classification of Tumors No 10. Geneva: World Health Organization, 1973. [Google Scholar]
- 11.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998; : 857–872. [DOI] [PubMed] [Google Scholar]
- 12.Mitropoulos D, Kiroudi-Voulgari A, Nikolopoulos P et al. Accuracy of cystoscopy in predicting histologic features of bladder lesions. J Endourol 2005; : 861–864. [DOI] [PubMed] [Google Scholar]
- 13.Cina SJ, Epstein JI, Endrizzi JM et al. Correlation of cystoscopic impression with histologic diagnosis of biopsy specimens of the bladder. Hum Pathol 2001; : 630–637. [DOI] [PubMed] [Google Scholar]
- 14.Mostafid H, Kirby R, Fitzpatrick JM et al. The Safe and Economical Care of Ta Bladder Cancer. Urol Pract 2014; : 176–183. [Google Scholar]
- 15.Kulkarni GS, Hakenberg OW, Gschwend JE et al. An updated critical analysis of the treatment strategy for newly diagnosed high-grade T1 (previously T1G3) bladder cancer. Eur Urol 2010; : 60–70. [DOI] [PubMed] [Google Scholar]
- 16.Engilbertsson H, Aaltonen KE, Bjürnsson S et al. Transurethral bladder tumor resection can cause seeding of cancer cells into the bloodstream. J Urol 2015; : 53–57. [DOI] [PubMed] [Google Scholar]
- 17.Ohguchi N, Sakaida N, Okamura A et al. Extravesical tumor implantation caused by perforation during transurethral resection of a bladder tumor: a case report. Int J Urol 1997; : 516–518. [DOI] [PubMed] [Google Scholar]
- 18.Bouchelouche K, Turkbey B, Choyke PL. PET/CT and MRI in Bladder Cancer. J Cancer Sci Ther 2012; : [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeuchi M, Sasaki S, Naiki T et al. MR imaging of urinary bladder cancer for T-staging: a review and a pictorial essay of diffusion-weighted imaging. J Magn Reson Imaging 2013; : 1,299–1,309. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi M, Sasaki S, Ito M et al. Urinary bladder cancer: diffusion-weighted MR imaging—accuracy for diagnosing T stage and estimating histologic grade. Radiology 2009; : 112–121. [DOI] [PubMed] [Google Scholar]
- 21.Rosenkrantz AB, Mussi TC, Melamed J et al. Bladder cancer: utility of MRI in detection of occult muscle-invasive disease. Acta Radiol 2012; : 695–699. [DOI] [PubMed] [Google Scholar]
- 22.Rosenkrantz AB, Haghighi M, Horn J et al. Utility of quantitative MRI metrics for assessment of stage and grade of urothelial carcinoma of the bladder: preliminary results. AJR Am J Roentgenol 2013; : 1,254–1,259. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson SB, Bonington SC, Kershaw LE et al. Dynamic contrast-enhanced MRI in patients with muscle-invasive transitional cell carcinoma of the bladder can distinguish between residual tumour and post-chemotherapy effect. Eur J Radiol 2013; : 2,161–2,168. [DOI] [PubMed] [Google Scholar]
- 24.Rajesh A, Sokhi HK, Fung R et al. Bladder cancer: evaluation of staging accuracy using dynamic MRI. Clin Radiol 2011; : 1,140–1,145. [DOI] [PubMed] [Google Scholar]