Abstract
Objective
People living with HIV (PLWH) have chronic immune activation and increased cardiovascular disease (CVD) risk. Activation of monocytes and T lymphocytes causes up-regulation of glucose transporter-1 (GLUT1) for efficient function. PLWH have an increased percentage of GLUT1-expressing monocytes and T lymphocytes, but it is unclear if these cells are associated with CVD. We evaluated expression of GLUT1 and CD38 on monocyte and T lymphocyte populations from HIV-infected women with subclinical CVD.
Methods
Participants with >75th percentile (n=15) and <25th percentile (n=15) age-adjusted intima-media thickness (IMT) at the right common carotid artery and bifurcation were identified from the Women's Interagency HIV Study. Groups were matched by age, race/ethnicity, smoking status, and CD4 count. All women were receiving suppressive antiretroviral therapy except for one high and one low IMT participant. Monocyte and T lymphocyte populations were evaluated for GLUT1 and CD38 expression using flow cytometry.
Results
Intermediate monocytes from high IMT women had significantly increased expression of GLUT1 (310 MFI vs. 210 MFI, p=0.024) (66.4% vs. 48.5%, p=0.031) and CD38 (339 MFI vs. 211 MFI, p=0.002) (10.5% vs. 3.8%, p=0.0002) compared to women with low IMT. High and low IMT participants showed no differences in GLUT1 or CD38 expression on classical monocytes, non-classical monocytes, CD4+ and CD8+ T lymphocytes.
Conclusion
GLUT1-expressing intermediate monocytes are elevated in HIV-infected women with subclinical CVD. These cells may contribute to development of CVD in PLWH and could be a novel target to limit inflammation.
Keywords: HIV, monocyte, T cell, GLUT1, cardiovascular disease
Introduction
Persons living with HIV (PLWH) can be effectively treated with combination antiretroviral therapy (cART); yet their lifespans may still be shorter than those of HIV-uninfected persons [1]. In both high and lower income settings the causes of death among PLWH are shifting from AIDS-related to non-AIDS-related diseases such as cardiovascular disease (CVD) [1,2].
CVD is a metabolic and inflammatory disease associated with activated monocytes and T lymphocytes [3,4]. The metabolism of monocytes and T lymphocytes can change dramatically upon activation, with both activated cell types upregulating surface expression of glucose transporter-1 (GLUT1) for efficient function [5–8]. The percentage of GLUT1-expressing monocytes and CD4 T lymphocytes is elevated in both untreated and cART-treated PLWH when compared to uninfected persons [5,6]. This increase in GLUT1-expressing monocytes and T lymphocytes could be an important source of inflammation leading to CVD as GLUT1-expressing mononuclear phagocytes have been shown to express proinflammatory cytokines [9]. It remains unclear if these GLUT1-expressing cells are associated with CVD in PLWH.
Monocytes are composed of several populations that can be characterized by the differential expression of CD14 and CD16 surface markers [10]. Classical monocytes (CD14++CD16-) express high levels of CD14, lack CD16, and represent the majority of monocytes in the blood. CD16-expressing monocytes are associated with increased production of TNF-α and are considered to be inflammatory. CD16-expressing monocytes are further subdivided into non-classical monocytes (CD14+CD16++) and intermediate monocytes (CD14++CD16+). The intermediate monocyte population, while only recently considered to be a distinct subset of monocytes, has been increasingly associated with inflammation as it relates to the increased risk of CVD [11]; increased levels of TNF-α [12], IL-1β [13], and D-dimer [14]; and a distinct role in antigen presentation and processing [15].
Both activated CD4+ and CD8+ T lymphocytes have been pathogenetically implicated in HIV –infected people with CVD. Activated CD8+ T lymphocytes have previously been linked to markers of CVD inflammation [16] and Kaplan et. al. identified an association between activated CD4+ T lymphocytes and the presence of carotid artery plaques [17]. However, other reports have shown that T lymphocyte activation was not associated with serious non-AIDS events, including CVD outcomes [18].
The aim of this study was to evaluate the expression of GLUT1 on monocyte and T lymphocyte populations in HIV-infected women with subclinical CVD to explore if it could contibute to CVD in PLWH.
Methods
High and low IMT groups were identified from the Women's Interagency HIV Study (WIHS), a longitudinal cohort study of women with HIV and a risk-set matched control group. The high IMT group was defined as having >75th percentile intima-media thickness (IMT) measured at the right common carotid artery (CCA) and bifurcation (BIF) (CCA-IMT range 0.779-1.488 mm, BIF-IMT range 0.920-1.621 mm) and low IMT group was defined as having <25th percentile intima-media thickness at the CCA and BIF (CCA-IMT 0.591-0.704 mm, BIF-IMT 0.614-0.772 mm). IMT was measured by high resolution B-mode carotid artery ultrasound of the far wall of the right common carotid artery (CCA) and bifurcation (BIF) according to the procedure of Hodis et al. [19]. IMT values were first age-adjusted because IMT and age are correlated. Groups were then matched by age, race/ethnicity, smoking status, and CD4 count. Thereafter, 15 pairs were identified, giving a total of thirty (30) participants. All participants except one case and one control were virally suppressed on cART.
Blood samples were collected in the WIHS at semiannual visits [20]. Twenty-seven (27) participants were fasting at the time of sample collection. The samples used in this study were collected between April 2012 and March 2015. Peripheral blood mononuclear cells (PBMCs) were processed and stored in liquid nitrogen at a minimum 6 million cells per aliquot [20]. Plasma (never previously thawed) was also collected from women at these visits and stored at -20°C.
Cryopreserved PBMCs were rested for 2 hours at 37°C prior to staining in RPMI-1640 medium (Corning CellGro) containing 10% fetal bovine serum (Gemini Bio-Products), 200 IU penicillin/200 μg/mL streptomycin (Sigma Aldrich) and 2 mM L-glutamine (Sigma Aldrich). PBMCs were stained on ice for LIVE/DEAD Fixable Aqua Stain (Life Technologies; catalog L34957), CD3–BV421 (BD Biosciences; catalog 562426), CD4–PE-CF594 (BD Biosciences; catalog 562281), CD8–APC-H7 (BD Biosciences; catalog 560179), CD38–APC (BD Biosciences; catalog 555462), HLA-DR–PE-Cy7 (BD Biosciences; catalog 560651), CD14–BV786 (BD Biosciences; catalog 563698), CD16–BV650 (BD Biosciences; catalog 563692), CD19–AF700 (BD Biosciences; catalog 557921), CD56–AF700 (BD Biosciences; catalog 557919) and GLUT1–PE (R&D Systems; catalog FAB1418P). Antibody concentrations were selected according to the manufacturer's recommendation. Cells were acquired on an LSRFortessa II (BD Biosciences) and analyzed using FlowJo Software (Tree Star Inc.). Samples were run daily with matched samples from the high and low IMT group on each day. SPHERO™ rainbow calibration particles (Spherotech Inc.; catalog RCP-30-5A) were used each day to check the sensitivity and linearity of the flow cytometer. The cryopreservation and thawing process was previously shown to have no effect on the expression of GLUT1 on cells [5].
T lymphocyte and monocyte subpopulations were identified by flow cytometry (Supplemental Figure 1). Briefly, scatter properties were used to determine initial monocyte and lymphocyte gates which were then gated to select live cells. CD3, CD4 and CD8 expression were used to distinguish T lymphocyte populations, while CD3, CD56 and CD19 were used to exclude T lymphocytes, NK cells and B cells, respectively, from the initial monocyte gate. Monocyte subpopulations were then distinguished using CD14 and CD16 expression. CD38 was used as an activation marker of T lymphocytes and monocytes [21,22]. Histograms depicting the expression of GLUT1 and CD38 on monocyte populations are shown in Supplemental Figure 1.
For cytokine detection, plasma was thawed and ELISA was performed according to the manufacturer's instructions for IL-6 (Human IL-6 Quantikine HS ELISA Kit, catalog HS600B; MDD < 0.70 pg/mL), sCD14 (Human sCD14 Quantikine ELISA Kit, catalog DC140; MDD = 125pg/mL) and sCD163 (Human CD163 Quantikine ELISA Kit, catalog DC1630; MDD = 0.177 ng/mL). All ELISA kits were obtained from R&D Systems.
The non-parametric Mann Whitney U test was used for comparison of unpaired data. The Spearman rank test was used to test for correlation. The Fisher's exact test was used for comparison of categorical data. P values less than 0.05 were considered significant. All analyses were conducted using GraphPad Prism (Graph Pad Software, version 6.07).
Results
Demographic and clinical characteristics of participants are summarized in Table 1. High IMT participants had significantly greater CCA-IMT (0.893 mm vs. 0.653 mm, p<0.0001) and BIF-IMT (0.984 mm vs. 0.701 mm, p<0.0001) than participants with low IMT. Participants with high IMT had higher hemoglobin A1c (6.0% vs. 5.5%, p=0.022), serum insulin (19.5 mIU/mL vs. 13.0 mIU/mL, p=0.039), triglycerides (138.5 mg/dL vs. 91.5 mg/dL, p=0.043), systolic blood pressure (126 mmHg vs. 114 mmHg, p=0.036) and lower HDL levels (50.0 mg/dL vs. 60.5 mg/dL, p=0.037) than women with low IMT. Plasma levels of sCD14, IL-6 and sCD163 showed no difference between women with high or low IMT.
Table 1. Clinical Characteristics of Study Groups.
| Variables | N | Groups | p | |
|---|---|---|---|---|
|
| ||||
| High IMT | Low IMT | |||
| Mean Age ± SD, years | 30 | 51.48 ± 6.14 | 51.68 ± 6.97 | 0.934 |
| Race (Black) % | 30 | 60 | 60 | |
| Median CD4 Count (IQR), cells/mm3 | 30 | 638 (244 - 793) | 630 (441 - 917) | 0.675 |
| Median CD8 Count (IQR), cells/mm3 | 30 | 764 (550 - 1108) | 719 (592 - 1122) | 0.488 |
| Median CD4/CD8 Ratio (IQR) | 30 | 0.6 (0.3 – 1.2) | 0.9 (0.5 – 1.3) | 0.272 |
| Viral Load (< 50 copies/mL), % | 28 | 100 | 73 | 0.102 |
| Smoking (Current Smokers) % | 30 | 60 | 60 | |
| Median BIF-IMT (IQR), mm | 30 | 0.984 (0.941 - 1.184) | 0.701 (0.643 - 0.740) | < 0.0001 |
| Median CCA-IMT (IQR), mm | 30 | 0.893 (0.843 – 1.001) | 0.653 (0.642 – 0.692) | < 0.0001 |
| Median Systolic BP (IQR), mmHg | 30 | 126 (113 – 140) | 114 (105 – 122) | 0.036 |
| Median Diastolic BP (IQR), mmHg | 30 | 72 (66 – 80) | 71 (69 – 76) | 0.472 |
| Median Triglycerides (IQR), mg/dL | 28 | 138.5 (101.5 - 164.5) | 91.5 (86.25 - 149.5) | 0.043 |
| Median Cholesterol (IQR), mg/dL | 28 | 191.5 (162.0 - 202.5) | 184.5 (135.5 - 237.0) | 0.473 |
| Median HDL (IQR), mg/dL | 28 | 50.0 (42.3 - 59.5) | 60.5 (47.8 - 82.8) | 0.037 |
| Median LDL (IQR), mg/dL | 28 | 110.0 (79.5 - 126.8) | 110.0 (65.5 - 136.3) | 0.424 |
| Median Plasma Glucose (IQR), mg/dL | 26 | 90.50 (87.75 - 107.00) | 95.00 (85.75 - 97.75) | 0.485 |
| Median Hemoglobin A1c (IQR), % | 29 | 6.00 (5.50 - 6.20) | 5.45 (5.28 - 5.80) | 0.022 |
| Median Serum Insulin (IQR), mIU/mL | 19 | 19.50 (10.75 - 25.50) | 13.00 (6.00 - 16.00) | 0.039 |
| Median IL-6 (IQR), pg/mL | 30 | 1.005 (0.599 - 1.223) | 0.917 (0.609 - 1.485) | 0.479 |
| Median sCD14 (IQR), ng/mL | 30 | 2330 (2075 – 3035) | 2286 (1968 – 2508) | 0.360 |
| Median sCD163 (IQR), ng/mL | 30 | 756.4 (403.2 - 1201) | 732.0 (474.6 - 1206) | 0.403 |
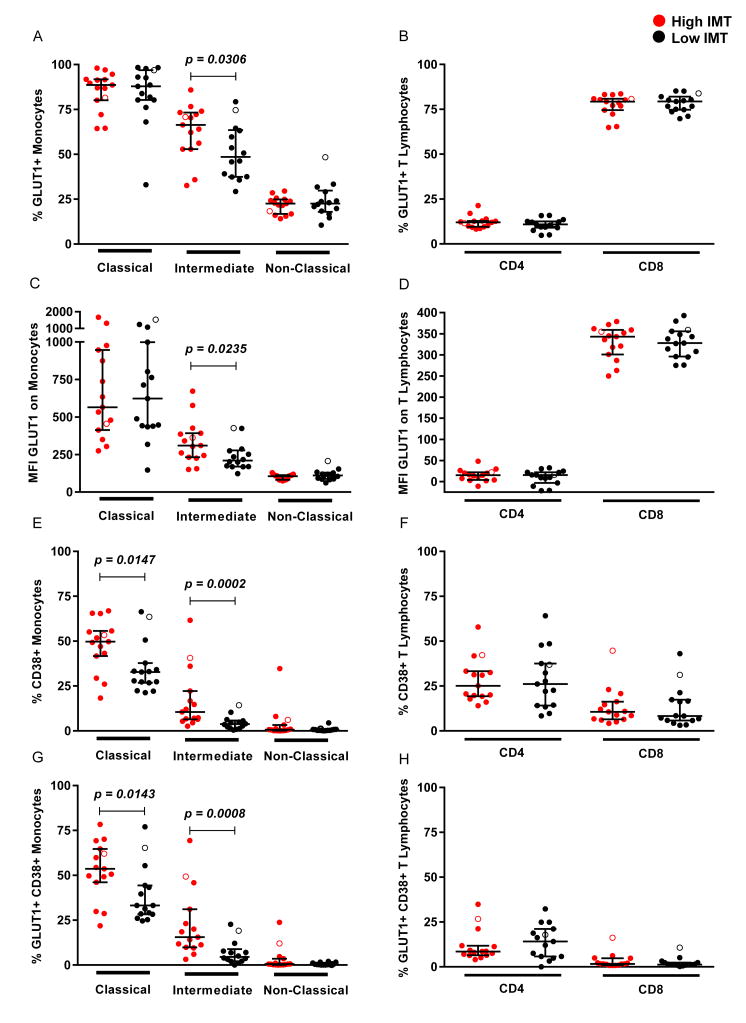
The percentage of intermediate monocytes expressing GLUT1 (66.4% vs. 48.5%, p=0.031) was higher in women with high IMT compared to those with low IMT while there were no differences between groups for non-classical monocytes, classical monocytes, and CD4 and CD8 T lymphocytes (Fig. 1a & b). Also, the expression of GLUT1 on intermediate monocytes was higher in women with high IMT (310 MFI vs. 210 MFI, p=0.024) with no differences in GLUT1 expression between groups for other monocyte subsets and CD4 and CD8 T lymphocytes (Fig. 1c & d). When assessing all samples, IMT correlated with both the percentage of GLUT1-expressing intermediate monocytes (r=0.390, p=0.037) and expression of GLUT1 on intermediate monocytes (r=0.398, p=0.032). There were no differences in the proportions of classical, non-classical and intermediate monocytes and CD4 and CD8 T lymphocytes between the two groups. The percentage of CD38 expressing intermediate monocytes was significantly elevated when comparing the high and low IMT groups (10.5% vs. 3.8%, p=0.0002) (Fig. 1e). There were no differences in the percentages of CD4 and CD8 T lymphocytes expressing CD38 when comparing high and low IMT groups (Fig. 1f). Similar to CD38 expression, the percentage of intermediate monocytes expressing both CD38 and GLUT1 was significantly higher in the high IMT group (15.5% vs. 4.5%, p=0.0008) (Fig. 1g), with no differences observed between groups for T lymphocytes (Fig. 1h). These data suggest an immunometabolic basis for monocyte activation.
Figure 1. Monocyte and T lymphocyte expression of GLUT1 and CD38 from women with high IMT and low IMT.
Monocyte and T lymphocyte populations were identified by flow cytometry as described in the Methods. The percentage of (A) monocyte and (B) T lymphocyte populations expressing GLUT1. The MFI of GLUT1 on the cell surface of (C) monocytes and (D) T lymphocytes. The percentage of (E) monocyte and (F) T lymphocyte populations expressing CD38. The percentage of (G) monocyte and (H) T lymphocyte populations expressing GLUT1 and CD38. Open circles represent women not on antiretroviral therapy at the time of sample collection.
Discussion
GLUT1 expression has been previously shown to be associated with activation of T lymphocytes and monocytes in treated and untreated HIV infection [5,6]. For CD4 T lymphocytes, the percentage of cells expressing GLUT1 is increased during untreated HIV infection; however, during treated HIV infection these levels are significantly decreased compared to untreated persons [5]. In contrast, the percentage of GLUT1-expressing monocytes is similar between untreated and treated HIV infection, indicating that even during treated HIV infection monocytes remain activated [6]. Consistent with these studies, we found that GLUT1-expressing intermediate monocytes, but not GLUT1-expressing T lymphocyte populations, are associated with subclinical CVD in a predominantly cART-treated population of HIV-infected women. Our results are also consistent with several recent reports showing that markers of activated monocytes are associated with CVD in PLWH [18,23,24].
The percentages of pro-inflammatory intermediate and non-classical monocyte subpopulations are similar between PLWH with uncontrolled viremia and HIV-uninfected persons with acute coronary syndrome [25], indicating that PLWH may have an inflammatory monocyte profile that contributes to a pro-atherogenic state. This is consistent with the data presented here demonstrating a higher percentage of GLUT1-expressing intermediate monocytes from HIV-infected women with high IMT compared to those with low IMT. These proinflammatory GLUT1-expressing intermediate monocytes are activated, as GLUT1 expression correlated with the activation marker CD38. This correlation between GLUT1 and CD38 suggests an interrelationship between metabolism and monocyte activation.
Immune stimuli present during HIV infection, such as the microbial translocation product LPS, can activate monocytes [26], causing an upregulation of GLUT1 [26,27]. Although we did not examine the cause of differential intermediate monocyte GLUT1 expression for the two IMT groups in this study, it is possible that increased levels of monocyte stimuli in some HIV-infected persons could lead to increased monocyte activation and GLUT1 expression [28,29]. We previously showed that monocytes stimulated with LPS upregulate GLUT1 expression and produce increased levels of TNF-α compared to unstimulated monocytes [6]. Similarly, Freemerman et. al. indicated that GLUT1 expression on mononuclear phagocytes facilitated their activation and was essential for proinflammatory cytokine production [9]. It is possible that these GLUT1-expressing intermediate monocytes contribute to an inflammatory state that increases CVD risk in PLWH.
While intermediate monocytes have only recently been defined as a subset of human monocytes [15,30], it has become increasingly apparent that this monocyte subset is linked to inflammation. A number of inflammatory diseases are selectively associated with the intermediate monocyte subset [11,31–33], and recently, intermediate monocytes were shown to be the major monocyte subset responsive to low level LPS injection in humans [34]. A high level of inflammatory and activation gene expression was identified in the intermediate monocyte subset [15], which may explain why this monocyte subset has been linked to many inflammatory diseases. However, mechanistic studies are lacking to explain why intermediate monocytes can be selectively associated with inflammatory disease.
We did not identify a statistical difference in GLUT1-expressing CD4+ or CD8+ T lymphocytes between HIV-infected women with high or low IMT. These results differ from some studies examining PLWH that showed an association between CVD and activated T lymphocytes [16,17]. However, GLUT1 expression was not examined in those studies. Also, our study population consisted of women with lower IMT levels than the noted previous studies, which could explain why activated T lymphocytes did not differ between IMT groups.
sCD14, sCD163 and IL-6 can be produced by activated monocytes and have been associated with IMT in some studies, but most studies show no association between these markers and IMT in HIV-infected persons [35]. In our study we found no differences in these markers between high and low IMT groups and no correlation was observed between any of the markers and GLUT1 intermediate monocytes. It is unclear why sCD14, sCD163 and IL-6 have only been associated with IMT in a small number of studies. Different study populations may explain the discrepant results. It is surprising that GLUT1 expressing intermediate monocytes are not correlated with sCD14, sCD163, or IL-6 as GLUT1 is associated with monocyte activation. It is possible that monocyte production of sCD14, sCD163 and IL-6 occurs through a pathway(s) independently of GLUT1 expression.
In summary, our data suggest a role for GLUT1-expressing intermediate monocytes in subclinical CVD observed during HIV infection. Pending replication, GLUT1-expressing intermediate monocytes could provide a way to predict CVD events in PLWH and may be a useful target for therapy to limit chronic immune activation and inflammation observed during HIV infection.
Supplementary Material
Acknowledgments
The study was supported by the Office of the Principal of the University of the West Indies, Mona and NIH grants U01 AI68636 to the AIDS Clinical Trial Group (Alan L Landay) and 1R01HL126543, 1R01HL095140, R21-HL-120394 (Robert C Kaplan). Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS). WIHS (Principal investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Mary Young and Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
Footnotes
Author contributions: T.R.B., C.S.P., A.L.L., and J.J.A. designed the study. T.R.B. carried out experiments. T.R.B and J.J.A. wrote the manuscript. A.L.L., D.B.H., R.C.K., S.M.C, and C.S.P. provided technical expertise and manuscript suggestions. All authors read and approved the manuscript.
References
- 1.Wada N, Jacobson LP, Cohen M, French A, Phair J, Muñoz A. Cause-Specific Life Expectancies After 35 Years of Age for Human Immunodeficiency Syndrome-Infected and Human Immunodeficiency Syndrome-Negative Individuals Followed Simultaneously in Long-term Cohort Studies , 1984 – 2008. Am J Epidemiol. 2013;177:116–25. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomfield GS, Khazanie P, Morris A, Rabadán-diehl C, Benjamin LA, Murdoch D, et al. HIV and non-communicable cardiovascular and pulmonary diseases in low- and middle-income countries in the ART era: What we know and best directions for future research. J Acquir Immune Defic Syndr. 2014;67:S40–53. doi: 10.1097/QAI.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woollard KJ, Geissmann F. Monocytes in atherosclerosis : subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansson GK, Libby P. The immune response in atherosclerosis: a double edged sword. Nat Rev Immunol. 2006;6:508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 5.Palmer CS, Ostrowski M, Gouillou M, Tsai L, Yu D, Zhou J, et al. Increased glucose metabolic activity is associated with CD4+ T-cell activation and depletion during chronic HIV infection. AIDS. 2013;28:297–309. doi: 10.1097/QAD.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer CS, Anzinger JJ, Zhou J, Gouillou M, Landay A, Jaworowski A, et al. Glucose Transporter 1 – Expressing Proinflammatory Monocytes Are Elevated in Combination Antiretroviral Therapy – Treated and Untreated HIV + Subjects. J Immunol. 2014;193:5595–603. doi: 10.4049/jimmunol.1303092. [DOI] [PubMed] [Google Scholar]
- 7.Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84:949–57. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer CS, Henstridge DC, Yu D, Singh A, Balderson B, Duette G, et al. Emerging role and characterization of immuno-metabolism: relevance to HIV pathogenesis, serious non-AIDS events and cure. J Immunol. 2016;196:4437–44. doi: 10.4049/jimmunol.1600120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, et al. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289:7884–96. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler-Heitbrock L. Blood monocytes and their subsets : established features and open questions. Front Immunol. 2015;39:923–38. doi: 10.3389/fimmu.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, et al. J Am Coll Cardiol. Vol. 60. Elsevier Inc; 2012. CD14++CD16+ Monocytes Independently Predict Cardiovascular Events: A Cohort Study of 951 Patients Referred for Elective Coronary Angiography; pp. 1512–20. [DOI] [PubMed] [Google Scholar]
- 12.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 13.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim Monocytes Patrol and Sense Nucleic Acids and Viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–86. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson EMP, Singh A, Hullsiek H, Gibson D, Henry WK, Lichtenstein K, et al. Monocyte-Activation Phenotypes Are Associated With Biomarkers of In fl ammation and Coagulation in Chronic HIV Infection. J Infect Dis. 2014;210:1396–406. doi: 10.1093/infdis/jiu275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zawada AM, Rogacev KS, Winter P, Marell R, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:50–62. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 16.Longenecker CT, Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, et al. Markers of inflammation and CD8+ T cell activation, but not monocyte activation are associated with subclinical carotid artery disease in HIV. HIV Med. 2013;14:385–90. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T Cell Activation and Senescence Predict Subclinical Carotid Artery Disease in HIV-Infected Women. J Infect Dis. 2011;203:452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble Markers of Inflammation and Coagulation but Not T-Cell Activation Predict Non – AIDS-Defining Morbid Events During Suppressive Antiretroviral Treatment. J Infect Dis. 2014;210:1248–59. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the Prevention of Atherosclerosis: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann Intern Med. 2001;135:939–53. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 20.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women ' s Interagency HIV Study : an Observational Cohort Brings Clinical Sciences to the Bench. Clin Diagn Lab Immunol. 2005;12:1013–9. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musso T, Deaglio S, Franco L, Calosso L, Badolato R, Garbarino G, et al. CD38 expression and functional activities are up-regulated by IFN-gamma on human monocytes and monocytic cell lines. J Leukoc Biol. 2001;69:605–12. [PubMed] [Google Scholar]
- 22.Funaro A, Spagnoli GC, Ausiello CM, Alessio M, Roggero S, Delia D, et al. Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J Immunol. 1990;145:2390–6. [PubMed] [Google Scholar]
- 23.Westhorpe C, Maisa A, Spelman T, Hoy J, Dewar EM, Karapanagiotidis S, Hearps A, et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol. 2014;92:133–8. doi: 10.1038/icb.2013.84. [DOI] [PubMed] [Google Scholar]
- 24.Zungsontiporn N, Tello RR, Zhang G, Mitchell BI, Budoff M, Kallianpur KJ, et al. Non-Classical Monocytes and Monocyte Chemoattractant Protein-1 (MCP-1) Correlate with Coronary Artery Calcium Progression in Chronically HIV-1 Infected Adults on Stable Antiretroviral Therapy. PLoS One. 2016;11:e0149143. doi: 10.1371/journal.pone.0149143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Funderburg NT, Zidar Da, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anzinger JJ, Butterfield TR, Angelovich TA, Crowe SM, Palmer CS. Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J Immunol Res. 2014;2014 doi: 10.1155/2014/569819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu YL, Maianu L, Melbert BR, Garvey WT. Facilitative glucose transporter gene expression in human lymphocytes, monocytes, and macrophages: a role for GLUT isoforms 1, 3, and 5 in the immune response and foam cell formation. Blood Cells, Mol Dis. 2004;32:182–90. doi: 10.1016/j.bcmd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Baroncelli S, Galluzzo CM, Pirillo MF, Mancini MG, Weimer LE, Andreotti M, et al. Microbial translocation is associated with residual viral replication in HAART-treated HIV+ subjects with <50copies/ml HIV-1 RNA. J Clin Virol. 2009;46:367–70. doi: 10.1016/j.jcv.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210:1228–38. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Derek N, et al. Nomenclature of monocytes and dendritic cells in blood Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:5–7. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 31.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, et al. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 32.Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012;64:671–7. doi: 10.1002/art.33418. [DOI] [PubMed] [Google Scholar]
- 33.Schauer D, Starlinger P, Reiter C, Jahn N, Zajc P, Buchberger E, et al. Intermediate Monocytes but Not TIE2-Expressing Monocytes Are a Sensitive Diagnostic Indicator for Colorectal Cancer. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0044450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thaler B, Hohensinner PJ, Krychtiuk KA, Matzneller P, Koller L, Brekalo M, et al. Differential in vivo activation of monocyte subsets during low-grade inflammation through experimental endotoxemia in humans. Sci Rep. 2016;6:30162. doi: 10.1038/srep30162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-Inflammatory Markers in Relation to Cardiovascular Disease in HIV Infection. A Systematic Review. PLoS One. 2016;11:e0147484. doi: 10.1371/journal.pone.0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.