Abstract
Background
Delayed diagnosis of childhood Takayasu arteritis (TA) is common due to its atypical symptoms. The objective of the present study was to summarize the clinical features of childhood TA to raise awareness and improve management.
Methods
Eleven children diagnosed with TA at our hospital were enrolled. Clinical information, diagnosis, treatment, and outcome were then examined retrospectively. The Pediatric Vasculitis Activity Score (PVAS) and the Indian Takayasu Clinical Activity Score (ITAS2010) were used to assess disease activity.
Results
Male-to-female ratio was 4:7. The mean age was 9.4 (1.4–14) years and the average time to diagnosis was 40.6 days (12–90 days). All patients suffered from hypertension and few had immunologic abnormalities. Two patients had low levels of autoantibodies and one had elevated immunoglobulin E levels. Aberrant (elevated) laboratory parameters included erythrocyte sedimentation rate (ESR) (9/10 patients, 90.0%), protein excretion (8/9 patients, 88.9%), renin-angiotensin-aldosterone system (RAAS) activity (5/5 patients, 100.0%), and serum lipid levels (3/5 patients, 60%). The common onset patterns were headache with convulsions (27.2%) and kidney damage (27.2%). The abdominal aorta (81.8%) and renal artery (72.7%) were the most commonly involved vessels. At presentation, the mean PVAS and ITAS2010 scores were 12.1 (6–26)/63 and 9.7 (5–14)/57, respectively. All patients were treated with glucocorticoids and antihypertensive agents; two underwent renal artery stent placement.
Conclusion
The diagnosis of TA should be considered in patients with pediatric hypertension and high expression of inflammatory markers or abnormal urine results. Doppler ultrasonography of major vessels may be helpful. PVAS and ITAS2010 both help to evaluate disease activity, and the PVAS is recommended for patients with kidney damage. Glucocorticoid and antihypertensive agents are effective. Interventional therapy can be an option for patients with persistent hypertension.
Electronic supplementary material
The online version of this article (doi:10.1186/s12969-017-0164-2) contains supplementary material, which is available to authorized users.
Keywords: Pediatric rheumatology, Takayasu’s arteritis, Diagnostic framework, Hypertension, Ultrasonography, Disease activity score
Background
Takayasu arteritis (TA) is a chronic type of systemic large vessel vasculitis, mainly involving the aorta and its main branches. Early symptoms include systemic inflammation and ischemia of involved organs [1].
Unfortunately, diagnosis of childhood TA is often delayed, particularly in children under 10 years old, a factor that contributes to cardiovascular damage and mortality [2, 3]. Therefore, many studies have attempted to identify new technologies that are both reliable and sensitive, for example, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT) [4]. Although such technologies have made diagnosis more timely and accurate, the most important factor that will yield an early diagnosis is an improvement in doctors’ clinical thinking and selection of the relevant tests/examinations.
Here, we performed a retrospective analysis of children diagnosed with TA at a single Chinese center and summarize the clinical features and follow-up data. The aim is to help clinicians reach an early diagnosis and to improve the management of chronic vasculitis.
Methods
The study enrolled 11 children diagnosed with TA at our hospital from 2000 to 2015. Demographic data, clinical manifestations, laboratory and imaging results, diagnostic and therapeutic processes, and outcomes were then analyzed retrospectively. All patients were followed up via regular clinic visits or by telephone interview.
Diagnostic criteria
Patients were assessed according to the 2008 EULAR/PRINTO/PRES criteria [5], which include angiographic abnormality (conventional, CT, or MRI) of the aorta or its main branches and pulmonary arteries (mandatory criterion) plus at least one of the following: (1) absence of the peripheral artery pulse or claudication induced by physical activity; (2) a >10 mm Hg difference in systolic BP in all four limbs; (3) Bruits over large arteries; (4) hypertension (when compared with age-matched healthy children); and (5) increased levels of acute phase reactants (erythrocyte sedimentation rate(ESR) and/or C reactive protein(CRP). Fibromuscular dysplasia or similar causes were excluded.
Evaluation of disease activity
Two tools were used for retrospective evaluation: the Pediatric Vasculitis Activity Score (PVAS) and the Indian Takayasu Clinical Activity Score (ITAS-2010) [6, 7]. The PVAS is derived from the Birmingham Vasculitis Activity Score (BVAS) and is specific for pediatric vasculitis. It assesses new or worsening features occurring during the last 4 weeks or symptoms that have persisted for 3 months. It is comprised of nine sections with a total score of 63. The ITAS-2010 is a tool specific for Takayasu arteritis and comprised of six organ-based systems. It evaluates new symptoms or symptoms that have worsened during the last 3 months, the maximum score is 51. The ITAS-A score is equivalent to the ITAS-2010 score plus the acute phase reactant (ESR and CRP) scores, which increases the maximum score to 57 [6, 7]. The brief comparison between PVAS and ITAS-2010 showed in Additional file 1 (not in the text).
Evaluation of treatment effects
A treatment was deemed effective if it resulted in improved clinical symptoms, a reduction in blood pressure, no radiographic progression, or a reduction in disease activity scores.
Data management
Statistical analysis was performed using SPSS version 19.0. Data were expressed as the median or mean, and the significance of differences was evaluated using Student’s t-test or the Mann-Whitney U-test as appropriate. Spearman’s rank correlation analysis was used to examine associations between the PVAS and ITAS.
Results
General data
The TA group was comprised of four boys (36.4%) and seven girls (63.6%). The mean age of disease onset was 9.4 ± 3.9 years (range, 1.4–14 years). One of the eleven was 1.4 and younger than 5 years of age, four were aged 5–10 years, and six were aged over 10 years. Girls showed a later age of onset (11.3 ± 1.7 years) than boys (6.1 ± 4.1 years) (p < 0.05).
The mean time to diagnosis was 40.6 ± 21.6 days (range, 12–90 days), with a mean of 59.6 days for boys and 29.6 days for girls (p < 0.05). None of the patients had a family history of vasculitis.
Clinical features
All 11 children had hypertension (nine at the first clinic visit and two at follow-up). The common manifestations are listed in Table 1.
Table 1.
Clinical manifestations
| Symptom | Number (%) | |
|---|---|---|
| Hypertension | 11 | (100%) |
| Fever | 5 | (45.4%) |
| BP discrepancy | 5 | (45.4%) |
| Vomiting | 5 | (45.4%) |
| Abdominal pain | 4 | (36.4%) |
| Headache | 3 | (27.2%) |
| Convulsion | 3 | (27.2%) |
| Bruits | 3 | (27.2%) |
| Weak pulse | 3 | (27.2%) |
| Abnormal urine output | 3 | (27.2%) |
According to the above data, six onset patterns can be summarized. The most common patterns were headache with convulsions and kidney damage, with three patients showing each of these patterns. Table 2 shows the onset pattern, initial presentation, and PVAS/ITAS-2010/ITAS-A scores at the first clinic visit.
Table 2.
Onset pattern, initial presentation, and disease activity scores
| Serial number | Age of initial presentation | Gender | Onset pattern |
Initial presentation | PVAS/ITAS/ITAS-A score at first clinic |
|---|---|---|---|---|---|
| 1 | 12.8y | M | Cardiac failure | Shortness of breath and chest tightness with pink frothy sputum | 10/5/6 |
| 2 | 5.3y | M | Headache with convulsions | Headache, convulsions, and intermittently febrile | 11/7/8 |
| 3 | 5.0y | M | Headache with convulsions | Headache, convulsions, and fever | 17/14/15 |
| 4 | 10.8y | F | Kidney damage | Dizziness, chest tightness, and palpitations | 20/13/18 |
| 5 | 1.4y | M | Kidney damage | Macro-albuminuria | 10/6/6 |
| 6 | 12.4y | F | Kidney damage | Precordial distress, vomiting, and oliguria | 18/7/- |
| 7 | 9.4y | F | Fever | Prolonged fever | 6/5/8 |
| 8 | 9.2y | F | Hypertension | Hypertensive at the preoperative physical examination; Symptoms of Henoch-Schönlein Purpura |
6/7/7 |
| 9 | 12.7y | F | Numbness of limbs | Numbness of the right limbs | 18/11/17 |
| 10 | 10.7y | F | Hypertension | Hypertensive on physical examination | 14/10/12 |
| 11 | 14.0y | F | Headache with convulsions | Cough, headache, convulsions, and fever | 11/8/12 |
In addition to TA, patient no. 1 had multiple lung cysts, patient no. 8 had Henoch-Schönlein Purpura, and patient no. 7 had autoimmune hemolytic anemia at the same time.
Laboratory and imaging results
Immune screening was almost normal, with only two patients (22.2%) showing low levels of autoantibodies (nine patients were tested). One patient had high serum IgE levels (10%; 10 patients tested), and seven tested patients were negative for anti-neutrophil cytoplasmic antibodies (ANCA). Inflammatory markers and other laboratory results are shown in Table 3.
Table 3.
Laboratory test results for TA children
| Test | Number (%) | |
|---|---|---|
| Elevated ESR | 9/10 | (90%) |
| Elevated CRP | 3/10 | (30%) |
| Elevated renin | 5/5 | (100%) |
| Elevated serum lipids | 3/5 | (60%) |
| Increased neutrophil percentage | 5/11 | (45.5%) |
| Low hemoglobin level | 4/11 | (36.4%) |
| Hypokalemia | 4/11 | (36.4%) |
| 24H protein excretion | 8/9 | (88.8%) |
| Urine routine | 6/11 | (54.5%) |
| Autoantibodies | 2/9 | (22.2%) |
All five tested patients showed increased renin levels, and four of nine patients tested showed evidence of prior tuberculosis infection: two were positive on sputum testing and imaging, whereas two were suspected cases (only the interferon-gamma release test was positive).
Computed tomographic angiography (CTA) revealed thickening of the arterial wall, along with lumen narrowing and vessel dilatation. Patient no. 1 showed radiographic evidence of aortic dissection. Ultrasonography revealed a rough inner wall or increased blood flow.
The abdominal aorta (nine patients, 81.8%) and renal artery (eight patients, 72.7%) were most frequently affected; The details are presented in Table 4.
Table 4.
Involved vessels
| Involved vessel | Number of patients (%) | Involved vessel | Number of patients (%) | ||
|---|---|---|---|---|---|
| Ascending aorta | 1 | (9.1%) | Abdominal aorta and its branches: | 11 | (100%) |
| Aortic arch and its branches: | 5 | (45.4%) | Abdominal aorta | 9 | (81.8%) |
| Aortic arch | 3 | (27.2%) | Celiac trunk | 5 | (45.4%) |
| Brachiocephalic trunk | 2 | (18.2%) | Superior mesenteric artery | 4 | (36.4%) |
| Common carotid artery | 3 | (27.2%) | Inferior mesenteric artery | 1 | (9.1%) |
| Subclavian artery | 2 | (18.2%) | Renal artery | 8 | (72.7%) |
| Radial artery | 1 | (9.1%) | Iliac artery | 1 | (9.1%) |
| Intracranial artery | 2 | (18.2%) | Femoral artery | 1 | (9.1%) |
| Thoracic aorta | 3 | (27.2%) | Coronary artery | 1 | (9.1%) |
| Pulmonary artery | 2 | (18.2%) | |||
Four of the eight patients with an involved renal artery also showed unilateral renal atrophy. The four patients with superior mesenteric artery involvement suffered no ischemic abdominal pain. Among the two patients showing intracranial artery involvement, patient no. 4 (anterior cerebral artery) felt dizzy and patient no. 9 (middle and posterior cerebral artery) suffered transient amaurosis. Two patients had pulmonary artery involvement, although none had pulmonary hypertension or pulmonary dysfunction. We identified two patients (of three patients examined) with funduscopic abnormalities (no. 4 and no. 8), although both had normal visual acuity.
According to the new angiographic classification of TA (International Conference of Takayasu’s arteritis in Tokyo, 1994) [8], the most common type of vessel involvement was type V (six patients; 45.5%), followed by type IV (four patients; 36.45%). Two patients showed type IIa and type III involvement, and none showed type I or type IIb involvement (Additional file 2).
Onset patterns and diagnostic process
The diagnostic and analysis framework for each onset pattern is shown in Fig. 1a and b.
Fig. 1.
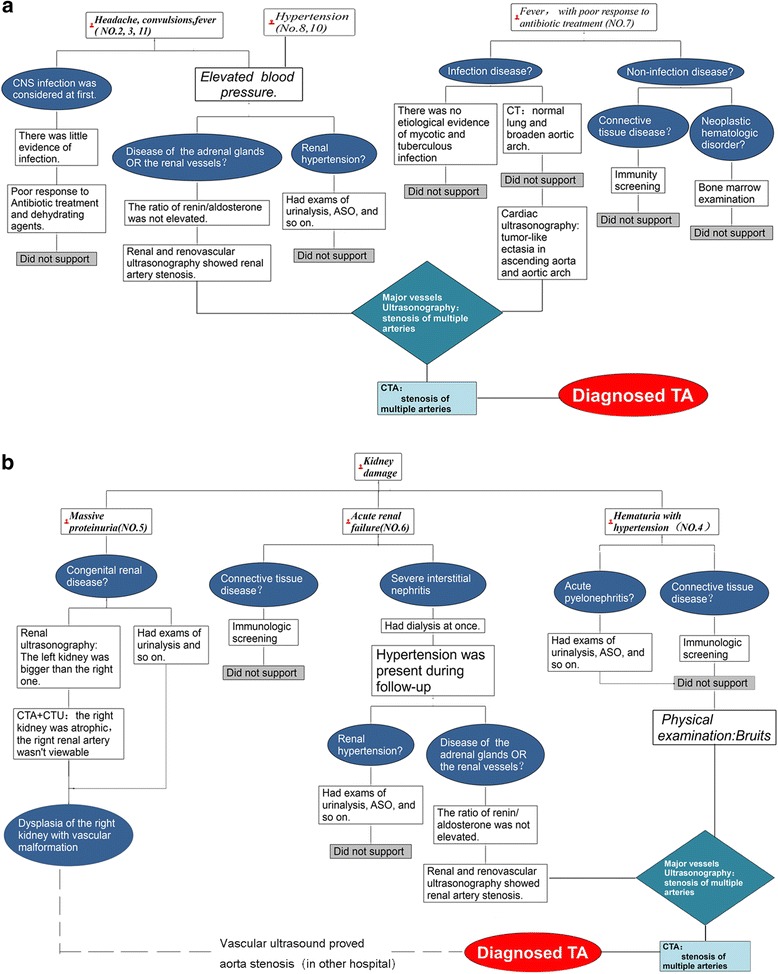
Diagnostic procedure and clinical framework of TA in cohort. Legend: a The onset patterns of headache with convulsions, Hypertension as well as fever. b The onset patterns of kidney damage
The example in Fig. 1a shows that the doctor firstly considered an intracranial infection in a child presenting with headache, convulsions, and fever. However, there was little evidence to support this diagnosis. Then doctors recognized that hypertension was a prominent symptom and carried relevant examinations. Further findings upon vessel ultrasonography and CTA enabled a final diagnosis.
Disease activity scores
At the time of presentation, the mean PVAS was 12.8/63 (range, 6–20); ITAS was 8.5/51 (range, 5–14); ITAS-A was 11.0/57 (range, 6-18). The PVAS showed a strong correlation with both the ITAS (r = 0.77; P = 0.002) and ITAS-A (r = 0.84; P = 0.002) (Fig. 2). The disease activity scores at first clinic visit are presented in Table 2.
Fig. 2.
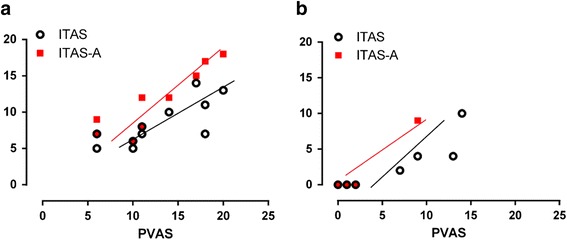
Correlation between Takayasu arteritis disease activity measures. Legend: a Correlation at time of diagnosis: ITAS-2010, r1 = 0.77, P = 0.002; ITAS-A, r2 = 0.84, P = 0.002. b Correlation at time of latest follow-up: ITAS-2010, r1 = 0.92, P < 0.001; ITAS-A, r2 = 0.72, P > 0.05. PVAS, Pediatric Vasculitis Activity Score; ITAS-2010, Indian Takayasu Arteritis Activity Score; ITAS-A, Indian Takayasu Arteritis Activity Score (containing the acute phase reactant item)
The average scores for the cardiovascular and renal items were highest. Meanwhile, the abdomen, skin, mucous membranes/eyes, and ENT (ear,nose,throat) all gained a score of 0 (Fig. 3).
Fig. 3.
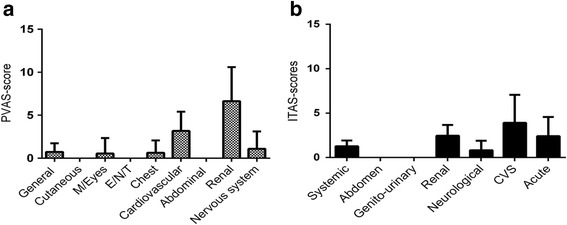
The mean score for each item in the PVAS (a) and the ITAS-2010 (b). Legend: M/Eyes: mucous membranes and eyes. E/N/T: ear/nose/throat. CVS: cardiovascular system
Treatments and outcome
All 11 children were treated with a combination of glucocorticoids (1–2 mg/kg), antihypertensive drugs, and antiplatelet drugs. One patient received intravenous cyclophosphamide pulses, six underwent several rounds of hemodialysis due to renal dysfunction during the hospitalization and follow-up, and two patients underwent angioplasty due to resistant hypertension.
Patiensts were followed up for a median 19 months (range, 2–54 months). The treatment response rate was 77.8% (7/9 patients; two patients were lost to follow-up). Figure 4 shows the changes in scores between first clinic visit and last follow-up.
Fig. 4.
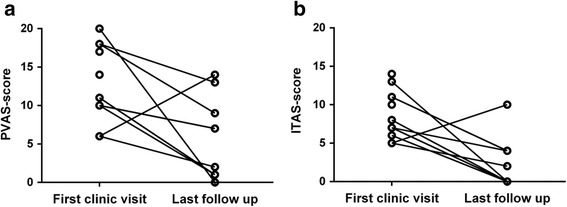
PVAS and ITAS at the first clinic visit and at the last follow-up. Legend: a PVAS score before and after therapy. b ITAS score before and after therapy
At last follow-up, patient no. 7 had an increased disease activity score (in both PVAS and ITAS-2010), suggesting no response of treatment. The scores for the other eight patients decreased, although patient no. 1 was still considered to have active disease due to edema and chest tightness.
Discussion
Pediatric TA is a common form of childhood chronic vasculitis that has various clinical manifestations, including fever, hypertension, kidney damage, and other inflammatory symptoms. Even today, the rate of under diagnosis and misdiagnosis is high due to atypical symptoms.
Demographic data and clinical presentation
The minimum age of onset was only 1.4 years old, which brings difficulties to our diagnosis. Most doctors would think it was not a rheumatic disease but a congenital disease for the patient’s (no.5) kidney damage. The youngest age reported was 1.5–4 months [3, 9, 10], suggesting that the onset age of TA may be younger than expected. In our cohort, the male-to-female ratio was 1:1.75. The incidence rate and average age of onset were higher in females than males, which is in keeping with the findings of previous studies [3, 9, 10]. However, the incidence in males and the average age of onset have risen in recent years [11]. Gender differences may be related to the role of estrogen or genetics [12]. The average time to diagnosis in our cohort was shorter than that reported previously [3, 9, 10, 13, 14], which may be due to pediatricians’ increased awareness of childhood TA in recent years, as well as regional differences. Tuberculosis infection is thought to be one factor underlying TA [15] and many researchers have reported the co-existence tuberculosis infection in those with TA [3, 14, 15]. The rate of TB infection of our patients was only 22.2%, lower than that in previous reports [3, 13]; this may be related to increased use of tuberculosis vaccines and improvement of medical care.
Here, we found that all cases had hypertension. The most common causes of pediatric hypertension are renal disease, coarctation of the aorta, and renal artery stenosis [16]. Thus, in cases of unexplained hypertension, especially those complicated by elevated levels of acute phase reactants and abnormal urine test results, doctors should monitor patients for BP discrepancies or vessel bruits. Also, when available evidence cannot be explained by organ-specific disease, TA should be considered. We recommend large vessel ultrasound as a screening tool.
Laboratory and imaging results
TA has no specific immunologic index (e.g., autoantibodies or ANCA) or inflammatory markers [13, 14]. The cohort examined herein was more likely to show an elevated ESR than elevated CRP levels (90% vs. 30%, respectively), which is consistent with previous reports [7, 9, 10]. The correlation between acute phase reactant levels and other measurement tools is controversial [3, 7, 10, 17, 18]. Some reports suggest that PVAS shows a better correlation with the ESR than with CRP levels [7]. One Japanese group suggested that an increase in the ESR at first diagnosis can lead to a better prognosis [2]. Here, PVAS showed a stronger correlation with ITAS-A (which includes the acute phase reactants item) than ITAS (which does not), suggesting that the ESR can be used to assess disease activity. However, because the ESR may not be an early indicator of systemic inflammation [7], it should be used in combination with clinical symptoms and imaging results. In recent years, studies have tried to identify acute phase reactants that could replace the ESR and CRP as evaluation indices; these include PTX-3, MMP-9 (matrix metalloproteinase-9), and IL-6 [19–21]. Some studies indicate that PTX-3 is a better indicator of vascular inflammation than CRP. However, multicenter studies are needed to confirm this.
Abnormal lipid metabolism plays a role in the pathogenesis of TA. Some adult TA patients showed dyslipidemia, which may be a risk factor for complicating atherosclerosis [22]. Also, dyslipidemia is suggestive of disease activity [23]. Here, dyslipidemia was identified in 60% (3/5) of cases, similar to that reported previously [22]; however, the disease activity scores for children with and without dyslipidemia were not statistically different. Also, blood lipids do not correlate with CRP and ESR. Thus, it is still unclear whether lipids can be used as a disease activity index for childhood TA.
All tested patients (5 children) had high levels of renin and renal artery stenosis on at least one side, suggesting that elevated renin levels were caused by reduced renal perfusion. This finding is consistent with the literature [13, 24], but it remains unclear whether RAAS can be used to assess the degree of renal artery involvement in TA.
One of 11 patients (9.1%) in our study showed evidence of arterial dissection. It is similar to a Canada study, suggesting the importance of careful vessel wall assessment [25].
Our finding that the most commonly involved vessels were the abdominal aorta and renal arteries is in agreement with previous reports [3, 9, 14]. However, there was no significant difference in the disease activity scores between patients with or without renal artery stenosis. This may be due to different degrees of renal artery stenosis in each patient, and to the small number of cases enrolled.
Assessment of disease activity
There are multiple tools that can be used to evaluate TA disease activity, including the BVAS and the DEI.Tak (Disease Extent Index-Takayasu); however, there is no recognized standard [26, 27]. Color Doppler ultrasound also has been used to evaluate disease activity yet [18].
PVAS is the special scale for vasculitis activity in children, whereas ITAS-2010 is a TA-specific assessment scale. The results revealed a good correlation between PVAS and ITAS-2010. Here, we found a higher proportion of TA children with renal impairment, which is in agreement with previous reports [9, 13]. The kidneys component of PVAS (12/63) is much more comprehensive than that of ITAS-2010 (3/51), indicating that the former includes a more detailed assessment of renal involvement. Therefore, we recommend that the PVAS scale be used to assess disease activity in TA children with kidney damage.
The rate of mesenteric artery involvement was high (45.5%); however, the abdominal item of PVAS and ITAS scored 0, because there was no evidence of intestinal ischemic pain. Therefore, we emphasize the importance of an overall assessment of the systemic vessels at each follow-up session.
Advances in treatment of TA
The main medical treatment was glucocorticoids combined with antihypertensive and antiplatelet drugs; this combination showed good efficacy in most cases. For glucocorticoid-resistant children, further immunosuppressive therapy (e.g., cyclophosphamide, azathioprine, or methotrexate) should be considered [28, 29]. Biological agents such as tocilizumab and tumor necrosis factor (TNF) antagonists are used mainly for refractory adult TA cases [30, 31]. TA children who received TNF antagonist therapy showed a satisfactory response in the short term [10, 32, 33], but long-term multicenter studies are lacking.
Blood vessel reconstruction helps to control blood pressure in half of patients with renal artery stenosis [34]. Vascular surgery and interventional surgery can reduce mortality and improve the long-term prognosis for both adults and children, but some patients require a second surgical intervention due to repeat stenosis [35, 36]. Here, two patients with renal artery stenosis had well-controlled blood pressure and TA symptoms after angioplasty, although the long-term prognosis can only be assessed after long-term follow-up.
Conclusions
The clinical manifestations of childhood TA vary widely. For children with hypertension combined with high levels of inflammatory markers and abnormal urine results, TA should be considered in the differential. Doppler ultrasound scanning of major vessels can be used as a screening tool for early TA diagnosis. PVAS and ITAS-2010 are both helpful for evaluating disease activity in pediatric TA. However, we recommend PVAS as a disease activity assessment tool for patients with kidney damage. Glucocorticoids and antihypertensive agents are effective treatments, and biological agents are being explored. Also, interventional therapy can be an option for those with TA and resistant hypertension.
This is the first study to examine the onset of childhood TA and the use of PVAS and ITAS to monitor disease activity in those with childhood TA in China. However, it should be noted that this study was retrospective in nature and the number of cases was small, raising the possibility of selection bias. Future studies will require a larger sample size and longer-term follow-up to provide more reliable clinical information about pediatric TA.
Additional files
Brief comparison of PVAS and ITAS-2010. (DOCX 16 kb)
Angiographic classification and percentages of 11 TA children. (DOCX 12 kb)
Acknowledgements
The authors gratefully acknowledge the technical supports by Department of Medical Records Management of Children’s Hospital of Chongqing Medical University, as well as understanding and support by all patients and their parents.
Funding
Chongqing Science and Technology Commission-[2012]-No. 27-cstc2012jjA10077.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
All authors contributed extensively to the work presented in this paper. FY finished the study design, data acquisition and analysis, and manuscript drafting and revisions. TX contributed to the conception and design of the study, data analysis, and manuscript drafting and revisions. LM and ZJ assisted with data interpretation and provided critical manuscript revision. LQ and ZX provided critical manuscript revision. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
We have obtained consent to publish from Children’s hospital of Chongqing Medical University.
Ethics approval and consent to participate
We have obtained consent to publish from Children’s hospital of Chongqing Medical University ethics committee, and we have obtained consent to the participants.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ANCA
Anti-neutrophil cytoplasmic antibody
- BVAS
Birmingham vasculitis activity score
- CTA
Computed tomographic angiography
- CRP
C reactive protein
- ESR
Erythrocyte sedimentation rate
- ENT
Ear/nose/throat
- ITAS2010
Indian Takayasu clinical activity score
- PVAS
Pediatric vasculitis activity score
- RAAS
Renin-angiotensin-aldosterone system
- TA
Takayasu arteritis
Contributor Information
Ye Feng, Email: feng_yearn@126.com.
Xuemei Tang, Phone: + 86 23 63630957, Email: tangxuemei2008@163.com.
Mingyue Liu, Email: 1245358291@qq.com.
Juan Zhou, Email: zhoujuan1112@gmail.com.
Xiaodong Zhao, Email: Zhaoxd530@aliyun.com.
Qiu Li, Email: liqiu_21@126.com.
References
- 1.Arend WP, Michel BA, Bloch DA, et al. The American college of rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–34. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa K, Maetani S. Long-term outcome for 120 Japanese patients with Takayasu’s disease. Clinical and statistical analyses of related prognostic factors. Circulation. 1994;90(4):1855–60. doi: 10.1161/01.CIR.90.4.1855. [DOI] [PubMed] [Google Scholar]
- 3.Clemente G, Hilario MO, Lederman H, et al. Takayasu arteritis in a Brazilian multicenter study: children with a longer diagnosis delay than adolescents. Clin Exp Rheumatol. 2014;32(3 Suppl 82):S128–33. [PubMed] [Google Scholar]
- 4.Mavrogeni S, Dimitroulas T, Chatziioannou SN, Kitas G. The role of multimodality imaging in the evaluation of Takayasu arteritis. Semin Arthritis Rheum. 2013;42(4):401–12. doi: 10.1016/j.semarthrit.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69(5):798–806. doi: 10.1136/ard.2009.116657. [DOI] [PubMed] [Google Scholar]
- 6.Misra R, Danda D, Rajappa SM, et al. Development and initial validation of the Indian Takayasu clinical activity score (ITAS2010) Rheumatology (Oxford) 2013;52(10):1795–801. doi: 10.1093/rheumatology/ket128. [DOI] [PubMed] [Google Scholar]
- 7.Dolezalova P, Price-Kuehne FE, Özen S, et al. Disease activity assessment in childhood vasculitis: development and preliminary validation of the paediatric vasculitis activity score (PVAS) Ann Rheum Dis. 2013;72(10):1628–33. doi: 10.1136/annrheumdis-2012-202111. [DOI] [PubMed] [Google Scholar]
- 8.Moriwaki R, Noda M, Yajima M, Sharma BK, Numano F. Clinical manifestations of Takayasu arteritis in India and Japan--new classification of angiographic findings. Angiology. 1997;48(5):369–79. doi: 10.1177/000331979704800501. [DOI] [PubMed] [Google Scholar]
- 9.Song Hongmei, Zhou Xiaoying, Li Fa n, e t al. Clinical analysis of 28 patients with Takayasu’ s arteritis in children. Chin J Appl Clin Pediatr. 2005;20(10):594–96.
- 10.Szugye HS, Zeft AS, Spalding SJ. Takayasu Arteritis in the pediatric population: a contemporary United States-based single center cohort. Pediatr Rheumatol Online J. 2014;12:21. doi: 10.1186/1546-0096-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe Y, Miyata T, Tanemoto K. Current clinical features of New patients with takayasu arteritis observed from cross-country research in Japan: Age and Sex specificity. Circulation. 2015;132(18):1701–9. doi: 10.1161/CIRCULATIONAHA.114.012547. [DOI] [PubMed] [Google Scholar]
- 12.Hong Z, Chen F, Fu W, Xu X. Effect of estrogen on hemodynamics and vascular remodeling with Takayasu arteritis. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2001;18(1):22–5. [PubMed] [Google Scholar]
- 13.Yue Ai-hong, Xiao Hui-jie, Zhong Xu-hui, Liu Xue-qin, Qi Jian-guang. Clinical Analysis of 10 Children with Takayasu’ s Arteritis. J Appl Clin Pediatr. 2010;25(1):31–33.
- 14.Eleftheriou D, Varnier G, Dolezalova P, McMahon AM, Al-Obaidi M, Brogan PA. Takayasu arteritis in childhood: retrospective experience from a tertiary referral centre in the United Kingdom. Arthritis Res Ther. 2015;17:36. doi: 10.1186/s13075-015-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lupi-Herrera E, Sánchez-Torres G, Marcushamer J, Mispireta J, Horwitz S, Vela JE. Takayasu’s arteritis. Clinical study of 107 cases. Am Heart J. 1977;93(1):94–103. doi: 10.1016/S0002-8703(77)80178-6. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Lavín M, Buendía A, Pineda C. Hypertension in children. N Engl J Med. 1997;336(23):1675–6. doi: 10.1056/NEJM199706053362312. [DOI] [PubMed] [Google Scholar]
- 17.Direskeneli H, Aydin SZ, Merkel PA. Assessment of disease activity and progression in Takayasu’s arteritis. Clin Exp Rheumatol. 2011;29(1 Suppl 64):S86–91. [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha D, Mondal S, Nag A, Ghosh A. Development of a colour Doppler ultrasound scoring system in patients of Takayasu’s arteritis and its correlation with clinical activity score (ITAS 2010) Rheumatology (Oxford) 2013;52(12):2196–202. doi: 10.1093/rheumatology/ket289. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Ma L, Yan F, et al. MMP-9 and IL-6 are potential biomarkers for disease activity in Takayasu’s arteritis. Int J Cardiol. 2012;156(2):236–8. doi: 10.1016/j.ijcard.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Tombetti E, Di CMC, Sartorelli S, et al. Systemic pentraxin-3 levels reflect vascular enhancement and progression in Takayasu arteritis. Arthritis Res Ther. 2014;16(6):479. doi: 10.1186/s13075-014-0479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishihara T, Haraguchi G, Tezuka D, Kamiishi T, Inagaki H, Isobe M. Diagnosis and assessment of Takayasu arteritis by multiple biomarkers. Circ J. 2013;77(2):477–83. doi: 10.1253/circj.CJ-12-0131. [DOI] [PubMed] [Google Scholar]
- 22.Guleria A, Misra DP, Rawat A, et al. NMR-based serum metabolomics discriminates Takayasu arteritis from healthy individuals: a proof-of-principle study. J Proteome Res. 2015;14(8):3372–81. doi: 10.1021/acs.jproteome.5b00422. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Chen B, Lv N, Liu Q, Dang A. Association of abnormal lipid spectrum with the disease activity of Takayasu arteritis. Clin Rheumatol. 2015;34(7):1243–8. doi: 10.1007/s10067-014-2819-4. [DOI] [PubMed] [Google Scholar]
- 24.Wiggelinkhuizen J, Cremin BJ. Takayasu arteritis and renovascular hypertension in childhood. Pediatrics. 1978;62(2):209–17. [PubMed] [Google Scholar]
- 25.Aeschlimann FA, Grosse-Wortmann L, Benseler SM, Laxer RM, Hebert D, Yeung RS. Arterial dissection in childhood Takayasu Arteritis: not as rare as thought. Pediatr Rheumatol Online J. 2016;14(1):56. doi: 10.1186/s12969-016-0115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham vasculitis activity score (version 3) Ann Rheum Dis. 2009;68(12):1827–32. doi: 10.1136/ard.2008.101279. [DOI] [PubMed] [Google Scholar]
- 27.Aydin SZ, Yilmaz N, Akar S, et al. Assessment of disease activity and progression in Takayasu’s arteritis with disease extent index-takayasu. Rheumatology (Oxford) 2010;49(10):1889–93. doi: 10.1093/rheumatology/keq171. [DOI] [PubMed] [Google Scholar]
- 28.Ozen S, Duzova A, Bakkaloglu A, et al. Takayasu arteritis in children: preliminary experience with cyclophosphamide induction and corticosteroids followed by methotrexate. J Pediatr. 2007;150(1):72–6. doi: 10.1016/j.jpeds.2006.10.059. [DOI] [PubMed] [Google Scholar]
- 29.Stern S, Clemente G, Reiff A, Ramos MP, Marzan KA, Terreri MT. Treatment of Pediatric Takayasu arteritis with infliximab and cyclophosphamide: experience from an American-Brazilian cohort study. J Clin Rheumatol. 2014;20(4):183–8. doi: 10.1097/RHU.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 30.Molloy ES, Langford CA, Clark TM, Gota CE, Hoffman GS. Anti-tumour necrosis factor therapy in patients with refractory Takayasu arteritis: long-term follow-up. Ann Rheum Dis. 2008;67(11):1567–9. doi: 10.1136/ard.2008.093260. [DOI] [PubMed] [Google Scholar]
- 31.Abisror N, Mekinian A, Lavigne C, Vandenhende MA, Soussan M, Fain O. Tocilizumab in refractory Takayasu arteritis: a case series and updated literature review. Autoimmun Rev. 2013;12(12):1143–9. doi: 10.1016/j.autrev.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Filocamo G, Buoncompagni A, Viola S, et al. Treatment of Takayasu’s arteritis with tumor necrosis factor antagonists. J Pediatr. 2008;153(3):432–4. doi: 10.1016/j.jpeds.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 33.Bravo MB, Perin F, Guez VDRMM, García SA, Alcázar RPP. Successful tocilizumab treatment in a child with refractory Takayasu arteritis. Pediatrics. 2012;130(6):e1720–4. doi: 10.1542/peds.2012-1384. [DOI] [PubMed] [Google Scholar]
- 34.Ladapo TA, Gajjar P, McCulloch M, Scott C, Numanoglu A, Nourse P. Impact of revascularization on hypertension in children with Takayasu’s arteritis-induced renal artery stenosis: a 21-year review. Pediatr Nephrol. 2015;30(8):1289–95. doi: 10.1007/s00467-015-3049-y. [DOI] [PubMed] [Google Scholar]
- 35.Kalangos A, Christenson JT, Cikirikcioglu M, et al. Long-term outcome after surgical intervention and interventional procedures for the management of Takayasu’s arteritis in children. J Thorac Cardiovasc Surg. 2006;132(3):656–64. doi: 10.1016/j.jtcvs.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 36.Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56(3):1000–9. doi: 10.1002/art.22404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Brief comparison of PVAS and ITAS-2010. (DOCX 16 kb)
Angiographic classification and percentages of 11 TA children. (DOCX 12 kb)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


