Abstract
Background
Multi drug resistant tuberculosis (MDR-TB) poses formidable challenges to TB control due to its complex diagnostic and treatment challenges and often associated with a high rate of mortality. Accurate and rapid detection of MDR-TB is critical for timely initiation of treatment. Line Probe Assay (LPA) is a qualitative in vitro diagnostic test based on DNA-STRIP technology for the identification of the M. tuberculosis complex and its resistance to rifampicin (RMP) and/or isoniazid (INH). Hain Lifescience, GmbH, Germany has improved the sensitivity of Genotype MTBDRplus VER 2.0 LPA for the detection of MDR-TB; with the possibility of applying the tool in smear negative sputum samples.
Method
A cross sectional study was conducted on 274 presumptive MDR-TB patients referred to the National TB Reference Laboratory (NTRL), Ethiopian Public Health Institute (EPHI) who submitted sputum samples for laboratory diagnosis of drug resistant-TB testing. Seventy-two smear and culture positive samples processed in smear positive direct LPA category and 197 smear negative sputum samples were processed for direct LPA. Among the smear negative samples 145 (73.6%) were culture negative and 26 (13.2%) were culture positive. All specimens were processed using NALC-NaOH method and ZN smear microscopy done from sediments. Genotype MTBDRplus VER 2.0 done from processed sputum sediments and the result was compared against the reference, BACTEC MGIT 960 culture and DST. Sensitivity, specificity, PPV and NPV of Genotype MTBDRplus VER 2.0 assay was determined and P-value <0.05 was considered as statistically significant.
Results
The sensitivity, specificity, PPV and NPV of Genotype MTBDRplus VER 2.0 LPA were 96.4, 100, 100 and 96.9%, respectively for the detection of MDR-TB from direct smear positive sputum samples. The sensitivity, specificity, PPV and NPV of Genotype MTBDR plus VER 2.0 LPA were 77.8, 97.2, 82.4 and 97.2%, respectively, for the detection of M. tuberculosis from direct smear negative sputum samples. Fourteen (53.8%) samples had valid results with LPA among the 26 smear negative culture positive samples. The remaining 8 (30.8%) and 4 (15.4%) were invalid and negative with LPA, respectively. The sensitivity and specificity of Genotype MTBDRplus VER 2.0 LPA were 100% for the detection of MDR-TB among 14 direct smear negative and culture positive sputum samples.
The most common mutations associated with RMP and INH resistance were S531L and S315TL, respectively. A single rare mutation (C15T/A16G) was detected for INH resistance.
Conclusion
The diagnostic performance of Genotype MTBDRplus VER 2.0 LPA in direct smear positive sputum sample was highly sensitive and specific for early detection of MDR-TB. However, the diagnostic performance of this molecular assay in direct smear negative sputum sample was low and showed a high level of invalid results for detection of M. tuberculosis and its resistance to RMP and/or INH so it is unlikely to implement Genotype MTBDRplus VER 2.0 for the detection of MDR-TB in direct smear negative sample in our routine settings. The sensitivity of the assay should be improved for detection of MDR-TB in direct smear negative sputum specimens.
Keywords: Performance, Genotype MTBDRplus VER 2.0, MDR-TB
Background
Tuberculosis (TB) remains a major global health problem, responsible for ill health among millions of people each year. TB ranks as the second leading cause of death from an infectious disease worldwide, after the HIV. The latest estimates included in this report are that there were 9.0 million new TB cases in 2013 and 1.5 million TB deaths (1.1 million among HIV-negative people and 0.4 million among HIV-positive people). TB mortality is unacceptably high given that most deaths are preventable if people can access health care for a diagnosis and the correct treatment is provided [1, 2].
According to the 2011 Ministry of Health report, TB is the eighth leading cause of hospital admissions and the third leading cause of hospital deaths in Ethiopia. In 1992, to prevent and limit the spread of the disease in Ethiopia, the government implemented the DOTS Strategy, the backbone of global TB control, whose objectives are the diagnosis of 70% of new smear positive TB cases and achieving 85% cure [3].
The first population-based national tuberculosis prevalence survey in Ethiopia which was done in 2010–2011 showed that the prevalence of smear-positive TB and bacteriologically confirmed TB were 108/100000 (95%CI 73–143), and 277/100000 (95%CI 208–347) respectively. The finding indicated that the actual TB prevalence in Ethiopia was much lower than the World Health Organization (WHO) estimates [3].
Globally in 2013, data from drug resistance surveys and continuous surveillance among notified TB cases suggest that 3.5% of newly diagnosed TB cases and 20.5% of those previously treated for TB had MDR-TB. The highest levels of MDR-TB are found in Eastern Europe and central Asia, where in some countries more than 20% of new TB cases and more than 50% of those previously treated for TB have MDR-TB [1].
The first countrywide anti-tuberculosis drug resistance survey in Ethiopia was carried out between 2003 and 2006. The survey reported the levels of MDR-TB in new and in previously treated patients, as 1.6% and 11.8% respectively [4].
The second round national anti-tuberculosis drug resistance surveillance in Ethiopia also done from November 2011 to June 2013. Multidrug resistant isolates were detected in 80 of the total 1651 samples from new and previously treated cases, making the overall prevalence of MDR-TB was 4.8%. The prevalence of MDR-TB among survey participants was 2.3% and 17.8% among new and previously treated cases, respectively [5].
Conventional drug susceptibility testing using a solid medium such as Lowenstein – Jensen is time consuming, whereas liquid medium based methods such as Mycobacterium Growth Indicator Tube (MGIT) system are sensitive but expensive. New assay such as Line Probe Assay (LPA) have been developed to detect resistance faster and reliable using genotype rather than phenotype and have been endorsed by WHO for the fast and reliable detection M. tuberculosis complex and its resistance to RMP and/or INH [6].
The Genotype Mycobacterium tuberculosis drug resistant (MTBDR) plus VER 2.0 is a qualitative in vitro test for the identification of the M. tuberculosis complex and its resistance to rifampicin and/or isoniazid from pulmonary smear positive and negative clinical specimens and cultivated isolates. This molecular genetic assay is based on DNA-STRIP technology and it includes DNA extraction, master mix preparation and addition, multiplex amplification with biotinylated primers and detection with reverse hybridization. The test is an aid in the rapid diagnosis of MDR-TB which is a prerequisite for the appropriate treatment initiation [6].
The aim of this study was to evaluate and provide information about the diagnostic performance of Genotype MTBDRplus VER 2.0 LPA for the detection of drug resistance TB in smear positive and negative sputum samples comparing against conventional liquid culture based reference standard method, BACTEC MGIT 960 culture and DST. This study also provides information on effective use of the test and/or for further recommendations in smear negative sputum samples in routine laboratory service. Evaluating new molecular tools such as Genotype MTBDRplus VER 2.0 LPA offer opportunity to scale up DST capacity in Ethiopia.
Methods
A cross sectional study was conducted on presumptive MDR-TB patients referred to the NTRL, Ethiopian Public Health Institute(EPHI) from April to August 2015 in our routine laboratory setting. The Genotype MTBDRplus VER 2.0 assay was compared against conventional liquid culture based reference standard method, BACTEC MGIT 960 culture and DST.
Sample size
Sputum samples were collected from Presumptive MDR-TB patients who were >15 years of age with consent based on non-probability convenience sampling technique [7] and calculated as followed below.
N = (zα/2)2p(1 − P)/d2
N = Number of individuals to be participated in the research,
z = standard normal distribution curve value for 95% confidence level, zα/2 = 1.96.
d = margin of error taken as 5%.
P = sensitivity of the assay, 97%(0.97).
N = 194, Sample size.
In standard culturing technique there is culture contamination and culture negative with acceptable range, However MGIT culture is more prone to culture contamination and there was low record that smear negatives turned to be culture positive so for each, 20% contingency added and this would compensate the samples that lost by contamination and culture negativity.
A total of 274 samples collected for the study considering contingency for samples that would be lost by culture contamination and culture negativity.
After AFB smear microscopy done from processed sediment, the sample would be categorized in to smear negative and smear positive sputum sample to process LPA accordingly. After smear microscopy we found 72 smear positive and 202 smear negative sputum sample which would be processed for direct smear positive LPA and smear negative LPA, respectively in the study.
Specimen collection, storage and transportation
Prior to collection of specimen, eligible study participants were signed on the consent form and basic demographic data and clinical information concerning the previous history of TB checked and obtained from the request form. Based on the criteria for Presumptive MDR-TB and Programmatic Management of Drug resistant Tuberculosis (PMDT) [8] who satisfied the study inclusion criteria were asked to provide one morning 5 ml sputum sample in a sterile screw cap universal disposable container provided for the routine diagnosis. The sputum samples were kept in a refrigerator at a maximum temperature of +40c until the specimens were processed for culture within two days of delivery.
Laboratory investigation
Sample processing, inoculation and smear microscopy
We processed 274 sputum samples for digestion, decontamination and concentration (Fig. 1). Equal volumes of sputum and reagents (NaoH-Nacitrate with NALC) added to 50 ml falcon tube up to 10 ml inside biological safety cabinet (BSC) and mixed with vortex for 20 sec incubated for 15 min. After addition of 35 ml of Phosphate buffer solution the mixture centrifuged for 15 min at 3000 g in 40c safety centrifuge. Smear preparation was made from the sediment with a sterile loop for AFB microscopy. Then the sediment was neutralized and resuspended in a 1 ml phosphate buffer solution and inoculated to Liquid media (Middle brook 7H9 broth base) in which PANTA and Supplement were added for the growth of the bacilli. MGIT 96o machine loaded with the inoculated media for the incubation and growth of the culture. For quality control, start and control sterile distilled water were processed as a sample to ensure the quality of sample processing using start and end control. Smears which is prepared from the sediment were stained with Zeihl Nelson and stained smears were read with light microscopy [9–11].
Fig. 1.
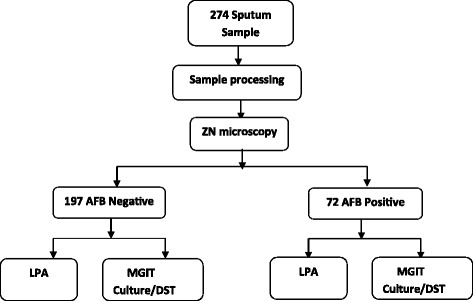
Flow diagram for AFB negative and AFB positive sample for laboratory investigation of LPA and MGIT culture and DST. *ZN-Zeihl Nelson, MGIT-Mycobacterium Growth Indicator Tube, LPA-Line Probe AssayNote: 5 samples lost during sample processing
Identification
To differentiate whether the growth is due to contamination with other microorganisms or true M. tuberculosis complex growth, we performed blood agar inoculation and ZN smear for MGIT positive growths. The Rapid TB anigen (SD Bioline) was tested to confirm the presence of organisms belonging to M. tuberculosis complex once growth is observed in MGIT. Known positive and negative control strains were tested for Rapid TB antigen per new batch [9–11].
Line probe assay
DNA was extracted from the appropriate sample using chemical A and B inside BSC. After final centrifugation the supernatant was taken as DNA extract. Master mix was prepared in a clean room to prevent contamination of molecular laboratory. In addition room, 5 μl of DNA extracts was added to the corresponding PCR tubes, 5 μl of DNA extract from H37Rv quality control strain to the positive control tubes and 5 μl of distilled water the negative control tube. After addition, the mixture with the polymerase chain reaction (PCR) tube placed in to PCR machine for amplification. After completion of PCR process, the amplicon was detected with a series of procedures by adding different reagents to the strip. The strips were formed color bands after addition of the final substrate reagent [6].
MGIT DST
We performed MGIT DST by inoculating two MGIT tubes with the test culture. A known concentration of a test drug was added to one of the MGIT tubes, and growth was compared with the MGIT tube without the drug (growth control). Growth was monitored by the BACTEC 960 instrument which automatically interprets results as susceptible or resistant. One H37RV sensitivity strain was run per batch of DST set for quality control purpose [9–11].
Data processing and statistical analysis
Statistical analysis was carried out using SPSS version 20 software packages. Sensitivity, specificity, PPV and NPV of the MTBDRplus VER 2.0 line probe assay was calculated among smear positive and negative sputum samples, comparing the results with the reference standard method, BACTEC MGIT 960 culture and DST and the results was interpreted based on 95% confidence interval, statistical significant was taken at p-value <0.05.
Results
Sociodemographic characteristics
A total of 274 subjects were included in this study. Among these 169 (61.7%) were male and the average age was 37 years. One hundred-thirty four (48.9%) had no previous history of TB treatment, 78 (28.5%) were relapse, 32 (11.7%) were return after default, 18 (6.5%) were treatment failure, 1 (0.4%) MDR-TB contact and 11 (4%) had unknown treatment history.
Performance of LPA from smear positive sample
Seventy-two samples were smear positive and culture positive. Results of LPA were compared with results of Mycobacterium Growth Indicator Tube (MGIT) first line DST (Fig. 1). The sensitivity, specificity, PPV and NPV of Genotype MTBDRplus VER 2.0 LPA were 88.2 (30/34) 89.5 (34/38), 88.2 (30/34) and 89.5 (34/38) %, respectively for the detection of RMP resistance directly from smear positive sputum sample. The sensitivity, specificity, PPV and NPV of Genotype MTBDRplus VER 2.0 LPA were 91.7 (33/36), 97.2 (35/36), 97.1 (33/34) and 92.1(35/38) %, respectively for the detection of INH resistance directly from smear positive sputum sample. The sensitivity, specificity, PPV and NPV of Genotype MTBDR plus VER 2.0 LPA were 96.4(27/28), 100 (31/31), 100 (27/27) and 96.9 (31/32) %, respectively for the detection of MDR-TB directly from smear positive sputum sample (Table 1).
Table 1.
Performance characteristics of Genotype MTBDRplus VER 2.0 LPA for detection RMP and INH resistance and MDR-TB in smear positive direct sample, 2015
| Detection of drug resistance | MGIT R | MGIT S | LPA R | LPA S | Sensitivity% | Specificity% | PPV% | NPV% |
|---|---|---|---|---|---|---|---|---|
| (95%* CI) | (95%* CI) | (95%* CI) | (95%* CI) | |||||
| *RMP | 34 | 38 | 34 | 38 | 88.2 (72.6–96.7) | 89.5 (75.2–97.1) | 88.2 (72.6–96.7) | 89.5 (75.2–97.1) |
| *INH | 36 | 36 | 34 | 38 | 91.7 (77.5–98.3) | 97.2 (85.5–99.9) | 97.1 (84.7–99.9) | 92.1 (78.6–98.3) |
| *MDR-TB (RMP&INH) | 28 | 31 | 27 | 32 | 96.4 (81.7–99.9) | 100 (88.8–100) | 100 (87.2–100) | 96.9 (83.8–99.9) |
*RMP Rifampicin, INH Isoniazid, MDR-TB Multidrug resistant Tuberculosis, R Resistant, S Susceptible, CI Confidence Interval
Performance of LPA from smear negative direct sputum sample
A total of 197 smear negative sputum samples were processed for direct LPA (Fig. 1) and from these 145(73.6%) were culture negative and 26(13.2%) were culture positive. Among 145 smear negative and culture negative samples, LPA results were negative in 139 (96%), Invalid in 3 (2%) and falsely detected in 3 (2%) of the sample (Fig. 2).
Fig. 2.
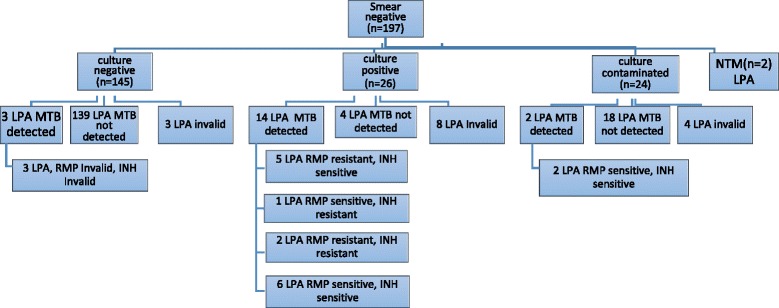
Performance of LPA from a total of 197 smear negative direct sputum sample. *LPA-Line Probe Assay, MTB - Mycobacterium Tuberculosis, NTM-Nontuberculous Mycobacterium
The sensitivity of Genotype MTBDRplus VER 2.0 LPA found to be 77.8 (14/18) % for the detection of M. tuberculosis from direct smear negative sputum sample (Table 2).
Table 2.
Performance characteristics of Genotype MTBDR plus VER 2.0 LPA for detection M. tuberculosis in smear negative sample, 2015
| MGIT positive | MGIT negative | LPA positive | LPA negative | Sensitivity% | Specificity% | PPV% | NPV% |
|---|---|---|---|---|---|---|---|
| (95%* CI) | (95%* CI) | (95%* CI) | (95%* CI) | ||||
| 18 | 142 | 17 | 143 | 77.8 (52.4–93.6) | 97.9 (94–99.6) | 82.4 (56.6–96.2) | 97.2 (93–99.2) |
*CI Confidence Interval
Among the 26 smear negative and culture positive sample the LPA had valid results in 14 of the samples for the detection of RMP and INH resistance. Low specificity and PPV was found for the detection of RMP resistance directly from smear negative and culture positive sputum sample. The sensitivity of Genotype MTBDR plus VER 2.0 LPA was low, 60 (3/5) % for the detection of INH resistance directly from smear negative and culture positive sputum sample. The sensitivity, specificity, PPV and NPV of Genotype MTBDRplus VER 2.0 LPA were 100 (2/2), 100 (5/5), 100 (2/2) and 100 (5/5) % respectively, for the detection of MDR-TB directly from smear negative and culture positive sputum sample (Table 3).
Table 3.
Performance characteristics of Genotype MTBDRplus VER 2.0 LPA for detection RMP and INH resistance and MDR-TB in smear negative and culture positive direct sample, 2015
| Detection of drug resistance | MGIT R | MGIT S | LPA R | LPA S | Sensitivity% | Specificity% | PPV% | NPV% |
|---|---|---|---|---|---|---|---|---|
| (95%* CI) | (95%* CI) | (95%* CI) | (95%* CI) | |||||
| *RMP | 3 | 11 | 7 | 7 | 100 (29.2–100) | 63.6 (30.8–89.1) | 42.9 (9.9–81.6) | 100 (59–100) |
| *INH | 5 | 9 | 3 | 11 | 60 (14.7–94.7) | 100 (66.4–100) | 100 (29.2–100) | 81.8 (48.2–97.7) |
| *MDR-TB (RMP&INH) | 2 | 5 | 2 | 5 | 100 (15.8–100) | 100 (47.8–100) | 100 (15.8–100) | 100 (47.8–100) |
*RMP Rifampicin, INH Isoniazid, MDR-TB Multidrug resistant Tuberculosis, CI Confidence Interval
Mutations associated with RMP and INH drug resistant TB
The frequency of mutations associated with RMP and INH drug resistant TB analysed among 35 results of Genotype MTBDRplus VER 2.0 and concordant with MGIT DST.
Twenty seven (77.1%) were missing of wild type 8 (530–533) and mutation S531L in rpoB gene and this was the most frequent mutation associated with RMP resistance. On the other hand 28 (80%) were missing wild type (315) and mutation S315TL in KatG gene and this was the most frequent mutation associated with INH resistance. A single rare mutation (C15T/A16G) in inhA gene was detected in this study (Table 4).
Table 4.
Mutations associated with RMP and INH drug resistant TB among concordant Resistant result with Genotype MTBDRplus VER 2.0 and MGIT DST
| RMP resistance | INH resistance | |||||
|---|---|---|---|---|---|---|
| rpoB gene | KatG gene | inhA gene | ||||
| WT1–8 missing | Mutant | WT missing | Mutant | WT1–2 missing | Mutant | Frequency |
| 530–533 (WT8) | S531L | 315 | S315TL | - | - | 23 |
| 530–533 (WT8) | S531L | 315 | - | - | - | 3 |
| 526–529 (WT7) | H526Y | 315 | S315TL | - | - | 1 |
| 530–533 (WT8) | S531L | 315 | - | - | - | 1 |
| 526–529 (WT7) | H526Y | 315 | S315TL | -15,-16 (WT1) | C15T | 1 |
| -8 (WT2) | A16G | |||||
| a510–513 (WT2) | - | 1 | ||||
| 516–519 (WT4) | ||||||
| 522–526 (WT6) | ||||||
| 526–529 (WT7) | ||||||
| a505–509 (WT1) | - | 1 | ||||
| 513–517 (WT3) | ||||||
| 526–529 (WT7) | ||||||
| 530–533 (WT8) | ||||||
| b315 | S315TL | - | - | 3 | ||
| b315 | - | -15,-16 (WT1) | - | 1 | ||
aMutations associated with RMP mono resistance, bMutations associated with INH mono resistance
Discussion
In this study the diagnostic performance of Genotype MTBDRplus VER 2.0 LPA was high for the detection of MDR-TB (sensitivity-96.4, specificity-100%) in smear positive sputum sample. Similar studies have reported high sensitivity and specificity for the detection of MDR-TB in smear positive sputum sample in Uganda by Albert et al. [12], India by Maduri et al. [13], Thailand by Anek et al. [14] and Moldova by Crudu et al. [15]. Only one sample (1.4%) detected as falsely susceptible for detection of MDR-TB and the same result was also detected from Xpert MTB/RIF assay. As both tests share a similar principle of molecular technique and there might be mutations present out of rpoB gene region, one falsely RMP sensitive result has been detected and this could be explained by the fact that, about 5% and 10% to 25% of resistant strains are thought to have mutations outside rpoB and, katG - inhA loci respectively [16].
In this study relatively high sensitivity and specificity was observed for the detection of RMP (sensitivity-88.2 and specificity-89.5%) and INH (sensitivity-91.7 and specificity-97.2%) resistance. Higher sensitivity and specificity reported for the detection of both RMP and INH resistance in a study done in India by Raizada et al. [17] and Germany by Hillemann et al. [18]. The reason for higher sensitivity and specificity could be due to large sample size used in the study by Raizada et al. [17] and gene sequencing used as reference standard to characterize the genotype of resistance mutations in the study by Hillemann et al. [18]. A systematic review and meta-analysis of 14 comparisons reviewed by Ling et al. [19] were also identified very high and consistent pooled sensitivity and specificity for the detection of RMP and INH resistance in smear positive sample. On the other hand lower sensitivity reported in Brazil by Maschmann et al. [16] and in India by Singhal et al. [20] for INH. This could be explained by the fact that, it is well known that about 10% to 25% of INH resistant strains are thought to have mutations outside katG and inhA loci [16].
The performance of LPA from smear negative sample showed low detection and similar report observed in the USA by Luetkemeyer et al. [21] that evaluated LPA for the detection of MTB and resistance to RMP and INH found very low sensitivity (44.1%) for AFB smear negative specimen however relatively high sensitivity (79.8%) reported in Brazil by Maschmann et al. [16]. This might be due to appropriate selection of the study population that used sputum of patients who had treatment failure or relapse in a routine outpatient setting in the study. In addition, in the study done in South Africa by Barnard et al. [22] to evaluate LPA with Xpert MTB/RIF from smear positive and negative sample reported that the diagnostic performance of the GenoType MTBDRplus VER 2 LPA was equivalent to that of the Xpert MTB/RIF assay. This could be retreatment cases were selected given their high risk of associated drug resistance and both assays were molecular techniques and share the same principle of testing that detect mutations conferring in rpoB gene region for the detection of RMP resistance.
Among the 26 smear negative sample and had culture positive growth, LPA detected valid result in 14(53.8%), negative in 4(15.4) and invalid in 8(30.8%) samples and this suggests that detection of M. tuberculosis and its resistance to RMP and/or INH prone high level of invalid results. In this study, high sensitivity and specificity observed in smear negative sample and which had confirmed culture growth for the detection of MDR-TB, however there were few number of valid results (2 for sensitivity and 5 for specificity) for comparison from other studies. In addition, high level of invalid and false negative results observed.
The most common mutation associated with RMP by Raizada et al. [17] was similar to the present study. This finding was also common with findings in a study done in Uganda by Albert et al. [12].
Conclusion
The diagnostic performance of Genotype MTBDRplus VER 2.0 LPA in direct smear positive sputum sample was highly sensitive and specific for early detection of MDR-TB. The diagnostic performance of Genotype MTBDRplus VER 2.0 LPA in direct smear negative sputum sample was low and showed a high level of invalid results for detection of M. tuberculosis and its resistance to RMP and/or INH so it is unlikely to implement Genotype MTBDRplus VER 2.0 for the detection of MDR-TB in direct smear negative sample in our routine settings. The sensitivity of the assay should be improved for detection of MDR-TB in direct smear negative sputum specimens.
Acknowledgment
We would like to thank National TB Reference Laboratory, Ethiopian Public Health Institute and Addis Ababa University, Department of Medical Laboratory Sciences for their technical and financial support.
Availability of data and materials
All raw data of sociodemographic and consent of study participants available and well documented. A document for ethical clearance from ethical review office also available. All data and materials that support the final result are available in excel sheet and also in printed result form. We can also access the result from the MGIT 960 instrument data base.
Authors’ contribution
AM designed and carried out the study and, wrote the manuscript. KD and AK advised all parts of the study, including in the writing of the manuscript. ZY and MG involved in the data analysis of the study. ZD, SM, BY, GD, HM, MT and DA participated in the laboratory analysis of sample processing, culture, MGIT culture and DST, LPA and Xpert MTB/RIF testing. All authors read and approved the final manuscript.
Competing interest
We declare that there is no any conflict of interest that might have influenced the performance and the presentation of the work described in this manuscript.
Ethics approval and consent to participate
The study was approved by Department of Research and Ethical Review Committee, Addis Ababa University. Written consent was obtained from the study participants. Test results were reported to the clinician through the routine system of NRL, EPHI and confidentiality were maintained.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- DNA
Deoxyribonucleic acid
- DST
Drug Susceptibility Testing
- EPHI
Ethiopian Public Health Institute
- HIV
Human Immunodeficiency Virus
- INH
Isoniazid
- LPA
Line Probe Assay
- M. tuberculosis
Mycobacterium tuberculosis
- MDR TB
Multi drug resistance Tuberculosis
- MGIT
Mycobacterium Growth Indicator Tube
- MTBDR
Mycobacterium tuberculosis drug resistant
- NPV
Negative Predictive Value
- NTRL
National Tuberculosis Reference Laboratory
- PMDT
Programmatic Management of Drug resistance Tuberculosis
- PPV
Positive Predictive Value
- RMP
Rifampicin
- TB
Tuberculosis
- WHO
World Health Organization
References
- 1.World Health Organization . Annual Report. Geneva: WHO; 2014. Global Tuberculosis Report. [Google Scholar]
- 2.Sinha N, Mishra GJ. Psycho-social problems of TB patients: a social work intervention. JHSS. 2015;20(4):81–85. [Google Scholar]
- 3.Kebede AH, Alebachew Z, Tsegaye F, Lemma E, Abebe A, Agonafir M, et al. The first population based national tuberculosis prevalence survey in Ethiopia. Int J Tuberc Lung Dis. 2014;18(6):635–639. doi: 10.5588/ijtld.13.0417. [DOI] [PubMed] [Google Scholar]
- 4.WHO Antituberculosis Drug Resistance in the world. Fourth Global Report. Geneva: WHO; 2008.
- 5.Eshetu L, Beniam F, Abebaw K, Muluwork G, Zelalem Y, Ribka F, et al. Second round national anti-tuberculosis surveillance. Capetown: ASLM 2nd Round Conference; 2014. [Google Scholar]
- 6.GGenotype MTBDRplus VER 2.0 instruction for use. Testing instruction. Germany: Hain Life Sciences; 2012.
- 7.TDR. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol. 2010:S17–29. [PubMed]
- 8.Guidelines for clinical and programmatic management of TB, Leprosy and TB/HIV in Ethiopia. Addis Ababa: Ministry of Health; 2012. 5th edition.
- 9.Kent PT, Kubica PG. Public Health Mycobacteriology: a guide for the level III laboratory. Atlanta: U.S. Deapartement of Health and Public Health Services, Centers for Disease Control; 1985.
- 10.Siddiqi SH, Gerdes SR. FIND MGIT procedure manual for Bactec MGIT 960 System. Maryland: BD Diagostics; 2006.
- 11.SD Bioline package insert for rapid TB antigen identification. Yongin: Standard Diagnostic; 2007.
- 12.Albert H, Bwanga F, Mukkada S, Nyesiga B, Ademun JP, Lukyamuzi G, et al. Rapid screening of MDR-TB using molecular line probe assay is feasible in Uganda. BMC Infect Dis. 2010;10:41. doi: 10.1186/1471-2334-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maduri K, Deshpande S, Dharmashale S, Bharadwaj R, et al. Utility of line probe assay for the early detection of multidrug-resistant pulmonary tuberculosis. J Global Infect Dis. 2015;7(2):60–65. doi: 10.4103/0974-777X.157237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anek R, Sinthuwattanawibool C, Podewils LJ, McCarthy K, Ngamlert K, Promsarin B, et al. Validation of the GenoType MTBDRplus assay for detection of MDR-TB in a public health laboratory in Thailand. BMC Infect Dis. 2010;10:123. doi: 10.1186/1471-2334-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crudu V, Stratan E, Romancenco E, Allerheiligen V, Hillemann A, Morarua N. First evaluation of an improved assay for molecular genetic detection of tuberculosis as well as Rifampin and Isoniazid resistances. J Clin Microbiol. 2012;5(4):1264–1269. doi: 10.1128/JCM.05903-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maschmann RA, Spies FS, Nunes LS, Ribeiro AW, Machado TM, Zaha A. Performance of the GenoType MTBDRplus assay directly on sputum specimens from Brazilian patients with tuberculosis treatment failure or relapse. J Clin Microbiol. 2013;51(5):1606–1608. doi: 10.1128/JCM.00364-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raizada N, Sachdeva KS, Chauhan DS, Malhotra B, Reddy K, Dave KP, et al. A multi-site validation in India of the line probe assay for the rapid diagnosis of multi-drug resistant tuberculosis directly from sputum specimens. PLoS One. 2014;9(2):e88626. doi: 10.1371/journal.pone.0088626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillemann D, Gerdes SR, Richter E. Evaluation of the GenoType MTBDRplus assay for Rifampin and Isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol. 2007;45(8):2635–2640. doi: 10.1128/JCM.00521-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J. 2008;32:1165–1174. doi: 10.1183/09031936.00061808. [DOI] [PubMed] [Google Scholar]
- 20.Singhal R, Arora J, Lal P, Bhalla M, Myneeedu VP, Behera D. Comparison of line probe assay with liquid culture for rapid detection of multi-drug resistance in Mycobacterium tuberculosis. Indian J Med Res. 2012;136:1044–1047. [PMC free article] [PubMed] [Google Scholar]
- 21.Luetkemeyer AF, Kendall MA, Wu X, Lourenço MC, Jentsch U, Swindells S. Evaluation of two line probe assays for rapid detection of Mycobacterium tuberculosis, tuberculosis (TB) drug resistance, and non-TB Mycobacteria in HIV-infected individuals with suspected TB. J Clin Microbiol. 2014;52(4):1052–1059. doi: 10.1128/JCM.02639-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnard M, Gey Van Pittius NC, Van Helden PD, Bosman M, Coetzee G, Warren RM. The diagnostic performance of the GenoType MTBDRplus version 2 line probe assay is equivalent to that of the Xpert MTB/RIF assay. J Clin Microbiol. 2012;50(11):3712–3716. doi: 10.1128/JCM.01958-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw data of sociodemographic and consent of study participants available and well documented. A document for ethical clearance from ethical review office also available. All data and materials that support the final result are available in excel sheet and also in printed result form. We can also access the result from the MGIT 960 instrument data base.


