Abstract
Neurological diseases and disorders (NDDs) present a significant societal burden and currently available drug- and biological-based therapeutic strategies have proven inadequate to alleviate it. Gene therapy is a suitable alternative to treat NDDs compared to conventional systems since it can be tailored to specifically alter select gene expression, reverse disease phenotype and restore normal function. The scope of gene therapy has broadened over the years with the advent of RNA interference and genome editing technologies. Consequently, encouraging results from central nervous system (CNS)-targeted gene delivery studies have led to their transition from preclinical to clinical trials. As we shift to an exciting gene therapy era, a retrospective of available literature on CNS-associated gene delivery is in order. This review is timely in this regard, since it analyzes key challenges and major findings from the last two decades and evaluates future prospects of brain gene delivery. We emphasize major areas consisting of physiological and pharmacological challenges in gene therapy, function-based selection of an ideal cellular target, available therapy modalities, and diversity of viral vectors and nanoparticles as vehicle systems. Further, we present plausible answers to key questions such as strategies to circumvent low blood-brain barrier permeability, most suitable CNS cell types for targeting. We compare and contrast pros and cons of the tested viral vectors in context of delivery systems used in past and current clinical trials. Gene vector design challenges are also evaluated in the context of cell-specific promoters. Key challenges and findings reported for recent gene therapy clinical trials, assessing viral vectors and nanoparticles, are discussed in the context of bench to bedside gene therapy translation. We conclude this review by tying together gene delivery challenges, available vehicle systems and comprehensive analysis of neuropathogenesis to outline future prospects of CNS-targeted gene therapies.
Keywords: adenoviral vectors, adeno-associated viral vectors, lentiviral vectors, polymeric nanoparticles, CNS-gene delivery, CNS-specific promoters
1. Introduction
A comprehensive global burden of disease study indicated that years lived with disability increased by 59% for neurological disorders and by 83% for cerebrovascular disease from 1990 to 2013 (Vos et al., 2015). These neurological disorders include Alzheimer’s disease (AD) and other dementias, Parkinson’s disease (PD), and multiple sclerosis (MS), while ischemic and hemorrhagic stroke constitute cerebrovascular disease. Neurological and cerebrovascular conditions are discussed in this review as neurological diseases and disorders (NDDs) in the context of therapeutic gene delivery. Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS) and human immunodeficiency virus (HIV)-associated neurocognitive disorders (HAND) are also included in the NDDs spectrum. Available drug- and biological-based therapeutic strategies are inadequate to treat or cure NDDs. Several studies are testing safe, effective, non-invasive, therapeutic strategies for NDDs by attempting delivery of drugs (Geldenhuys et al., 2011), proteins (Chaturvedi et al., 2014), and genes (Su et al., 2009) to cure disease or to slow its development. NDD progression could potentially be halted or reversed by targeting a single cell type, for instance neurons or astrocytes. Gene therapy is highly promising in this regard as it could be tailored for transient or long-term gene expression in specific cell types.
Researchers have actively investigated gene-based NDD therapies in the last two decades, and genes have been delivered successfully to central nervous system (CNS) cells (Bevan et al., 2011; Morris and Labhasetwar, 2015). Conventionally, challenges towards brain-targeted therapeutic delivery include low blood-brain barrier (BBB) permeability, brain heterogeneity, route of administration and dosing. Though these challenges are common for drugs and genes, unique modifications to gene delivery vehicles could potentially circumvent the challenges (Section 2). In addition to tackling the physiological and pharmacological problems, the principal questions related to gene delivery have revolved around obtaining an ideal cellular target, suitable therapeutic gene(s), and an efficient gene delivery vehicle. Oligodendrocytes and microglia play key role(s) in the neuropathogenesis of NDDs, yet treatments have centered on neurons and astrocytes. We have analyzed the functional features and disease roles of these cell types to ascertain their suitability as cellular target(s) (Section 3). Gene therapy modalities increased after the advent of silencing by RNA interference (RNAi) technology and genome editing endonuclease systems in addition to augmentation. The pros and cons of each approach are discussed here from the perspective of NDDs (Section 4). Vectors derived from adenoviruses, adeno-associated viruses (AAV), and lentiviruses as well as non-viral transport systems, such as polymeric nanoparticles (NPs) and lipid complexes, are under investigation as vehicles for CNS gene delivery. Evaluating expression efficiency, duration, and targeting specificity is critical to validate a suitable delivery system (Section 5). Therapeutic genes driven by cell-specific promoters have been utilized to restrict expression. The selection of promoter during vector design process has evolved over time and unique trends for neural cell types have been examined (Section 6). Recent gene therapy clinical trials are evaluating safety and efficacy of novel viral vectors and nanoparticles. The findings reported thus far for these trials must be analyzed in the context of ‘bench to bedside translational’ challenges to postulate directions for future gene therapy research (Section 7). To conclude, we present a synopsis of the overall trends observed for each aspect of CNS-targeted gene delivery systems and propose future directions (Section 8).
2. Challenges towards CNS-targeted gene delivery
Effective gene delivery could restore normal cell function during NDDs by providing transient or long-term expression of the gene of interest, eliminating the need for frequent drug administration and continual penetration of the BBB. An ideal gene delivery system development would involve in vitro testing of delivery vehicle under consideration of biocompatibility, efficacy, dose-time kinetics, and sustained expression. Subsequently, in vivo studies would evaluate dosing, route of administration, and immune responses in addition to the same in vitro assessments followed by transition into clinical trials. However, each step of this process contains its own challenges. For example, our ongoing studies have revealed surprising difficulty in polymeric NP-mediated gene delivery to primary human neurons and astrocytes, let alone targeting them in vivo. Currently, we are looking into the NP-uptake mechanisms in these cells to determine feasible alternatives and to overcome gene expression challenges (unpublished data). Further, transgenic animal models [Reviewed by (Jucker, 2010; Gorantla et al., 2012)] fail to mimic the complexities of the NDD-associated mechanisms making therapeutic assessments difficult. Diverse hosts (mouse, rat, rabbit, human), coupled with age differences have resulted in varying gene expression levels in preclinical studies (Gray et al., 2011; Gholizadeh et al., 2013). Finally, the immunologically privileged status of the brain makes reaching brain cells difficult, given the low permeability of the intact BBB and complexity of brain structure. These barriers along with obstacles to routes of administration for gene therapies, and the difficulties in bench to bedside translation will be discussed in this section.
2.1 Low blood-brain barrier permeability
In the absence of trauma or disease, tight junctions between brain microvascular endothelial cells (BMVECs) covered by astrocyte foot processes seal the majority of the brain to peripheral immune surveillance and passive diffusion of water-soluble drugs (Abbott et al., 2006). The intricate vasculature of the brain promotes global perfusion since all brain cells are within 20 μm of a blood capillary (Pardridge, 2002). Therefore, intravascular gene delivery systems could permeate the entire brain if the BBB can be traversed. Low permeability of the BBB remains the primary physical challenge and rate-limiting step for targeting brain cells. An ideal vascular gene delivery system would have very small size, high lipophilicity, and low plasma protein binding (Pardridge, 2002).
Severe neuroinflammation compromises the BBB by activating BMVECs and astrocytes, increasing cellular adhesion molecule expression and decreasing tight junction and extracellular matrix protein levels. Thus, BBB permeability varies in health and disease (Xia et al., 2004). Based on this, delivering gene(s) during NDDs, such as AD and PD, may be easier since chronic neuroinflammation enhances BBB permeability. However, compromised BBB during ischemia may not be helpful for gene therapies since the therapeutic window is small and a rapidly acting therapy is required (Jaffer et al., 2011). According to a report, gene delivery was efficient in fetal mice compared to older mice, attributed underdeveloped BBB (Gholizadeh et al., 2013). As classic NDDs (e.g. AD) are diagnosed and treated in older patients, improved gene delivery older mice will be more relevant. Disruption of BBB to enhance delivery has been explored via microbubbles, ultrasound (Tan et al., 2016) and chemicals (e.g. mannitol) (Kwon et al., 2010; Gray et al., 2011); but the clinical implications of such approaches are not known. Arginine-modified polyplexes (Morris and Labhasetwar, 2015), transactivator of transcription-conjugated NPs (Rao et al., 2008) and AAV vector serotype 9 (Foust et al., 2009) crossed the BBB when injected intravenously in rodent models, indicating that tailor-made NPs with surface modifications and genetically modified viral vectors with particular capsid sequences can overcome the BBB. Further, off-target gene expression in peripheral organs could be prevented by inclusion of cell-specific promoters (discussed in Section 6).
Gene vectors have been injected directly into the brain to circumvent the BBB (Do Thi et al., 2004; Yang et al., 2013). The innate difficulty and risk during brain administration makes this method less applicable over long-term clinical trials. Convection-enhanced delivery, in which one or more catheters are carefully placed in the brain parenchyma for therapeutic delivery, could be a potential solution. While elaborate, this technology is currently in Phase III clinical trials for glioblastoma treatments (Debinski and Tatter, 2009) and could be a feasible delivery mechanism for future gene-based NDD therapies.
2.2 Brain structure complexity
The heterogeneity of the brain rules out a ‘universal’ delivery system for genes. In addition, unique pathogenesis of each NDD requires a tailored vehicle system designed to deliver a gene of interest to a certain cell type for a given disease. Though genes have been injected or infused directly in the brain (Do Thi et al., 2004; Yang et al., 2013; Mastorakos et al., 2015), obtaining global gene expression has been difficult -(Lisovoski et al., 1997; Arregui et al., 2011). This is attributed to low gene or vehicle diffusion within brain parenchyma (Mastorakos et al., 2015), less viral vector transduction efficiency (Lisovoski et al., 1997), and reduced gene expression driven by weak promoters (Sandhu et al., 2009). Additional difficulties ensue in diseases where a precise region is to be targeted within the heterogeneous brain structure, e.g. the substantia nigra (SN) in PD. Age contributes to brain complexity in the context of the extent of BBB development (Gholizadeh et al., 2013) and the ratios of glia to neurons throughout the brain parenchyma.
Some viral vectors demonstrate select cell type and brain region tropism (Nomoto et al., 2003; Aschauer et al., 2013). For instance, AAV2 preferentially transduced neurons while AAV5 transduced astrocytes in vitro. In vivo, AAV2-mediated expression was restricted to pyramidal and granular cells and AAV5 transduced only granular cells (Nomoto et al., 2003; Aschauer et al., 2013). Further, neurons and astrocytes could respond dissimilarly to the same biomolecules, such as heme oxygenase (Benvenisti-Zarom and Regan, 2007), requiring evaluation of off-target effects. Tagging specific cell surface receptor ligands on DNA-vehicle complex can target neurons or astrocytes (Discussed further in Section 6). Thus, thorough knowledge of neuropathology, cellular responses to particular stimuli, intrinsic cell tropisms of delivery systems, and gene vector or vehicle system modifications are critical to tackle the brain heterogeneity challenge in the treatment of each NDD.
2.3 Route of administration
Pharmacokinetic and pharmacodynamic profiles of drugs are studied to obtain optimal dose(s) and route of administration. Gene delivery is complicated because dose and frequency of administration depends on both the gene and the delivery system. Intranasal (Kim et al., 2012), intracarotid (Gray et al., 2011), intravitreal (Aartsen et al., 2010), intrathecal (Milligan et al., 2006) and intramuscular (Towne et al., 2010) routes of administration have been explored for brain delivery. Currently, intravenous (Yurek et al., 2009) and intracerebral (Drinkut et al., 2012) administrations are frequently used; yet, neither is an effective ‘gold standard’ for CNS gene delivery. Intracerebral delivery was shown to be efficient (Yurek et al., 2009), but had brain diffusion issues (Drinkut et al., 2012). Low diffusion is a noted disadvantage of intracerebroventricular (ICV) delivery as well (Pardridge, 2002). Depending on the therapeutic strategy though, limited diffusion could be beneficial when a local effect is desired (Drinkut et al., 2012). Intrathecal delivery revealed promising results in rodents with spinal cord injury (SCI) (Milligan et al., 2006); however, it led to severe neurotoxic effects in monkeys (Samaranch et al., 2014). Overall, the route of administration must be tailored to the therapeutic usage, while reducing associated risks and optimizing ease of use and efficacy. Intravenous and intranasal routes offer ease of administration compared to intracerebral or intrathecal routes. Therefore, research should be directed towards developing systems that could be delivered via intravenous or intranasal routes.
Age, weight, gender, and genetics of the examined species and the route of administration determine the NPs or viral vector dosing strategy. The transition from in vitro to in vivo studies and from mice to non-human primates (NHPs) is difficult as the delivery system design and dosing paradigms vary significantly between models (Gray et al., 2011). Additionally, there are logistical concerns over bulk production and handling. Thorough dose-time kinetics experiments will be required to establish the therapeutic window of gene delivery for individual NDD. For instance, gene therapies for stroke would need a rapid onset of expression, or be prophylactically administered to those with a significant risk. Potential palliative measures for would require dose concentrations and frequencies tailored to particular patients based on relative risk and symptoms. Taken together, it can be discerned that the route of administration issue can be partly attributed to low BBB permeability (Section 2.1) and complex brain structure (Section 2.2); while, an optimal administration method may overcome the BBB and the brain heterogeneity challenges.
3. Selecting an ideal CNS cellular target for NDD therapy
Neuroinflammation and neurodegeneration are hallmarks of NDDs (Dhar et al., 2006; Begum et al., 2008; Hirsch and Hunot, 2009; Silvestroni et al., 2009; Beers et al., 2011). These hallmarks and intracellular processes that precede or succeed them involve a crosstalk between neurons, astrocytes, microglia and peripheral blood monocytes (Carson et al., 2006). Thus, all of these cell types could potentially serve as cellular targets for NDDs treatment.
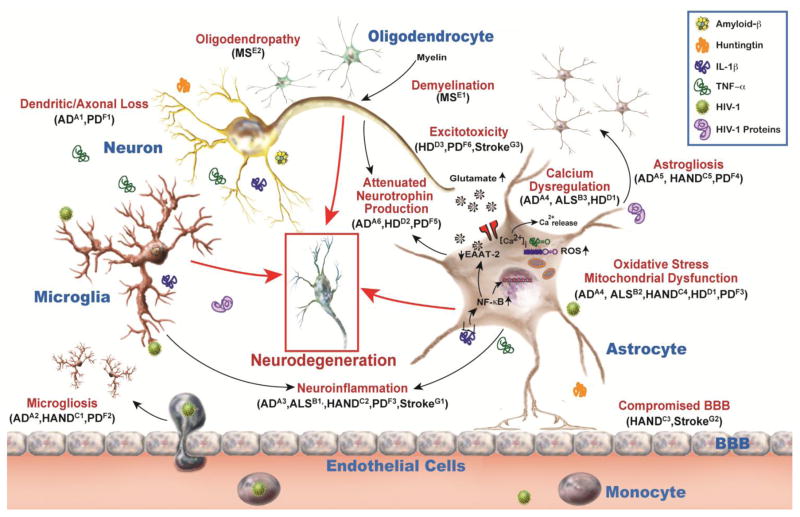
Gene delivery challenges discussed in Section 2 could be attributed to physical properties of the vehicle, i.e. viral vectors or NPs. Structural or physical modifications of vehicles could overcome the challenges to offer a feasible and efficient gene delivery. On the other hand, an equally important question would be which cell type would make an ideal target? In this section, we discuss some principal types of CNS cells in the context of their functions and their role in NDD pathogenesis to ascertain suitable cellular targets. A schematic of key underlying processes during NDDs and all discussed cell types along with their functional relevance is depicted in Fig. 1.
Fig. 1. Common cellular mechanisms implicated in neurological diseases and disorders (NDDs).
NDDs are associated with cellular dysfunctions of principal CNS cells including neurons, microglia, oligodendrocytes, and astrocytes. Neuronal damage includes dendritic and axonal loss, and reduced neurotrophin secretion. Microglial activation occurs during brain injury and resultant proinflammatory cytokines induce neuroinflammation along with microgliosis due to extravasation of peripheral monocytes. Oligodendrocyte dysfunctions include demyelination and oligodendropathy, i.e. death of oligodendrocytes. During injury, astrocytes contribute to excitotoxicity, and neuroinflammation by reduced glutamate uptake and increased release of proinflammatory cytokines, respectively. They likely undergo oxidative stress, mitochondrial dysfunction, calcium dysregulation, attenuated neurotrophin production and astrogliosis. Additionally, altered BBB permeability can increase neuroinflammation and contribute to disease. These mechanisms precede or succeed neurodegeneration and overlap in diseases such as Alzheimer’s disease (AD) [A1(Uylings and De Brabander, 2002), A2 (Wake et al., 2013), A3 (Wyss-Coray and Rogers, 2012), A4 (Alberdi et al., 2013), A5 (Fuller et al., 2009), A6 (Allen and Barres, 2009)], Amyotrophic lateral sclerosis (ALS) [B1 (Evans et al., 2013), B2 (Manfredi and Xu, 2005), B3 (Grosskreutz et al., 2010)], HIV-associated neurocognitive disorders (HAND) [C1 (Lu et al., 2011), C2 (Cisneros and Ghorpade, 2012), C3 (Persidsky et al., 2000), C4 (Steiner et al., 2006), C5 (Vartak-Sharma et al., 2014)], Huntington’s disease (HD) [D1 (Wang et al., 2013), D2 (Giralt et al., 2010), D3 (Fan and Raymond, 2007)], Multiple Sclerosis (MS) [E1 (Franklin and Kotter, 2008), E2 (Popescu and Lucchinetti, 2012)], Parkinson’s disease (PD) [F1 (Van Spronsen and Hoogenraad, 2010), F2 (Hu et al., 2008), F3 (Zinger et al., 2011), F4 (Niranjan, 2014), F5 (Drinkut et al., 2012), F6 (Ambrosi et al., 2014)], and stroke [G1 (Ceulemans et al., 2010), G2 (Xia et al., 2004), G3 (Lai et al., 2014)].
3.1 Neurons
Structurally and functionally neurons remain the most investigated CNS cell type and therefore neuronal changes and biomarkers associated with NDDs have been well characterized. Neuronal numbers reduce in healthy aging and there is a rapid loss of certain brain region neurons during NDDs. For instance, loss of neurons in the frontal cortex, SN, and striatum occurs during AD (Uylings and De Brabander, 2002), PD (Anglade et al., 1997), and HD (DiFiglia et al., 1997), respectively.
Clinically, neuronal death is associated with dendritic loss, synaptic reduction and cognitive and/or motor impairment (Uylings and De Brabander, 2002). Synaptic transmission, the principal function of neurons, is altered during AD and PD (Van Spronsen and Hoogenraad, 2010). Neuritic plaques, containing the 43-amino acid peptide amyloid-β (Aβ), and neurofibrillary tangles, containing tau protein, are pathological hallmarks of AD (Andrade-Moraes et al., 2013). Accumulation of interneuronal cytoplasmic ‘Lewy’ bodies, composed of α-synuclein, parkin, ubiquitin, and neurofilaments, remain a diagnostic criterion for PD, in addition to dopaminergic neuron loss (Emerit et al., 2004). In HD, huntingtin (htt) protein aggregates build up inside neurons (DiFiglia et al., 1997). Despite precise neuropathogenesis of every NDD, intracellular processes preceding or succeeding neuronal loss may overlap including mitochondrial and oxidative stress (Emerit et al., 2004), synaptic transmission dysfunction (Van Spronsen and Hoogenraad, 2010), altered neurotransmitter levels (Tekin and Cummings, 2002) and changes in stimuli preventing or promoting neuronal cell death (Emerit et al., 2004). Disrupted synaptic transmission of the excitatory neurotransmitter glutamate leading to excitotoxicity has been implicated in ischemic stroke, epilepsy, MS, and HAND (Arundine and Tymianski, 2003; Cisneros and Ghorpade, 2014). Axonal damage and subsequent impairment of axonal transport is linked to ALS (Boillée et al., 2006) and MS (De Stefano et al., 2003) pathologies. Importantly, astrocyte or microglia or oligodendrocyte dysfunctions can cause neuronal damage by inducing the same processes listed above. Additionally, reduced expression of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) and glial cell-derived neurotrophic factor (GDNF), is also implicated in NDDs and replenishing their levels is considered a potential therapeutic strategy, discussed in detail in section 4.1.
Neuroprotection remains the ultimate aim of all therapeutic strategies either by preventing or reversing damage. Currently available palliative NDD treatments involve drugs for AD (e.g. acetylcholinesterase inhibitors (von Bernhardi et al., 2003) and PD (e.g. dopamine agonists (Schapira, 1999), immunosuppressive therapy for MS (Stangel et al., 2006), blood thinners for stroke (e.g. tissue plasminogen activator (Jaffer et al., 2011), rehabilitative care and physical therapy. Additionally, neural stem cell-based transplantations and other surgical procedures are also included in the NDD treatment repertoire. Unfortunately, these treatments are inadequate making gene therapy a promising alternative.
Neuronal gene delivery investigations have shown varying degrees of success to reverse pathogenesis (Nakajima et al., 2007; Chen et al., 2012). However, targeting neurons still presents certain limitations. The neuronal population is always reducing since they die during healthy aging, in addition to rapid loss during disease. Synaptic transmission is fastidious and must be tightly regulated. As pointed out by Drinkut and colleagues, if neurons secrete exogenous biomolecules from various cell parts, including the soma and synaptic sites, it could produce off-target, undesirable effects since axonal endings terminate on other neurons or peripheral organs (Drinkut et al., 2012). Since NDDs are not diffuse or random but involve damage to a precise subset of neurons, neuronal gene delivery has always been challenging (Callaway, 2005). However, if gene expression could be restricted to a brain region and a strong, neuron-specific promoter is used, a low dose of exogenous DNA for neuronal expression may prove beneficial.
3.2 Microglia
Comprising ~10 to 15% of the total brain cells, microglia are the resident immune cells of CNS (Carson et al., 2006). Resting microglia perform normal brain surveillance (Polazzi and Monti, 2010; Aguzzi et al., 2013). In acute conditions, activated microglia provide neuroprotection by releasing neurotrophic and anti-inflammatory factors including NGF, BDNF, neurotrophin-3 and GDNF, providing innate immunity, facilitating repair through guided migration of stem cells (Garden and Möller, 2006; Block et al., 2007) and phagocytizing dead neurons and debris (Polazzi and Monti, 2010).
During normal aging, microglia produce a chronic, mild inflammatory environment by secreting inflammatory cytokines interleukin (IL)-1β, tumor necrosis factor (TNF)-α, CCL2, CXCL8 and reactive nitrogen species such as nitric oxide, a potential risk factor for NDDs (Von Bernhardi et al., 2015). Triggers include extracellular adenosine triphosphate, serum factors (e.g. plasma thrombins), microbial agents (e.g. lipopolysaccharides) and pathological proteins (e.g. Aβ) induce microglial activation (Garden and Möller, 2006). Microgliosis is another key process in chronic inflammation, which is attributed to extravasation of circulating monocytes post-BBB disruption (Wake et al., 2013). Neurons could inhibit the microglial activation via receptor-ligand interaction; an example is neuronal CD200 and its receptor on microglia (Aguzzi et al., 2013); however, disrupting such interactions could lead to chronic neuroinflammation.
Microglia cause neurotoxicity either as a response to external pro-inflammatory stimuli or through activation post-neuronal injury, eventually eliciting neurodegeneration by secretion of neurotoxic substances (Block et al., 2007). Since their responses differ drastically during mild, acute injury versus chronic inflammation, microglia can act as friend or foe depending on the stage and extent of injury (Aguzzi et al., 2013). Therefore, an ideal therapeutic approach would consist of weakening microglial responses instead of silencing them entirely.
Microglia cleared Aβ plaques in AD immunotherapy preclinical trials (Lobello et al., 2012). Non-steroidal anti-inflammatory drugs such as cyclooxygenase-2 inhibitors are being investigated to alleviate microglial activation (Rock and Peterson, 2006). Few studies reported that genes were successfully delivered to neurons, astrocytes, and oligodendrocytes as well as microglia (Zhao et al., 2003; Hendriks et al., 2007).
The window of opportunity to target microglia is very unpredictable, especially in chronic NDDs (Polazzi and Monti, 2010), and would require timely diagnosis of particular disease and degree of microglial involvement. The extent of peripheral extravasation of monocytes during injury would be necessary to examine before targeting microglia (Wake et al., 2013). Early detection of microglial activation will be essential to inhibit their neurotoxic effects and subsequent neuronal loss. To achieve this, sophisticated techniques such as positron emission tomography will be essential (Block et al., 2007).
3.3 Oligodendrocytes
Oligodendrocytes, a type of glial cells, are responsible for myelination of axons in the brain and spinal cord (McTigue and Tripathi, 2008). They originate as pre-progenitors in the subventricular zone (SVZ) and then migrate, proliferate, mature and start producing myelin sheaths. These processes are regulated by platelet-derived growth factors (PDGF), fibroblast growth factor 1 and 2, insulin-like growth factor-1 (IGF-1), transforming growth factor-β, neurotrophin-3, and ciliary neurotrophic factor (CNTF) (McMorris and McKinnon, 1996). Oligodendrocytes electrically insulate sodium channel-clustering axons, which ensures saltatory nerve conduction (Bradl and Lassmann, 2010).
Metabolic changes in oligodendrocytes attributed to genetic defects, infections, or toxins lead to flawed myelination or demyelination (Chen et al., 1998). Remyelination, i.e. formation of new myelin sheaths by oligodendrocytes, remains the intrinsic response. Remyelination does not occur in MS due to unknown causes (Franklin and Kotter, 2008). Further, a variable loss of oligodendrocytes occurs in all MS subtypes, which includes apoptotic death in type III and non-apoptotic death in type IV. The underlying pathways linked to oligodendrocyte loss include oxidative and mitochondrial stress (Bradl and Lassmann, 2010), proinflammatory cytokine signaling, free radicals, and complement-facilitated injury (Merrill and Scolding, 1999). Clinically, lack of myelin associated protein and 2,3-cyclic nucleotide 3-phosphodiesterase indicate oligodendropathy (Popescu and Lucchinetti, 2012). Oligodendrocytes are implicated in other demyelinating diseases including Marburg disease and Devic’s disease (Popescu and Lucchinetti, 2012).
Preventing oligodendrocyte loss and increasing myelination are the two possible approaches to treat dysfunctional oligodendrocytes. Currently investigated MS treatments including immune-, cell-based-, and gene- therapies have direct implications/effects in oligodendrocytes (Rodgers et al., 2013). Proposed strategies to prevent oligodendropathy include upregulating growth factor gene expression, which regulate their maturation. However, a single growth factor is not involved in that process, posing a potential multiple gene delivery challenge. Oligodendrocyte-targeted gene delivery has been tested in studies investigating SCIs (Zhao et al., 2003; Tuinstra et al., 2012). Overall, demyelination diseases constitute a small fraction of NDDs spectrum and oligodendrocytes are one of the least abundant cell types in the brain. Hence, they may not be the best choice to attain global CNS gene delivery.
3.4 Astrocytes
Astrocytes, principal glial cells of the CNS (Wang and Bordey, 2008), are structural components of tight junctions of the BBB and form ‘tripartite’ synapses with pre- and post-synaptic neurons (Halassa et al., 2007). Astrocytes produce neurotrophic factors, including BDNF, NGF, GDNF (Cabezas et al., 2016) and tissue inhibitor of metalloproteinases-1 (Gardner and Ghorpade, 2003; Ashutosh et al., 2012). They regulate neurotransmission and synaptic activity by sequestering synaptic potassium and neurotransmitters, including glutamate. Astrocytes communicate with neighboring astrocytes via calcium waves and gap junctions (Barres, 2008), and also by secreting a number of cytokines and chemokines including CCL2 (Kiyota et al., 2009), CXCL8 (Zheng et al., 2008), IL-1β and TNF-α (Niranjan, 2014) that play critical roles in NDD pathogenesis. The astrocyte neurotrophic repertoire also includes antioxidant defense and metabolic support.
Neuroinflammation induces reactive gliosis whereby reactive astrocytes proliferate and migrate towards injury, leading to glial scar formation (Carson et al., 2006; Buffo et al., 2010; Colangelo et al., 2014; Anderson et al., 2016). While this response is an attempt to repair the initial damage, both astrocyte and neuronal functions are compromised by chronic inflammation. Similar to microglia, astrocytes secrete neuromodulatory molecules, which can be either protective or damaging depending on the stage of injury, making astrogliosis a ‘double-edged’ sword (Buffo et al., 2010). Other astrocyte intracellular processes that contribute to NDD pathologies include oxidative and mitochondrial stress (Manfredi and Xu, 2005), reduced excitatory amino acid transporter (EAAT)2 levels that lead to excitotoxicity (Ambrosi et al., 2014), calcium dysregulation (Alberdi et al., 2013) and attenuated neurotrophin secretion (Giralt et al., 2010).
Astrocytes could be central targets for NDD gene therapies as they interact with other cell types including neurons, microglia, BMVECs, and ependymal cells throughout the brain contributing to the disease and recovery processes (Wang and Bordey, 2008). Their foot processes are present at the interface between the periphery and the brain, giving them preferential access to therapeutics delivered via vascular route. A phosphodiesterase inhibitor, Ibudilast, prevents glial activation; and clinical trials testing its potential for chronic migraine, and substance abuse disorders are underway (Institut and Health, 2016). Simultaneously, preclinical studies have been performed to develop astrocyte-directed gene delivery systems (Gray et al., 2011; Gholizadeh et al., 2013). Clinical trials have been conducted in which astrocytes are cellular targets (Section 7). The majority of these preclinical and clinical trials intended to overexpress neurotrophin genes with or without astrocyte-specific promoter thereby mimicking inherent astrocyte neuroprotective function.
Before, focusing on astrocytes as a suitable cellular target for global CNS gene delivery, some potential issues must be discussed. Genes delivered via viral vectors, for example AAV, and NPs remain episomal. During chronic inflammation, these delivered genes could get diluted or lost due to astrocyte proliferation presenting astrogliosis as a “self-limiting” factor (Drinkut et al., 2012). Additionally, abundant interconnected astrocytes may not be ideal for PD and stroke therapy where site-restricted gene expression is required. Nevertheless, the fundamentally neuroprotective functions of astrocytes make them relevant. Considering the limitations stated in Section 3.1 for neurons, targeting astrocytes might prove a beneficial strategy.
4. Gene delivery modalities
Gene delivery strategies for NDD therapies are categorized into three approaches: 1. Augmentation 2. Silencing and 3. Editing. The goal of such therapies would be to alter specific gene expression and correct disrupted CNS homeostasis. Increasing neuroprotective measures, for instance, secreted neurotrophins, or reversing neurotoxic mechanisms, such as excitotoxicity, could restore CNS homeostasis. Thus, an ideal CNS-targeted gene delivery method would increase neurotrophin levels or reduce proinflammatory biomarkers. Hereditary NDDs, such as HD, depict distinct gene mutations and require therapies to permanently correct the mutated gene, which can be achieved by gene editing. Though the names of gene therapy modalities are self-explanatory, it is essential to compare and contrast between these methods to determine their applicability in the context of NDDs.
4.1 Gene augmentation
Gene augmentation would be applicable when an insertion or missense mutation produces nonfunctional protein or when certain genes are downregulated owing to disease pathology. For CNS diseases, it has been employed for replenishing neurotrophin levels, for example BDNF, GDNF, and NGF. All neurotrophins, except BDNF, are constitutively expressed in the brain. Neurotrophin levels are reduced in NDDs and exogenous administration is not possible since they cannot cross the BBB (Allen et al., 2013). Therefore, a gene augmentation modality could be used to increase or maintain neurotrophin levels. Neuroprotective and neurorestorative effects of this approach have been reported in AD (Furman et al., 2012), PD (Choi-Lundberg et al., 1997), and HD (Giralt et al., 2010). Many gene therapy trials for neurodegeneration are testing neurotrophin augmentation (Section 7).
A notable exception to the above theme includes the delivery of 7ND, a dominant negative analogue of CCL2. Overexpression of 7ND competitively inhibited CCL2-facilitated proinflammatory effects in an amyloid precursor protein/presenilin-1 rodent model of AD (Kiyota et al., 2009). Additionally, genes of neurotoxic or neuroinflammatory proteins have been augmented to mimic disease pathology in preclinical studies. Examples of such studies include mutated htt gene delivery to study downstream effects on astrocyte function in HD. Mutated htt overexpression led to downregulation of astrocyte-BDNF indicating neurotrophin levels are critical to HD pathogenesis (Wang et al., 2012). Similarly, inflammatory cytokines IL-1β (Ferrari et al., 2004) and TNF-α (Ezcurra et al., 2010) were overexpressed in rat brains to determine subsequent effects in demyelination and PD, respectively. Gene augmentation is the oldest and principally tested gene delivery modality.
4.2 Gene silencing
Gene silencing can be useful for nonsense or repeat mutations that produce abnormally functioning proteins or to reduce levels of neurotoxic proteins that increase neuroinflammation. Gene silencing alters gene expression by post-transcriptional regulation of a gene by the RNAi technique. It has been employed for NDD-associated studies where small interfering RNA (siRNA) (Kim et al., 2010), microRNA (miRNA) (Tuinstra et al., 2012), and short hairpin RNA (shRNA) (Desclaux et al., 2009) specific to the gene of interest were delivered successfully to CNS cells.
Gene silencing inquiries involve studying disease mechanisms, evaluating cellular or protein function, and testing therapeutic potential. An early work testing RNAi in brain cells investigated the function of astrocyte-aquaporin (AQP) 4 water channels using siRNA. Reduced AQP4 resulted in reduced membrane water permeability, subsequently altering astrocyte morphology to maintain surface-volume ratio and water influx. Knocking down AQP4 also reduced ischemia-related protein expression indicating a possible therapeutic option (Nicchia et al., 2003). Therapeutic potential of shRNA for glial fibrillary acidic protein (GFAP) and vimentin was determined in primary astrocyte cultures. Both GFAP and vimentin are markers astrocyte markers activated during glial inflammation and their knockdown expression resulted in reduced glial activation and migration (Desclaux et al., 2009).
Lack of cell-specific restriction is a potential pitfall of using RNAi for therapy. Detrimental off-target effects could result from miRNA binding to multiple messenger RNAs. Exogenously produced siRNA and shRNA bind with single mRNAs; however, careful assessments must be performed during preclinical studies to avoid any off-target effects.
4.3 Gene editing
Gene editing is the least explored delivery modality due to its relatively novel nature compared to previously discussed modalities. Gene or genome editing is done with engineered nucleases composed of sequence-specific DNA-binding domains fused to a non-specific DNA cleavage module. These nucleases are capable of modifying DNA precisely and efficiently by inducing precise DNA double-strand breaks, which stimulates cellular DNA repair mechanisms. Such endonucleases include zinc-finger nucleases, transcription activator-like effector nucleases, and clustered regulatory interspaced short palindromic repeats (CRISPR)/CRISPR-associated system 9 (Cas9) (CRISPR-Cas9) RNA guided system (Gaj et al., 2013). Genome editing components are delivered to the target cell with overexpression vectors and have the same delivery and specificity limitations. In HD, a mutated htt gene contains increased CAG repeats at the 5′ end of the gene, making it an ideal NDD for gene editing. Since normal htt functions are not well established, knocking it down may not be a suitable approach. The number of CAG repeats is linked to disease severity, thus, gene editing methods that precisely remove excessive CAG repeats may attenuate disease pathology (Aronin and DiFiglia, 2014). Recently, CRISPR-Cas9 successfully eradicated HIV-1 proviral DNA from latently infected human CD4+ T cells and cells derived from microglia, monocytes, and T cells (Hu et al., 2014; Kaminski et al., 2016). During HAND, neurons and oligodendrocytes are not infected, 2 to 20% of astrocytes are nonproductively infected, and microglia are productively infected with HIV-1 (Ghafouri et al., 2006). Though anti-retroviral therapy reduces productive infection, HIV-1 proviral DNA is incorporated into microglia and astrocyte genome. These latently infected cells, astrocytes and microglia, become viral reservoirs in the brain, continually releasing viral proteins that cause neurotoxicity and neurocognitive decline. CRISPR-Cas9 technology could prove useful in eliminating latent infection in brain cells (Hu et al., 2014). Since the complete inactivation or removal of proviral DNA from infected cells has been a critical hurdle for HIV therapies, this approach is a giant leap toward a cure and for therapeutic gene editing in general.
5. Delivery Systems
Development of sophisticated systems has enabled successful gene delivery to CNS cells. Widely used gene delivery systems include, but are not limited to, viral vectors (Tables 1, 2, and 3), and NPs (Table 4). Based on the nature of the gene, delivery system investigations can be categorized into ‘proof of concept’ and ‘therapy-based’ inquiries. Reporter genes are used for ‘proof-of concept’ experiments that establish and validate delivery system’s efficiency, for example, evaluating AAV9 vector’s brain gene delivery potential using green fluorescent protein (GFP) reporter (Foust et al., 2009). Therapy-based studies deliver genes to alleviate disease progression based on NDD pathogenesis. For example, low BDNF levels during disease led to testing BDNF gene therapy for HD (Arregui et al., 2011). The same gene and/or delivery system could result in varied outcomes in two studies based on differences in gene delivery route, targeted cell type, and in vitro or in vivo models.
Table 1.
Adenoviral vectors for brain-targeted gene delivery
| Gene | Disease/Mechanism | Transduced Cell Types | In Vitro Cell Source | In Vivo | Result | Reference |
|---|---|---|---|---|---|---|
| Adrenomedullin | Stroke/ischemia | Neurons, Astrocytes | Sprague-Dawley (SD) rats | Middle cerebral artery occlusion (MCAO) in SD rats | In vitro: reduced apoptosis, increased migration, nitric oxide (NO), B-cell lymphoma (Bcl)-2 levels, and Akt, GSK-3β phosphorylation; In vivo: decreased infarct size and astrocyte migration to ischemic core | (Xia et al., 2004) |
| Brain-derived neurotrophic factor (BDNF) | Huntington’s disease (HD) | Astrocytes | R6/2 mice | Sustained expression, improved behavior, induced astrocyte motor phenotype | (Arregui et al., 2011) | |
| BDNF | HD | Not specified (N/S) | SD rats | Quinolinic acid (QA)-induced SD rats | Improved protection of striatal neurons | (Bemelmans et al., 1999) |
| Ciliary neurotrophic factor (CNTF) | Astrogliosis | Neurons, Astrocytes | Chicken embryo neural cells | SD rats | Hypertrophied astrocytes expressing gene for over 5 weeks | (Lisovoski et al., 1997) |
| CNTF, Glial cell line-derived neurotrophic factor (GDNF) | Stroke/Ischemia | Astrocytes | B6 mice | Prior intrastriatal delivery increased viable neurons post-ischemia, Reduced injured cells and DNA fragmentation, GDNF-induced effects better than CNTF | (Hermann et al., 2001a) | |
| Genetically encoded, fluorescent calcium indicator protein (G-CaMP2) | Astrocytes | Mice type N/S | Calcium signaling recorded in Bergmann glia and protoplasmic astrocyte | (Kuhn et al., 2011) | ||
| GDNF | Focal cortical trauma | Astrocytes | Cold injured SD rats | Reduced lesions attributed to reduced inducible nitric oxide synthase, caspase 3, and DNA fragmentation | (Hermann et al., 2001b) | |
| GDNF | Parkinson’s disease (PD) | Astrocytes | SD rats | 6-hydroxydopamine (OHDA)-treated SD rats | Improved nigral dopamine (DA) neuron survival and behavior | (Do Thi et al., 2004) |
| Heme oxygenase-1 | Oxidative injury | Astrocytes | BALB/c x 129/Sv mice | Reduced cell death post Hemin exposure | (Benvenisti-Zarom and Regan, 2007) | |
| Insulin-like growth factor-1 | Glial inflammation | Astrocytes | Caesarean derived (CD)-1 mice | Reduced toll-like receptor 4 expression, reduced NF-κB translocation | (Bellini et al., 2011) | |
| LacZ | Neurons, Astrocytes, Microglia | SD rats | Astrocytes and other cells expressed reporter | (Liu et al., 1997) | ||
| LacZ | Spinal cord injury (SCI) | Neurons, Astrocytes | Rats type N/S | Dorsal side astrocytes and ventral side neurons transduced, temperature-sensitive vector-mediated expression remained longer than controls | (Romero and Smith, 1998) | |
| LacZ | Neurons, Astrocytes | SD rats | High-capacity vector maintained gene expression over 6 months, prevented immune cells infiltration in the brain post-peripheral injection | (Thomas et al., 2000) | ||
| Secretory leukocyte protease inhibitor | Focal stroke | Neurons, Astrocytes | MCAO-in Hypertensive rats, SD rats | Reduced ischemic lesion size with neuroglial gene expression peak after 2 days | (Wang et al., 2003b) |
Table 2.
Adeno-associated viral vectors for brain-targeted gene delivery
| Type | Gene | Disease/Mechanism | Transduced Cell Types | In Vitro Cell Source | In Vivo | Result | Reference |
|---|---|---|---|---|---|---|---|
| AAV1 | Galactocerebrosidase (GALC) | Neurons, Astrocytes, Oligodendrocytes | Humans, mice | Twitcher (Twi) mice | Stable expression in vivo, improved myelination, symptom reduction, and increased life span | (Rafi et al., 2005) | |
| AAV1 AAV2 |
Green fluorescent protein (GFP) | Neurodegenerative diseases and disorders (NDDs) | Neurons, Astrocytes, Oligodendrocytes, Microglia | C3H/HeJ mice | AAV1 transduced multiple cell types, higher efficiency and better distribution than AAV2 | (Wang et al., 2003a) | |
| AAV1 AAV2 AAV8 |
CNTF | Myelin disorders | Sensory neurons, Schwann cells | CD-1 mice | AAV8: preferential Schwann cells transduction, lower immunogenicity, increased myelin and neuronal proteins associated with axonal regeneration | (Homs et al., 2011) | |
| AAV1 AAV5 AAV9 AAVrh10 |
GFP | SCI | Neurons,
Astrocytes Oligodendrocytes |
SD rats | AAVrh10: Neuronal transduction efficiency was similar to other vectors but also transduced other cell types | (Petrosyan et al., 2014) | |
| AAV1 AAV8 AAV9 |
GFP | Neurons, Astrocytes, Purkinje Cells | B6 mice | AAV9 most effective and sustained global expression for 18 months | (Miyake et al., 2011) | ||
| AAV2 | B-cell lymphoma 2-like 2 (a.k.a. Bcl-w) | Cerebral ischemia | Neurons, Astrocytes, Ependymal Cells | MCAO in SD rats | Global expression, infarct size reduction, improved neurological function | (Sun et al., 2003) | |
| GFP | Neurons, Microglia | SD rats | Neuronal expression 3 days post-infusion | (Bartlett et al., 1998) | |||
| GFP | Neurons, Astrocytes | N/S | Wistar rats | Human synapsin promoter: neuronal expression in vitro, in vivo Cytomegalovirus promoter: glial expression in vitro, thalamic neurons in vivo | (Kügler et al., 2003) | ||
| GFP | Neurons, Astrocytes | Cynomolgus monkeys | Neuronal expression and astrocyte activation | (An et al., 2016) | |||
| GFP | Oligodendrocytes | Rats type N/S | B6 mice | Expression in white matter in vivo, oligodendrocytes in vitro due to inclusion of myelin basic protein promoter | (Chen et al., 1998) | ||
| AAV2 AAV5 |
GFP, Luc | NDDs | Neurons, Astrocytes | Kainate-treated SD rats | Kainate-induction reduced transduction efficiency; AAV5 better than AAV2; AAV5 glial transduction in seizured brains | (Weinberg et al., 2011) | |
| LacZ | Neurons, Astrocytes | Wistar rats | Gerbils | In vitro: AAV2 neuronal tropism, AAV5 astrocyte tropism; In vivo: higher AAV5-mediated hippocampal expression | (Nomoto et al., 2003) | ||
| LacZ, Red fluorescent protein (RFP) | NDDs | Microglia | Wistar rats | SD rats | F4/80 promoter provided the best specificity compared to CD11b, CD68 promoters | (Cucchiarini et al., 2003) | |
| AAV2, AAV6 | GFP | Astrocytes, Muller cells | Humans, rats | Fischer 344 rats | Higher efficiency in vitro, astrocyte, muller glia transduction ability in vivo of AAV variants generated by loop replacement | (Koerber et al., 2009) | |
| GFP | Astrocytes | Human retinas, Crb1−/− mice | Astrocyte-expression with glial fibrillary acidic protein (GFAP) promoter | (Aartsen et al., 2010) | |||
| AAV2 AAV9 |
GFP, L-amino acid decarboxylase | Astrocytes | SD rats, Cynomolgus monkeys | AAV2, but not AAV9, mediated protein expression without immune response | (Ciesielska et al., 2013; Samaranch et al., 2014) | ||
| AAV4 | GFP, LacZ | Astrocytes, Neuronal progenitor cells | C57/BL6 mice | Expression in ependymal cells, astrocytes of subventricular zone, rostral migratory stream | (Liu et al., 2005) | ||
| AAV5 | GDNF | PD | Astrocytes | MPTP-induced B6 mice, 6-OHDA-treated Wistar rats | Expression specific to the injected hemisphere, no off-target effects; same efficacy as neuron-derived GDNF; increased DA synthesis, behavioral improvement | (Drinkut et al., 2012) | |
| VIVIT, a peptide that interferes with the calcineurin/nuclear factor of activated T-cells (NFAT) signaling pathway | Alzheimer’s disease (AD) | Astrocytes | Amyloid precursor protein/presenilin-1 mice | Reduced glial activation, amyloid levels, improved cognitive and synaptic function | (Furman et al., 2012) | ||
| AAV8 AAVcy5 AAVrh20 AAVrh39 AAVrh43 |
GFP | Neurons, Astrocytes, Oligodendrocytes | SD rats | Preferential astrocyte and oligodendrocyte transduction with AAV8 and AAVrh43 using cell-specific promoters | (Lawlor et al., 2009) | ||
| AAV8, AAV9 | GFP, mCherry | Neurons, Astrocytes | B6 mice | AAV8 astrocyte tropism and AAV9 neuronal tropism; single complementary (sc) AAV better than single strand (ss) AAV | (Aschauer et al., 2013) | ||
| AAV9 | Erythropoietin (EPO) | PD | Neurons, Astrocytes | 6-OHDA treated SD rats | Prior intramuscular injection reduced intrastriatal transduction efficiency due to circulating antibodies to AAV and EPO | (Yang et al., 2013) | |
| GFP | Amyotrophic lateral sclerosis | Neurons, Astrocytes | B6 mice | Crosses blood-brain barrier, neonatal mice: neuronal tropism, adult mice: astrocyte tropism | (Foust et al., 2009) | ||
| GFP | Spinal muscular atrophy (SMA) | Motor neurons, Astrocytes | Survival motor neurons Δ7 and B6 mice | Intramuscular injection transduced spinal cord motor neurons, astrocytes; increased median lifespan | (Benkhelifa-Ziyyat et al., 2013) | ||
| GFP | SMA | Neurons, Astrocytes, Oligodendrocytes | Cynomolgus monkeys | Global CNS expression at different ages; selective CNS expression by cerebrospinal fluid delivery | (Bevan et al., 2011) | ||
| GFP | Neurons, Astrocytes | BALB/C mice, Rhesus macaques | Higher neuronal expression in rodent, higher glial expression in NHPs, scAAV efficient than ssAAV | (Gray et al., 2011) | |||
| GFP | Neurons, Astrocytes | MF1 mice | Neuronal tropism in fetal mice, astrocyte tropism in neonatal mice; ssAAV efficient than scAAV | (Rahim et al., 2011) | |||
| GFP | Neurons, Astrocytes | B6 mice | Neonatal mice: astrocyte tropism; young mice: neuronal tropism | (Gholizadeh et al., 2013) | |||
| GFP | Neurons, Astrocytes | B6 mice | Intrathecal delivery efficient than intravenous delivery | (Schuster et al., 2015) | |||
| GFP | Neurons, Astrocytes | SD rats, farm mice | Dose-dependent increase in global transduction, no astrogliosis | (Donsante et al., 2016) | |||
| Glutamine synthetase, excitatory amino acid transporter, micro RNA targeting adenosine kinase (miADK) | Temporal lobe epilepsy | Astrocytes | Kainate-treated SD rats | miADK reduced seizure duration | (Young et al., 2014) | ||
| β-galactosidase | GM1-gangliosidosis | N/S | GM1 mice | Reduced astrogliosis, improved behavior and median survival, moderate CNS expression | (Weismann et al., 2015) | ||
| AAV9 AAVrh10 |
GFP | Neurons, Astrocytes | B6 mice | Efficient transduction without inflammatory response | (Zhang et al., 2011) | ||
| AAVrh8 AAVhu32 AAVhu37 AAVpi2A AVhu48R3 |
GFP | Neurons, Astrocytes, Oligodendrocytes | C3H/HeOuJ mice | Neuronal transduction by all serotypes, astrocyte and oligodendrocyte transduction by certain serotypes | (Cearley et al., 2008) |
Table 3.
Lentiviral vectors for brain-targeted gene delivery
| Gene | Disease/Mechanism | Transduced Cell Types | In Vitro Cell Source | In Vivo | Result | Reference |
|---|---|---|---|---|---|---|
| CNTF | Excitotoxicity | Neurons | SD rats | Quinolinic acid (QA)-induced Lewis rats | Hypertrophied astrocytes with increased glutamate aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1) expression, improved glutamate handling | (Escartin et al., 2006) |
| GDNF | NDDs | Neurons, Astrocytes | Mouse N2a neural cells, ventral mesencep halon cells | Gene expression for 3+ weeks, neuroprotection from 6-OHDA insult mediated by GDNF and glutathione together | (Sandhu et al., 2009) | |
| GDNF | PD | Astrocytes | 6-OHDA treated SD rats | Efficient, sustained expression, improved DA neuronal survival, improved behavior | (Lu-Nguyen et al., 2014) | |
| GFP | PD | Neurons, Astrocytes | Lewis rats | Astrocyte tropism by lymphocytic choriomeningitis virus (LCMV)- and Moloney murine leukemia virus (muLV)-enveloped | (Cannon et al., 2011) | |
| GFP | Neural scar formation | Neurons, Astrocytes, Oligodendrocytes precursors, Microglia | Wistar rats | Wistar rats | In vitro astrocyte and meningeal cells transduction, lack of transduction at injury/injection site in vivo with peripheral transduction of several cell types except meningeal cells | (Hendriks et al., 2007) |
| GFP | Neurons, Astrocytes | B6 mice | Astrocytes transduced with neuron promoter | (Lai and Brady, 2002) | ||
| GFP | Neurons, Astrocytes | SD rats | Vesicular stomatitis virus glycoprotein (VSV-G)-pseudotyped vector transduces glia and neurons, promoter-dependent selectivity | (Jakobsson et al., 2003) | ||
| GFP | Neurons, Astrocytes | SD rats, MF1 mice | Astrocyte tropism by gp64-enveloped | (Rahim et al., 2009) | ||
| GFP | Neurons, Astrocytes | Cynomolgus monkeys | Higher astrocyte transduction without activation over AAV2 | (An et al., 2016) | ||
| LacZ | Demyelination | Astrocytes, Oligodendrocytes, Oligodendrocyte progenitors | SD rats | Expressed in glial cells of spinal cord white matter, microglia and macrophages at injection site | (Zhao et al., 2003) | |
| LacZ | Neurons, Astrocytes, Microglia | SD rats | C3H mice | Different brain regions transduced by muLV, LCMV, Mokola virus, VSV-G enveloped, lack of transduction with Ebola virus enveloped | (Watson et al., 2002) | |
| LacZ | Neurons | Fisher rats | Neurons ~90% of transduced cells at the injection site | (Blömer et al., 1997) | ||
| miR124, shRNA GLAST, dsRednuc, mCherry | Astrocytes | BAC-GLT1-eGFP, BAC-GLAST-DsRed, B6 mice | Neuron-specific silencing, used other miRNAs; astrocyte-specific promoters and tetracycline-inducible systems for astrocyte-targeting and regulation | (Merienne et al., 2015) | ||
| miR124T, GLAST, LacZ | Neurons, Astrocytes | SD rats | B6 mice | miR124T blocked neuronal expression and reporter expressed in astrocytes | (Colin et al., 2009) | |
| miRNA chondroitin polymerizing factor or chondroitin synthase-1 | Astrocytes | Neu7 rats, chicken embryo dorsal root ganglia | Reduced chondroitin sulphate proteoglycans and neurite outgrowth inhibition | (Tuinstra et al., 2013) | ||
| Neurotro phin-3, BDNF | SCI | Astrocytes, Schwann cells | Long-Evans rats | Transduced multiple cell types, increased axonal growth and myelination | (Tuinstra et al., 2012) | |
| Small hairpin RNA (shRNA) GFAP, vimentin | SCI | Astrocytes | B6 mice neuroglia | Reduced glial scarring, improved neuronal survival; axonal growth increased or not affected by shGFAP | (Desclaux et al., 2009) |
Table 4.
Polymeric nanoparticles targeting different cell types in central nervous system
| Polymeric Nanoparticle | Gene | Disease/Mechanism | Transfected Cell Types | In Vitro Cell Source | In Vivo | Result | Reference |
|---|---|---|---|---|---|---|---|
| Arg-polyamidoamine (PAMAM) dendrimer | Green fluorescent protein (GFP), luciferase (Luc), small interfering RNA (siRNA) (High mobility group box 1 protein, HMGB1) | Neurons, Astrocytes, Oligodendrocytes, Microglia | Mice | Lower toxicity compared to polylactic acid or lipofectamine, transfected multiple cell types | (Kim et al., 2006) | ||
|
|
|||||||
| siRNA (HMGB1) | Stroke/ischemia | Neurons, Astrocytes | Mouse neuroglial cells | Sprague -Dawley (SD) rats | In vitro: siHMGB1 reduced H2O2-and N-methyl-D-aspartate-induced apoptosis; In vivo: decreased infarct volume | (Kim et al., 2010) | |
|
| |||||||
| Carbosilane dendrimers | siRNA (HIV-1 p24 and Nef) | HIV-associated neurocognitive disorders | Astrocytes | Humans, U87 glioma | Sustained siRNA release, transcytosis across blood brain barrier in vitro, lower viral replication | (Jiménez et al., 2010) | |
|
| |||||||
| DMAP-BLP:DSPC: cholesterol: PEG-DMG [3-(dimethylam ino)propyl (12Z,15Z)-3-[(9Z,12Z)-octadeca-9,12-dien-1-yl] henicosa-12,15-dienoate distearoylph osphatidylcholine: cholesterol: polyethylene glycol-(1,2-dimyristoyl-sn-glycerol)] | siRNA (glutamate receptor, ionotropic, N-Methyl D-aspartate 1, GRIN1; phosphatase and tensin homolog, PTEN) | Neurons | SD rats | SD rats | In vitro and in vivo testing, apolipoprotein E (ApoE) regulated NP uptake, knocked down both targets (GRIN1, PTEN) | (Rungta et al., 2013) | |
|
| |||||||
| Lipid nanoparticle-ss-pH-activated, lipid-like materials (LNPssPalm) vitamin A (A), vitamin E (E), myristic acid (M) | Luc, LacZ, mCherry | Astrocytes | KT-5 cells | ICR/HaJ Mice | In vitro: ApoE3-dependent uptake; In vivo: higher efficiency with LNPssPalmA, LNPssPalmE | (Akita et al., 2015) | |
|
| |||||||
| Lipofect-amine® | LacZ, HIV Nef | Astrocytes | Humans,Rats, U87 glioma | Lipofection effective than modified vaccinia Ankara-vector and calcium phosphate precipitation | (Ambrosini et al., 1999) | ||
|
|
|||||||
| Tyrosine hydroxylase | Parkinson’s disease (PD) | Astrocytes | 6-hydroxy dopamine (OHDA)-treated SD rats | Expression in astrocytes, behavioral recovery | (Segovia et al., 1998) | ||
|
| |||||||
| Organically-modified silica (ORMOSIL) | (CAG)20 or (CAG)127 fused to human influenza hemagglutinin-tag | Huntington’s disease (HD) | Neurons | R6 mice, Wistar rats | (CAG)127 delivery activated astrocytes in mice and rats | (Klejbor et al., 2007) | |
|
| |||||||
| PEG-polyethynein amine (PEI) | GFP, Luc | Neuro-degenerative disorders | N/S | Rats, Rabbits type N/S | Fischer rats | Higher efficiency and viability than PEI | (Mastorakos et al., 2015) |
|
|
|||||||
| Arg-PEI-PEG | Luc | Neurons | Rats type N/S | Nude mice | Low cytotoxicity and hemotoxicity compared to PEI. Crosses BBB | (Morris and Labhasetwar, 2015) | |
|
| |||||||
| PEG L-cysteinyl-poly-l-lysine (PLL) | Glial cell line-derived neurotrophic factor (GDNF) | Aging, PD | Astrocytes | SD rats | 6-OHDA treated SD rats | Higher expression in the denervated striatum, in older animals due to increased gliosis | (Yurek et al., 2015) |
|
| |||||||
| PEG-Liposomes | Luc, LacZ | Astrocytes | BALB/c mice | Astrocyte-specific expression with GFAP promoter | (Shi et al., 2001) | ||
|
|
|||||||
| PEI, Lipofectamine® | GFP, Luc | Astrocytes, Schwann cells | Lewis rats, Fisher rats | Comparable efficacy in vitro, decreased viability with PEI | (Rao et al., 2015) | ||
|
|
|||||||
| LacZ | Astrocytes | Rat glioma, Astrocytes, Hippocampal progenitors | Lipofectamine-higher number of cells transfected, PEI-more gene expression per cell | (Tinsley et al., 2004) | |||
|
| |||||||
| PEI-methacrylate knot polymer | GDNF | PD | Astrocytes | Rats type N/S | Lower cytotoxicity and higher expression than PEI; increased neurite outgrowth in neuronal co-cultures | (Newland et al., 2013) | |
|
| |||||||
| pFastBac1, (with PEI for in vitro) | Luc | Astrocytes | Wistar rats | Wistar rats | With modified promoter sequences: higher expression in vitro, improved astrocyte-specific expression in vivo | (Wang and Wang, 2006) | |
|
| |||||||
| PEI, poly-lactic-glycolic-acid (PLGA) | Interleukin (IL)-10 | Spinal cord injury (SCI) | Astrocytes | SD rats | Low intrathecal dose required compared to naked pDNA | (Milligan et al., 2006) | |
|
|
|||||||
| PEI, cholesterol-PLGA | Luc, vascular endothelial growth factor | SCI | Neurons, Astrocytes | Mouse neural stem cells | SD rats | Lower in vitro, in vivo cytotoxicity than PEI, higher efficiency in vivo with angiogenesis, motor function recovery | (Gwak et al., 2016) |
5.1 Viral vectors
Viral vectors are preferred for gene delivery to brain cells as well as other cell types including muscles (Wang et al., 2014), cardiac cells (Katz et al., 2013), and cancer cells (Cerullo et al., 2010). Viral vectors exploit the ability of a virus to infect mammalian cells and use of host machinery to produce viral proteins. Viral vector construction involves replacing immunogenic viral genome segments with the gene of interest. Structural viral proteins required for viral capsid and host genome integration are included as trans-acting factors. Literature on viral vectors derived from adenoviruses (AVs) (Table 1), AAVs (Table 2), and lentiviruses (LVs) (Table 3), major research breakthroughs and failures for each vector type are discussed here.
5.1.1 Adenovirus
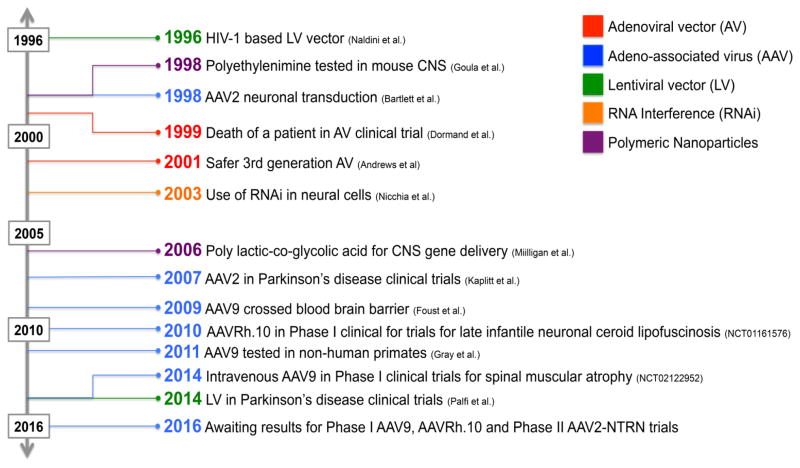
In 2012, About 23% of gene clinical trials utilize AVs (Ginn et al., 2013); despite a setback in 1999 due to the death of a patient in an AV clinical trial (Andrews et al., 2001). Since then, replication-restricted or deficient AVs have been developed by deleting non-essential viral DNA (Andrews et al., 2001; Do Thi et al., 2004; Dormond et al., 2009). Consistent AV-mediated brain gene expression has been found at three through thirty days, and was detectable at six months in Sprague-Dawley (SD) rats (Thomas et al., 2000). Adenoviruses do not integrate in the host genome and have been shown to preferentially transduce neurons in vivo (Lisovoski et al., 1997) and astrocytes in vitro (Kuhn et al., 2011). When AV-LacZ was injected into the dorsal side spinal cord of SD rats, predominantly astrocytes were transduced on the dorsal side while neurons were transduced on ventral side (Romero and Smith, 1998).
In ischemia therapy, AV-GDNF and AV-CNTF were delivered via intrastriatal injections a week prior to middle cerebral artery occlusion (MCAO)-induced ischemia in rats. These neurotrophins were primarily expressed in astrocytes and prevented neuronal damage compared to controls. At sites distant to the injection site, GDNF effects were stronger than CNTF (Hermann et al., 2001b). Apart from neurotrophins, an astrocyte-specific molecule, secretory leukocyte protease inhibitor, expression led to neuroprotection indicated by reduced ischemic lesion size and lowered neurological deficits (Wang et al., 2003b). Similarly, adrenomedullin expression reduced apoptosis and astrocyte migration to the ischemic core in vivo. Compromised BBB could enhance the vector penetration (Xia et al., 2004). AV-delivered heme oxygenase-1 expression increased astrocyte, but not neuronal survival, during hemin-induced apoptosis, post-hemorrhagic injury (Teng et al., 2004; Benvenisti-Zarom and Regan, 2007) indicating that cell-type-associated protective mechanisms must be studied while designing of cell-specific therapies.
Neurotrophin genes delivered via AV have been effective in certain NDD rodent models. Adenoviral vector-mediated GDNF gene delivery was tested in 6-hydoxydopamine- (6-OHDA) and cold-lesioned rats, modeling PD and brain trauma, respectively (Hermann et al., 2001a; Do Thi et al., 2004). Lesions and apoptotic cells reduced in the brain trauma model (Hermann et al., 2001a), and dopaminergic neuronal loss decreased and motor function improved in the PD study (Do Thi et al., 2004). When AV delivered BDNF to excitotoxic rats (Bemelmans et al., 1999), Quinolinic acid-induced lesion size was reduced and striatal neuron survival increased (Bemelmans et al., 1999). In transgenic HD mice (Arregui et al., 2011), AV-BDNF delivery improved behavior tests and motor phenotype appearance (Arregui et al., 2011). Thus, neurotrophin expression alleviated disease outcomes despite the differences in targeted cell-type, associated disease, and species models reaffirming their applicability as NDD therapeutics.
In addition to high transduction efficiency, first generation AVs elicited an inflammatory response and caused striatal shrinkage at injection sites (Liu et al., 1997; Bemelmans et al., 1999). Subsequent peripheral AV immunizations led to reduced CNS gene expression, increased macrophage and T cell infiltration into the brain, microglial activation, and demyelination (Byrnes et al., 1996; Hermens and Verhaagen, 1998; Thomas et al., 2000). Second and third generation AV with deleted early regions (E1, E3, and E4) depicted low immunogenicity (Do Thi et al., 2004). When Bellini and coworkers delivered IGF-1 to rat spinal cords, the resulting mild inflammatory response was attributed to IGF-1 reducing AV immunogenicity (Bellini et al., 2011). Taken together, certain segments of the AV genome, peripheral AV immunizations and gene of interest alter AV immunogenicity. Though AVs remain an efficient viral gene delivery system, they are not being tested in NDD clinical trials due to immunogenic potential and subsequent inflammatory outcomes.
5.1.2 Adeno-associated virus (AAV)
Low immunogenicity of AAV vectors and ability to transduce terminally differentiated neurons as well as dividing astrocytes, make them suitable for brain gene delivery (Peel and Klein, 2000). Larger size gene constructs could significantly reduce uptake and the kinetics of gene delivery (Dong et al., 1996). The smaller AAV genome compared to adenoviruses (4.7 kilobase (kb) versus 36 kb) facilitates gene uptake and delivery. CNS-targeted AAV vector studies can be divided into three main groups based on the serotype they investigate 1. AAV2 (Bartlett et al., 1998; Kügler et al., 2003), 2. AAV9 (Foust et al., 2009; Gray et al., 2011), and 3. Other AAVs, which include AAV4, 6, 8, rh10, rh39, rh43 (AAV43), etc. (Lawlor et al., 2009; Zhang et al., 2011). While AAV2 remains the prototype vector, some AAV serotypes are generated by pseudotyping, i.e. an AAV2 genome is packaged with a different capsid sequence such as AAV9. The majority of serotypes, other than AAV2, tested in the cited literature are designed this way. Contradicting results are documented on transduction efficiencies of self-complimentary AAVs (scAAV) and classical single-stranded AAVs (ssAAV). Smaller size scAAV (2.3 kb versus 4.7 kb) transduced effectively compared to ssAAV (Gray et al., 2011; Aschauer et al., 2013); yet, the inverse may also be true (Rahim et al., 2011).
5.1.2.1 AAV2
Early studies demonstrated an AAV2-mediated immediate neuronal transduction. However, astrocytes were not transduced and microglial transduction was short-lived (24 hours) (Bartlett et al., 1998). However, in a later study, cytomegalovirus (CMV) or human (h) synapsin (Syn) promoter-driven AAV2 specifically transduced astrocytes and neurons, respectively (Kügler et al., 2003). Higher AAV2 infectivity and binding was reported in human astrocytes in vitro and was attributed to stronger interactions between AAV2 and secondary protein receptors on astrocytes facilitating endocytosis (Koerber et al., 2009). When AAV2 and AAV5 were compared, AAV2 preferentially transduced neurons and AAV5 transduced astrocytes (Nomoto et al., 2003). While, kainate-induced limbic seizure activity reduced transduction efficiency, preferential cell type-specific expression persisted for both serotypes. Further, AAV5 transduced more efficiently than AAV2, potentially due to astrocyte activation, dying neurons or AAV2 neutralizing antibodies (NAbs) (Weinberg et al., 2011). One study also reported an exclusive neuronal transduction (Shevtsova et al., 2005). Recently preferential neuronal transduction by AAV2 and astrocyte activation was documented (An et al., 2016). When tested in oligodendrocytes, AAV2 encoding myelin basic protein (MBP) promoter led to exclusive oligodendrocyte transduction in vitro and in vivo with rat brain cells and mouse model, respectively, indicating therapeutic potential (Chen et al., 1998). Similarly for microglia, in vitro and in vivo targeting was achieved by including murine F4/80 promoter in AAV2 and AAV5 in a rat model (Cucchiarini et al., 2003).
Therapy-based enquiries for ischemia (Sun et al., 2003), PD (Kaplitt et al., 2007), and AD (Furman et al., 2012) have employed AAV2. In a cerebral focal ischemia rodent model, AAV2-mediated B-cell lymphoma-w (Bcl-w) expression in neurons, astrocytes and endothelial cells reduced infarct size, and improved neurological function (Sun et al., 2003). Literature indicates neurons are the preferred target of AAV2, but potential expression in other cell types and effects on the disease prognosis remain to be seen. Additionally, the presence of anti-AAV2 NAbs in a significant percentage (~72%) of the human population (Boutin et al., 2010) could become a key factor affecting therapeutic efficacy.
5.1.2.2 AAV9
Attention shifted from AAV2 to AAV9 for CNS gene delivery after its BBB crossing ability was reported (Foust et al., 2009). Faust and colleagues first documented AAV9 neuronal transduction in neonatal mice and astrocyte transduction in adult mice (Foust et al., 2009). Later reports found that AAV9 tropism shifted from neurons in fetal mice to astrocytes in neonatal mice (Rahim et al., 2011), from astrocytes in neonatal mice to neurons in young mice (Gholizadeh et al., 2013), and exhibited astrocyte tropism in NHPs of all ages (Bevan et al., 2011). Thus, literature presented conflicting data regarding AAV9-mediated preferential transduction of astrocytes or neurons. It can be discerned that, age of tested animals could be critical contributor of AAV9 cell-tropism (Foust et al., 2009; Rahim et al., 2011; Gholizadeh et al., 2013).
Similarly, variations in route of administration (Gray et al., 2011; Aschauer et al., 2013), and tested species (Bevan et al., 2011; Gray et al., 2011) have shown changes in AAV9 cell-tropism. In adult mice, intramuscular injection led to reporter expression in spinal cord neurons and astrocytes (Benkhelifa-Ziyyat et al., 2013), and intravenous administration showed higher neuronal transduction in brain (Gray et al., 2011). A study in NHPs showed brain parenchymal injection led to exclusive neuronal expression and intravenous injection resulted in preferential astrocyte transduction. Better access to astrocytes, potential AAV9-blood protein interactions, and presence of NAbs were attributed as contributing factors affecting gene expression post-intravenous administration (Gray et al., 2011). With ICV injection, AAV9 was effective for neurons (Aschauer et al., 2013). Thus, Gray and Aschauer study findings consistently showed AAV9-mediated neuronal expression when injected in the brain. In contrast, intracerebral scAAV9 injection showed a dose-dependent increase in neuron and astrocyte transduction efficiency without astrogliosis in mice (Donsante et al., 2016). Literature also showed the highest transduction efficiency and astrocyte tropism of AAV9. Despite widespread neuronal transduction, astrocytes were the predominantly transduced cell type (Zhang et al., 2011) and reporter gene expression remained for over 18 months (Miyake et al., 2011).
Therapeutically, AAV9 has been used to deliver human erythropoietin (hEPO) (Yang et al., 2013), EAAT2, glutamine synthase (GS), miRNA against adenosine kinase (Young et al., 2014), and β-galactoside (Weismann et al., 2015). In an experimental PD model, a single intrastriatal dose of AAV9-hEPO was preceded by an additional intrastriatal or intramuscular injection to evaluate effects on immunogenicity and transduction efficiency. Prior intramuscular injection reduced transduction, increased major histocompatibility complex (MHC) class I and II expression, CD4 and CD8 T cells infiltration in the brain, and circulating NAbs for both AAV9 and hEPO in the blood. Alternatively, prior intrastriatal injection did not cause severe inflammation or reduced transduction efficiency, but increased peripheral blood cells (Yang et al., 2013). Though intrastriatal or intramuscular might not be preferred routes of administration, these findings highlight the importance of assessing dosing route and frequency. AAV9-EAAT2 and -GS delivery to rat hippocampal astrocytes did not alter kainate-induced seizures, while AAV9-miRNA against adenosine kinase reduced seizure duration suggesting a possible therapeutic usage (Young et al., 2014). Intravascular injection of AAV9-β-galactoside led to CNS and peripheral organ transduction. It partially reduced GM1-gangliosidosis by improving reactive astrogliosis in a rodent disease model (Weismann et al., 2015).
Since high peripheral organ transduction has been reported for AAV9 when injected intravenously, consequent studies investigating AAV9 immunogenicity in the CNS may have used intrastriatal or intrathecal routes. Immunogenic potential of AAV9 has been analyzed in comparison with AAV2. Human aromatic L-amino acid decarboxylase (AADC) was injected intrastriatally using AAV2 and AAV9 vectors and their effects on immune response and antigen-presenting cell activation were tested. In addition to higher brain expression compared to AAV2, AAV9 led to immune activation over time. Glia transduced by AAV9 revealed increased MHC class II expression within few days of infusion, which was absent with exclusively neuron-transducing AAV2 (Ciesielska et al., 2013). A follow-up paper found AAV9 encoding GFP (foreign-protein) elicited a stronger immune response compared to AAV9 encoding aromatic L-AADC (self-protein) in NHPs suggesting need for syngeneic and non-functional reporter protein testing. Intriguingly, AAV2-GFP did not induce a significant immune response (Samaranch et al., 2014). When AAV9 was delivered via intrathecal and intravenous routes, there was no global immune response, but higher reporter-expressing cell nuclei lacked Nissl bodies, an indication of neurotoxicity (Schuster et al., 2015). It is possible to avoid off-target effects by including brain-cell specific promoters to restrict expression, which is discussed in detail in Section 6. Since ongoing AAV9-based Phase I clinical trials involve intravenous or intrathecal injections (Table 5), findings about AAV9 immunogenicity and peripheral expression are clinically relevant and must be taken into considerations for future trials.
Table 5.
Gene delivery- and NP-based clinical trials for neurological diseases and disorders (NDDs)
| Disease | Phase | Therapeutic Intervention | Institution/Company | ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| Alzheimer’s Disease | I | Autologous intracerebral transplantation of skin fibroblasts genetically modified to produce nerve growth factor (NGF) | The Shiley Family Trust, California, USA | NCT00017940 |
| I, II | Bilateral stereotactic injections CERE-110 (Adeno-associated virus (AAV) 2-β-NGF) to basal forebrain region containing the nucleus basalis of Meynert (NBM) | Ceregene, San Diego, CA, USA | NCT00087789, NCT00876863 | |
| Batten Disease | I, II | Intrathecal single dose self-complementary AAV9 encoding ceroid-lipofuscinosis neuronal (CLN) 6 gene (scAVV9.CB.CLN6) | Nationwide Children’s Hospital, Columbus, OH, USA | NCT02725580 |
| Giant Axonal Neuropathy | I | Intrathecal administration of self-complementary AAV9 encoding gene for gigaxonin (scAAV9/JeT-GAN) | National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, MD, USA | NCT02362438 |
| Leber Hereditary Optic Neuropathy | Not available | Single intravitreal injection of recombinant AAV2-nicotinamide adenine dinucleotide phosphate dehydrogenase, subunit 4 (complex I) (rAAV2-ND4) | Bin Li, Huazhong University of Science and Technology, Wuhan, Hubei, China | NCT01267422 |
| Late Infantile Neuronal Ceroid Lipofuscinosis | I | Brain delivery of an AAV2 encoding human CLN2 cDNA (AAV2CUhCLN2) | Weill Medical College of Cornell University, Ithaca, NY, USA |
NCT00151216 NCT01414985 NCT01161576 |
| I, II | Brain delivery of an AAV2 encoding human CLN2 cDNA (AAV2CUCLN2) | |||
| I | Brain delivery of an AAVrh.10 encoding human CLN2 cDNA (AAVRh.10CUhCLN2) in two separate doses | |||
| Metachromatic Leukodystrophy | I, II | Multiple intracerebral doses of AAVrh.10 encoding arylsulfatase A (ARSA) enzyme (AAVrh.10cuARSA) | Institut National de la Santé Et de la Recherche Médicale, Paris, France | NCT01801709 |
| Parkinson’s Disease | I | Convection enhanced delivery of AAV2-Glial Cell-Line Derived Neurotropic Factor (AAV2-GDNF) | NINDS Bethesda, MD, USA | NCT01621581 |
| I, II | Stereotactic intrastriatal injection of ProSavin delivering genes of three key enzymes involved in the synthesis of dopamine | Oxford BioMedica, Oxford, United Kingdom | NCT00627588 | |
| I | Continuously infused intracerebral recombinant-methionyl GDNF (r-metHuGDNF) | National Center for Research Resources (NCRR) Bethesda, MD, USA | NCT00115427, NCT00111982, NCT00006488, NCT00148369 | |
| I, II | Bilateral surgical infusion of AAV-mediated glutamic acid decarboxylase (AAV-GAD) gene transfer to subthalamic nuclei | Neurologix, Inc., Fort Lee, NJ, USA | NCT00643890, NCT00195143, NCT01301573 | |
| I, II | Intraputaminal/striatal infusion of AAV-human aromatic L-amino acid decarboxylase | Voyager Therapeutics & Genzyme, Cambridge, MA, USA; Jichi Medical University, Shimotsuke, Tochigi, Japan | NCT01973543, NCT00229736, NCT02418598 | |
| I, II | Intraputaminal and intranigral administration of CERE-120 (AAV2-Neurturin) | Ceregene, San Diego, CA, USA | NCT00985517, NCT00400634, NCT00252850 | |
| Progressive Supranuclear Palsy | I | Continuously infused intracerebral r-metHuGDNF | NINDS, Bethesda, MD, USA | NCT00005903 |
| Schizophrenia, Cognition | I, II | Curcumin nanoparticles to improve cognitive dysfunction | VA Greater Los Angeles Healthcare System, Los Angeles, CA, USA | NCT02104752 |
| Spinal Muscular Atrophy Type 1 | I | Intravenous delivery of AVXS-101 i.e. self-complementary AAV9 encoding the spinal motor neuron gene under the control of a hybrid CMV enhancer/chicken-β-actin promoter | AveXis, Inc, Bannockburn, IL, USA | NCT02122952 |
5.1.2.3 Other AAV
Intrastriatal AAV1 injections resulted in preferential and higher neuronal expression than AAV2 control (Wang et al., 2003a). When AAV1 delivered galactocerebrosidase (GALC) to a twitcher mouse model of globoid cell leukodystrophy, the GALC gene was predominantly expressed in neurons and some astrocyte processes. Though oligodendrocyte precursors were effectively transduced in vitro, this was not reproduced in vivo. Leukodystrophy symptoms reduced, while increasing myelination and lifespan on GALC expression (Rafi et al., 2005).
Astrocytes in the SVZ and rostral migratory stream were transduced when AAV4 was delivered directly to the SVZ (Liu et al., 2005). In another study, AAV4 showed a higher astrocyte binding but low transduction efficiency highlighting the importance of post-binding barriers that play a key role in effective transduction. The same study also generated AAV2 and AAV6 variants by engineered peptide loop replacement that presented increased specificity and transduction efficiency in astrocytes and Muller glial of the retina (Koerber et al., 2009). Neurons and astrocytes were successfully transduced by AAV5 encoding a reporter gene driven by hSyn or CMV promoter, respectively. However, in vitro results were reproduced only for neurons in vivo (Shevtsova et al., 2005).
Adeno-associated virus serotype 6 was shown to transduce only neurons in rats and also astrocytes in NHPs with higher MHC I and II expression (San Sebastian et al., 2013). Astrocyte restricted gene expression was tested using AAV8 and AAV43 containing GFAP promoters (Lawlor et al., 2009). After being injected in sciatic nerve, AAV8 was found to be Schwann cells-tropic in mice and AAV8-CNTF delivery led to increased levels of myelin protein P0 and PMP22 indicating its therapeutic potential (Homs et al., 2011). Recently, AAV8 encoding GFAP promoter-driven achaete-scute complex homolog-like 1 converted dorsal midbrain astrocytes into functional neurons in vitro and in vivo in mice (Liu et al., 2015). Neuron and oligodendrocyte transduction has been shown with AAVrh8 (Yang et al., 2014) and AAVrh10 (Petrosyan et al., 2014; Yang et al., 2014) with an efficiency comparable to AAV9 (Miyake et al., 2011; Zhang et al., 2011). Both AAVrh8 and AAVrh10 crossed the BBB when injected intravenously in mice with minimal peripheral tropism as compared to AAV9 (Yang et al., 2014). Currently, AAVrh10 is under investigation in a Phase I clinical trial (Table 5).
Few AAV serotypes have successfully transitioned into clinical trials for NDD therapy. While inclusion of cell-specific promoters restricted AAV gene expression, mechanisms responsible for intrinsic cell tropisms of AAV serotypes have not been investigated well. Some AAV receptors are known, for example, PDGF receptor has a role in AAV5 transduction (Aschauer et al., 2013). We infer that abundance of such AAV receptors on cell surface must have a key role in uptake, endocytosis and expression efficiency of AAV-mediated transduction. Yet, due to availability of alternatives and ease of manipulation, the overall focus has been on testing serotypes that present higher efficiency and intrinsic tropism for intended cellular target instead of elucidating AAV serotype transduction mechanism.
5.1.3 Lentivirus
Lentiviruses, from the retroviridae family, integrate in the host genome, unlike adenovirus and AAV. Therefore, LVs are capable of producing longer, more stable gene expression in diverse cell types (Blömer et al., 1996; Naldini et al., 1996). Lai and Brody depicted LV-mediated gene expression in neurons and astrocytes (Lai and Brady, 2002). When injected in rat dorsal funiculus, LVs transduced astrocytes, oligodendrocytes, and their progenitors in spinal cord white matter (Zhao et al., 2003). During SCI in rats, LV injected at a lesion site led to high peripheral expression in astrocytes with lower transduction of neurons, oligodendrocytes precursors, and microglia (Hendriks et al., 2007). When compared with AV and AAV, early investigations involving intrastriatal and intrahippocampal injections of LV showed comparable CNS transduction efficiency at two weeks and higher efficiency from six to twenty four weeks in mice. About 90% of the transduced cells were terminally differentiated neurons (Blömer et al., 1997) indicating intrinsic neuronal tropism. Two decades later, a conflicting report demonstrated preferential astrocyte tropism of LV compared to AAV in NHPs (An et al., 2016).
Most investigated LVs are HIV-1-derived and are pseudotyped, a process by which viral genome is packaged with glycoproteins derived from a nonpathogenic virus such as vesicular stomatitis virus-G (VSV-G). Pseudotyping is performed to increase transduction efficiency and specificity i.e. cell-specific tropism. Vectors packaged with envelope proteins of murine leukemia virus (muLV), lymphocytic choriomeningitis virus (LCMV), rabies-related Mokola virus, and VSV-G transduced striatum, thalamus, and white matter in mice brains. Additionally, both VSV-G- and Mokola-LV were effective in oligodendrocytes and their precursors in vitro. In mixed neuroglial cultures, VSV-G-LV-facilitated gene expression was higher in astrocytes (Watson et al., 2002). In contrast, VSV-G-LV has been shown to preferentially transduce neurons in adult rats and embryonic mice, while glycoprotein 64-enveloped-LV mediated expression was seen in astrocytes of adult rats (Rahim et al., 2009). Also, astrocyte preference of LCMV- and muLV-pseudotyped LV was depicted post- infusion into SN (Cannon et al., 2011). On the other hand, an earlier study demonstrated VSV-G-LV-mediated exclusive neuronal or astrocyte-transduction including cell-specific promoters depicting promoter dictated restriction and not pseudotyping (Jakobsson et al., 2003).
To deliver therapeutic genes via LV, neurons were targeted by LV-CNTF in embryonic neuroglial cultures and astrocytes became activated with altered membrane distribution of highly glycosylated forms of glutamate transporter (GLAST) and glutamate transporter-1. Improved astrocyte glutamate clearance ability in these studies suggested translation potential in excitotoxicity-associated disease (Escartin et al., 2006). Lentiviral vector encoding GDNF cDNA downstream of the GFAP or CMV promoters showed similar neuroprotection indicating the GFAP promoter, which produced only 1/10 the GDNF of the CMV promoter, was enough to impart neuroprotection (Sandhu et al., 2009).
For RNAi investigations, LV has been the vector of choice. Compared to AV and AAV, more studies combined LV and RNAi. Colin and colleagues adapted a unique gene silencing approach to target astrocytes. They designed a LV construct pseudotyped with Mokola-G-LV and encoding miR124T to exclusively silence the ‘off-target’ neuronal gene expression. GLAST gene or miRNA regulating GLAST encoded downstream of miR124T in the above construct was delivered exclusively to astrocytes in vitro. Specificity of this system was shown in vivo using a LacZ reporter (Colin et al., 2009). In a follow-up paper, the LV construct described above was modified further to include an astrocyte-specific GS1 promoter, an additional sequence of miR124T and a regulatory tetracycline response element to increase specificity and add a regulatory component (Merienne et al., 2015). High complexity of this construct could limit its therapeutic applications, although certain vector modification approaches tested in this study can be utilized to improve targeted gene delivery.
Other RNAi-associated studies employed LVs to silence astrocyte marker molecules GFAP and vimentin (Desclaux et al., 2009), chondroitin polymerizing factor (CPF) and chondroitin synthase-1 (CS-1) (Tuinstra et al., 2013), as well as miR145 (Wang et al., 2015). Astrocyte intermediate filaments, GFAP and vimentin, are upregulated during inflammation adversely affecting axonal and neurite regeneration post-injury. Thus, LV-shGFAP and shVimentin delivery decreased astrogliosis, astrocyte migration in scratch assay and improved neuronal survival in co-cultures (Desclaux et al., 2009). Astrocyte-secreted chondroitin sulfate proteoglycans were activated by LV-miCPF and -miCS-1 delivery during CNS injury, which increased the neurite outgrowth in neuroglial cultures (Tuinstra et al., 2013). A negative regulator of reactive astrogliosis, miR145, delivered with LV reduced astrocyte activation, proliferation, and migration in an in vitro SCI model (Wang et al., 2015).
In contrast to adenoviruses, LV-administration did not elicit significant immune response (Blömer et al., 1996; Naldini et al., 1996). Microglial and macrophage accumulation at the injection site was attributed to injection toxicity and not LV (Zhao et al., 2003). Research later reported that prior peripheral LV immunization elicited an immune response to a subsequent CNS-injected LV only when both LVs encoded the same transgene (Abordo-Adesida et al., 2005). Since LVs are integration proficient, there’s a risk of insertional mutagenesis, and therefore integration-deficient vectors have been used as well (Lu-Nguyen et al., 2014). Recently, a first-ever LV-based Phase I PD clinical trial was completed (Table 5). The trial results are not only encouraging for its transition into Phase II and III clinical trials, but also for the promise of increased LV therapies in the future (discussed in Section 7).
5.2 Polymeric nanoparticles
Research and clinical applications of nanotechnology have increased in recent years. Formulations <100 nm in at least one dimension, which may enable crossing BBB, qualify as NPs. Effects of metallic NPs containing iron (Geppert et al., 2011; Pilakka-Kanthikeel et al., 2013), silver (Luther et al., 2011) or silica (Klejbor et al., 2007) have been evaluated in brain cells, but non-metallic polymeric NPs have been predominantly studied for gene delivery (Wong et al., 2012). Types of NPs including polymeric, solid-lipid, nanoemulsion, and liposomes have been well documented in the literature along with strategies to optimize delivery and expression efficiency (Wong et al., 2012; Shah et al., 2013; Tosi et al., 2013; Kreuter, 2014). Investigations associated with CNS gene delivery have used neuronal or neural stem cell lines such as PC12 (Park et al., 2007), C17.2 (Liu et al., 2013), and Neuro2a (Malhotra et al., 2013). There are fewer studies, which utilize primary brain cells or animal models. Also, microglia and oligodendrocytes were less frequently targeted with NP-mediated gene delivery compared to neurons and astrocytes.
Cationic polyethylenimine (PEI)-DNA ‘polyplexes’ are avidly used for gene delivery (Goula et al., 1998; Rao et al., 2015). Polyamidoamines (PAMAM) and poly(lactic-co-glycolic) acid (PLGA) have also been frequently examined. The positive charge on NPs allows electrostatic interaction with a negatively charged cell membrane and exogenous DNA to improve delivery and prevent systemic degradation of plasmid DNA (pDNA) (Midoux et al., 2008). After cellular uptake, titratable amine groups on PEI and PAMAM increase chloride concentration leading to endosomal swelling and higher endosomal escape. Therefore, PEI and PAMAM have better transfection efficiencies compared to other positively charged polymers (Sonawane et al., 2003).
Gene delivery inquiries involving NPs revolve around increasing transfection efficiency and reducing NP toxicity. Complexing NP with polyethylene glycol (PEG), or PEGylation, increased transfection efficiencies, reduced toxicity, and imparted “stealth” properties (Xin et al., 2012; Rungta et al., 2013; Morris and Labhasetwar, 2015; Yurek et al., 2015). Convection-enhanced delivery also increased efficiency of NP administration (Mastorakos et al., 2015). Arginine addition enhanced the cellular uptake and delivery across the BBB (Kim et al., 2006; Morris and Labhasetwar, 2015), and tagging liposome NPs with transferrin receptor ligand facilitated transcytosis across the BBB and endocytosis into CNS cells (Shi et al., 2001).
Lipid containing substances are frequently used for CNS delivery (Rao et al., 2015). Early investigations showed liposome-mediated gene delivery, i.e. lipofection, was more efficient than calcium phosphate transfection in fetal human and rat astrocytes, and was comparable to that of modified vaccinia Ankara viral transduction in fetal spinal cord astrocytes (Ambrosini et al., 1999), and PEI in primary rat astrocytes (Rao et al., 2015). Neutrally charged, 120–150 nm liposomal NPs containing palmitic acid, vitamin A, or vitamin E preferentially transfected astrocytes close to the injection site after ICV delivery. In vitro, apolipoprotein E (ApoE) co-treatment increased uptake and transfection efficiency lipid NP derived from vitamin A and E in astrocyte-derived cells (Akita et al., 2015).
For mammalian cell transfections, PEI is considered the gold standard. But it has exhibited some toxicity (Lu et al., 2015). Therefore, polymers with lower toxicity and comparable efficiency including C2-(dimethylamino)ethyl methacrylate-based cyclized knot polymer (Newland et al., 2013), arginine-modified PEI derivative (Lu et al., 2015; Morris and Labhasetwar, 2015), heavily PEGylated PEI (Mastorakos et al., 2015), arginine-modified PAMAM (Kim et al., 2010), and PLGA (Gwak et al., 2016) are preferred. In our collaborative work, arginine-PEI-PEG polyplexes formulated with luciferase-expressing plasmid DNA transfected rat primary astrocytes and neurons in vitro and in vivo following intravenous injection (Morris and Labhasetwar, 2015).
As a PD therapy, 8–11 nm diameter PEGylated poly-l-lysine nanorods delivered the GDNF gene to rats via intracerebral injections. Neurons and glia were transfected and sustained expression for over 11 weeks (Yurek et al., 2009). Transfection efficiency was higher in denervated striatum of 6-OHDA-induced mice and old mice, highlighting their disease sensitivity and age as a factor in gene delivery (Yurek et al., 2015). A cyclized-knot polymer delivered GDNF to primary rat astrocytes and the astrocyte cell line Neu7 that led to functional effects in co-cultured dorsal root ganglion cells (Newland et al., 2013). As a PD therapy option, GFAP-promoter driven-tyrosine hydroxylase (TH) gene-loaded liposomes were delivered intrastriatally (Segovia et al., 1998) or intravenously (Zhang et al., 2004) to a 6-OHDA-induced rat model. Rotational behavior was improved in both studies, but TH expression was restricted to either astrocytes (Segovia et al., 1998) or nigrastriatal neurons (Zhang et al., 2004).
For SCI, IL-10 (Milligan et al., 2006) and vascular endothelial growth factor (VEGF) (Gwak et al., 2016) gene therapies were evaluated using PLGA NPs. Anti-inflammatory cytokine IL-10 could reduce gliosis during neuroinflammation due to astrocyte-specific receptor expression. Dose of IL-10 pDNA reduced significantly when delivered via NPs compared to naked pDNA to achieve similar expression (Milligan et al., 2006). Cholesterol-modified PLGA NPs efficiently transfected the VEGF gene in both neurons and astrocytes post spinal cord injection (Gwak et al., 2016).
Gene silencing strategies have been reported using polymeric NPs (Jiménez et al., 2010; Kim et al., 2010; Rungta et al., 2013). Carbosilane dendrimers successfully delivered siRNA specific to HIV-1 negative regulatory factor (Nef) to primary astrocytes and related cell lines (Jiménez et al., 2010; Serramía et al., 2015). An arginine-modified PAMAM derivative delivered high mobility group protein 1 siRNA to cultured primary mouse cortical cells and led to a reduction in infarct volume in rat brains post-ischemia (Kim et al., 2006). Rungta and coworkers synthesized distearoylphosphatidylcholine/cholesterol/PEG-DMG containing lipid NPs to deliver siRNA targeting the neuronal GluN1 subunit of the N-methyl-D-aspartate (NMDA) receptor, reducing NMDA-based currents, but not α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-induced currents (Rungta et al., 2013). These NPs could be used to treat neurons, while co-delivering an astrocyte-specific gene.
Polymeric NPs are non-metallic and non-pathogenic. Their synthesis process can be better controlled than producing viral vectors in bulk. They can be optimized to increase transfection efficiency and BBB penetrability. Currently, PLGA is approved by the food and drug administration for therapeutic use indicating polymeric NPs are better accepted as delivery vehicles than viral vectors.
6. CNS-targeted gene delivery using cell-specific promoters
Low BBB permeability and brain structure complexity have already been discussed as major challenges in designing a delivery system for any neural cell type. In theory, cell-specific promoters could be used to target gene delivery to particular cells. Well-known cell-specific markers are neuron-specific enolase (NSE) for neurons, GFAP for astrocytes, F4/80 for microglia, and MBP for oligodendrocytes; which in turn represent the widely used methodology of restricting exogenous gene expression to specific cell types.
As discussed earlier in the review, neurons and astrocytes are targeted more frequently than microglia and oligodendrocytes. Targeting a precise neuronal subtype is essential during NDD treatment since that subtype often expresses genes linked to a disease; for instance, dopaminergic neurons express TH gene in PD. Yet few neuron-focused papers discuss gene vector design to improve expression specificity. Our observations suggest that neuronal targeting has been a tacit approach for numerous CNS gene delivery studies and implications of off-target gene expression in astrocytes or oligodendrocytes were not discussed. Nevertheless, NSE, hSyn, and PDGF are the frequently reported neuron-specific promoters.
When NSE and PDGF promoter-driven GFP encoding AAV2 were injected into the mid-cervical region of rat spinal cords, exclusive neuronal expression was obtained with at 15 and 45 weeks post-injection (Peel et al., 1997). Later studies reported that inclusion of NSE promoter achieved relatively precise but not exclusive neuronal expression (Navarro et al., 1999; Kügler et al., 2003). Among uncommon promoters, LV encoding a 1.6 kb segment of ‘homeobox 9’ promoter was used for motor neuron targeting in mice spinal cord (Peviani et al., 2012). Also, Thy1.2 and α-CAMKII promoter were shown to be specific for pyramidal neurons (Dittgen et al., 2004). An AV, encoding multiple copies of a cis-regulatory element of human dopaminergic β-hydroxylase promoter, selectively transduced noradrenergic neurons (Hwang et al., 2001).
Macrophage-specific promoter sequences, such as human CD11b, CD68, and murine F4/80, were evaluated for in vitro and in vivo microglial targeting employing AAV constructs. Murine F4/80 offered the highest expression efficiency and restriction in SD rats (Cucchiarini et al., 2003). Oligodendrocytes and Schwann cells in the CNS abundantly express MBP. An AAV2 vector encoding a MBP promoter precisely targeted oligodendrocytes in vitro and in vivo, in rat cells and mice, respectively (Chen et al., 1998). Inclusion of the MBP promoter in LV-GFP also led to exclusive oligodendrocytes expression (McIver et al., 2005).
Contrary to neurons, astrocyte-targeted studies have focused on one promoter, i.e. GFAP, and modified it to reduce size and increase efficiency (Brenner et al., 1994; Lee et al., 2008; Meng et al., 2015). Aldehyde dehydrogenase 1 L1 and GLAST/EAAT1 are other astrocyte-specific markers, but the GFAP promoter remains the ‘promoter of choice’ in gene delivery approaches (Regan et al., 2007; Lee et al., 2008; Adam et al., 2012). Brenner and colleagues identified an essential 2.1 kb promoter segment (gfa2), in the 5′ region of GFAP promoter capable of driving gene expression (Brenner et al., 1994). It has been utilized to restrict gene expression (Shi et al., 2001; Furman et al., 2012), in transgenic models (Kim et al., 2003), and to study astrocyte function (Nolte et al., 2001; Kim et al., 2003; Giralt et al., 2010). This promoter has also been used with viral vectors (von Jonquieres et al., 2013) and NPs (Shi et al., 2001). In spite of the widespread acceptance for gfa2, some reports have debated over the promoter specificity. An early report documented that using only 5′ flanking promoter sequence produced tissue restricted expression while both 5′ and 3′ flanking sequences were essential for cell-specific expression (Galou et al., 1994). In agreement with the findings, GFAP-driven TH was expressed exclusively in nigrastriatal neurons and not in astrocytes (Zhang et al., 2004).
Immunogenicity is a major problem with viral vectors, especially AVs. Gerdes and colleagues showed that using a strong promoter like major immediate early murine CMV reduced vector dose and immunogenicity (Gerdes et al., 2000). However, this approach is not suitable for cell-specific targeting. On the other hand, gfa2 promoter has been considered a weak promoter that resulted in lower expression (Benvenisti-Zarom and Regan, 2007) and its efficiency changed with age in vivo (von Jonquieres et al., 2013). Thus, gfa2 has been modified over a decade to improve transgene expression. Recently, a shorter, 1740 kb gfa2 promoter driven AAV-GFP was delivered to cortical astrocytes (Meng et al., 2015). Stronger promoter sequences, such as CMV and/or inverted terminal repeat sequences were appended upstream of the gfa2 promoter to increase expression. These modifications led to higher expression as compared to CMV promoter in vitro and in vivo, when injected intrastriatally (Wang and Wang, 2006). Brenner laboratory followed up their original work on gfa2 promoter by concluding that a 681 kb segment of the GFAP promoter, gfaBC1D, is optimal to obtain promoter-driven gene expression. They suggested that gfaBC1D enhanced the expression efficiency due to its smaller size (Lee et al., 2008). Since then, GfaBC1D was used to drive expression of the pleckstrin homology domain of phospholipase C-like protein p130 using AAV2/5 (Xie et al., 2010). Other astrocyte-specific promoters; aldehyde dehydrogenase 1L1, which is active in all mature astrocytes with broader expression pattern and GLAST/EAAT1; have been utilized to a lesser extent (Regan et al., 2007; Barres, 2008)
In addition to promoters, certain cellular receptors could be used for targeting. For instance, the rabies virus enters neurons via nicotinic acetylcholine receptor and a bacterial protein, namely tetanus toxin, binds with neurons through the triasialoganglioside receptor, GT1b (Kwon et al., 2010). Hence rabies virus glycoprotein or tetanus toxin could thus be tagged to exogenous DNA-vector complex to improve endocytosis. Additionally, a 12 amino acid peptide, Tet-1, has been identified and has been successfully used to target neurons (Kwon et al., 2010). Similarly, DNA-vehicle complex was tagged with neurotensin and NGF fragments for neuron-specific delivery (Rogers and Rush, 2012).
Another way to increase cell-targeting specificity is by including elements that respond to a biomarker characteristic of the neuropathology. This concept was utilized by flanking hypoxia response element with the VEGF gene sequence as a therapy for focal ischemia. Since hypoxia-inducible factor-1 binds to hypoxia response element, VEGF expression was enhanced during stroke (Shen et al., 2008). Uptake and transfection efficiency of lipid NP uptake and transfection efficiency increased in the presence of ApoE suggesting that it could be utilized for astrocyte targeting (Akita et al., 2015).
The perpetual process of designing and optimizing CNS-directed gene delivery system has remained a translational research priority. Though literature agrees on inclusion of a cell-specific promoter, standard constructs for each cell type have not been established. Going forward, comprehensive efforts must be directed toward delivery system design to enable BBB permeability, cell-specific targeting, and increasing expression of weak promoters.
7. Bench to bedside translation
One of the major highlights in gene delivery research has been its transition into clinical trials. Several viral vectors are currently being investigated in Phase I/II clinical trials (Table 5) for treatment of NDDs including AD, PD, and lysosomal storage disorders. Phase I clinical trial for AD treatment involved stereotactic injections of AAV2-NGF to the basal forebrain region of containing the nucleus basalis of Meynert. The study was conducted in ten patients with three different doses of AAV2-NGF to determine the highest safe dose for a Phase II study. Patients were monitored for over two years to ascertain treatment safety. All patients survived the procedure with few adverse events reported. AAV2-NGF therapy was evaluated to be safe and well tolerated (Rafii et al., 2014). Currently, a multicenter Phase II study evaluating the treatment efficacy is underway.
Several clinical trials have been completed or being carried out for PD treatment administering genes associated with either enzymes involved in dopamine synthesis, such as AADC, or neurotrophic factors, such as neurturin (NTRN) and GDNF. Most of these studies have successfully completed Phase I trials and have proven safety (Kaplitt et al., 2007; Bartus et al., 2014). Phase II clinical trials evaluating efficacy are currently ongoing and have shown mixed results thus far. AAV-glutamate decarboxylase trial has been terminated due to financial constraints despite improvements observed during Phase II (LeWitt et al., 2011). On the other hand, AAV-NTRN Phase II study results promised long-term safety but conclusive results on efficacy were not obtained (Kordover and Bjorklund, 2013). Additionally, results from first-ever clinical trial employing a LV have been reported. It involved stereotactic injections of a tri-cistronic vector expressing genes for TH, AADC, and cyclohydrolase-1 with the intention of stimulating non-dopaminergic, striatal neurons to secrete dopamine, replenishing levels. Motor improvements and long-term tolerability four years post-injection was established (Palfi et al., 2014). Currently, methods to increase effectiveness of this therapy are being investigated.
In vitro and preclinical investigations conducted so far have reported that presence of preexisting NAbs could be responsible for varied gene delivery efficiencies and immune responses between rodent models, NHPs, and humans. A study conducted with over 200 human serum samples indicated presence of AAV-NAbs in 40% to 70% samples for various AAV subtypes. In a preclinical study, presence of pre-existing NAbs reduced AAV9 transduction efficiency in NHPs (Gray et al., 2011). Consistent with these findings, completed clinical trials discussed above have also reported presence of AAV antibodies or VSV-G antibodies for AAV- and LV- associated trials, respectively. While none of the published data report adverse events or immune responses; it must be noted that the primary endpoint of Phase I trials is safety and are conducted with small (<100) patient cohorts. It will be important to evaluate if presence of NAbs will reduce efficacy in larger patient cohorts. According to Palfi et al. the presence of NAbs should not interfere with LV transduction efficacy since NAbs were detected in some patients three months post-gene delivery; and thus, LVs would be integrated by the time NAbs appear (Palfi et al., 2014).
An ongoing clinical trial involves intravenous injections of an AAV9 encoding survival motor neuron gene to six to nine month old infants diagnosed with spinal muscular atrophy. The study involves monthly monitoring until death or continuous respiratory support for over 16 hours. The final results of the study are not available; however, preliminary data presented at scientific conferences and provided on the trial sponsor’s website indicates safety and some motor function improvements (AveXis, 2016).
NPs are another major system being evaluated as gene delivery vehicles. Special characteristics of NPs must be considered before transitioning to preclinical or clinical trials. NPs have higher surface area resulting in higher toxicity in vitro compared to in vivo studies, leading to inconsistent results, a concept known as ‘nanomaterial paradox’ (Joris et al., 2013). NPs that are toxic at low doses in vitro could be delivered at relatively higher dose in vivo without toxicity. Therefore, it is essential to perform in vivo experiments with NPs, even if in vitro findings indicate toxicity. Currently, no ongoing or completed clinical trials have used polymeric NPs for NDDs. It must be noted that polymeric NPs are being used in cancer drug delivery studies and can soon be expected to transition into clinical trials for gene delivery based on the translational data available in literature (Jensen et al., 2013; Guerrero-Cázares et al., 2014; Mangraviti et al., 2015). All gene delivery systems require careful and comprehensive analyses for route, dose, and frequency of administration, in addition to immunological profiling of the animal models to improve the transition from in vitro to in vivo and further into clinical trials.
8. Conclusions and future prospects
In this review, we appraised key elements of the ever-growing field of CNS-targeted gene delivery focusing on the past two decades. Challenges encountered in therapeutic strategies targeting the brain, such as low BBB permeability and brain structure complexity were carefully analyzed, specifically in the context of gene delivery. Furthermore, we critically evaluated functional aspects of principal CNS cell types implicated in NDD pathogenesis. Taken together, we infer that targeting neurons and astrocytes will have significantly broader implications in the context of CNS gene delivery, than microglia and oligodendrocytes. Ultimately, astrocytes will emerge as the most appealing therapeutic targets as compared to terminally differentiated neurons, with their complex structure and fastidious synaptic transmission. We thus anticipate robust development of astrocyte-focused delivery systems in the near future.
Of all the delivery systems investigated thus far, there are pros and cons to each, and clearly, the quest for an ideal gene delivery system continues. Among the viral gene delivery approaches, higher immunogenicity limits the application of AVs despite their better transduction efficiency compared to other viral vectors; AAVs have an edge over other approaches with their successful progression into clinical trials and consistent results over the years; whereas LVs require further comprehensive investigations for clinical translation. A parallel line of investigation comprises of concurrent development of polymeric NPs to successfully tackle the BBB and target specific CNS cell types. The non-viral nature of NPs renders these formulations far more amenable for acceptance by the general population. All together, the sheer diversity of viral vectors and NPs ensures that suitable vehicles for CNS cellular targets will soon be available despite issues such as varying gene expression efficiency, distinct cell tropisms and immunogenicity. Considering these significant challenges and caveats encountered, NDD gene therapy has made impressive strides. Fig. 2 summarizes the milestones of this exciting journey over the past two decades.
Fig. 2. Milestones and breakthroughs in gene delivery over two decades.
Significant progress was made in the last twenty years of gene therapy research. Considering the time taken by any therapy to transition from bench to bedside, we can expect additional clinical gene therapy applications in the coming years with the help of cutting-edge technology.
The litmus test for any therapeutic strategy is its success in the bench to bedside translation. In this regard, we have included viral vector- and NP-associated clinical trials for NDDs in our review (Table 5) (Health, 2016). However, the enigma of the brain as the ultimate challenge is reflected in the clinical trial numbers as well. In 2012, only 2% of the clinical trials were associated with NDDs as compared to 64.4% for cancer, 10% for monogenic disease and 7.5% for cardiovascular diseases (Ginn et al., 2013). Without a doubt, safety of gene therapy for NDDs is well established given the results of multiple successful Phase I clinical trials. However, based on the literature available thus far, no studies have provided conclusive data substantiating improved efficacy of gene therapy over current treatments. Thus, although gene therapy has shown long-term safety, transitions into Phase II to evaluate efficacy and fine-tuning the route, dose and frequency of administration will be critical.
The majority of the gene therapy investigations thus far have focused on validating the concept of gene delivery in attempts to overcome diverse multifactorial challenges. Consistent with this notion, most clinical trials that spearheaded these investigations employed classically known disease biomarker genes for first line investigations. One notable example would be clinical trial testing of L-AADC, which converts L-DOPA to dopamine, as a therapy for PD (Eberling et al., 2008). Going forward, emerging trends both in discovery of novel biomarkers and early diagnostic techniques, will pave the way for the next generation gene therapy approaches.
While significant research and resources have been invested in the last few decades establishing the basics of gene augmentation and silencing, these specific modalities may soon become obsolete with development of genome editing technology. Regardless, key challenges facing successful delivery of these technologies to the brain will remain the same, and past lessons learnt will thus help guide strategies for successful translation of novel gene editing techniques, such as CRISPR-Cas9, to the clinic.
The overall progress in gene delivery approaches promotes a brighter future for NDD management and a strong potential for the development of effective, personalized gene therapy. In conclusion, gene therapy has come of age with bits of both successes and failures thus far, yet, the lessons from past and current research promise that cell- and disease-specific, safe and effective CNS gene delivery will be a reality in the not too distant future.
Acknowledgments
The authors would like to acknowledge Ms. Kathleen Borgmann for her help with critical reading and scientific writing of the manuscript, tables, and figures. Ms. Lenore Price and Mr. Timothy Van Treuren proofread the manuscript. Dr. Irma Cisneros and Dr. Richa Pandey helped in making figures. The authors also thank all other members of Ghorpade laboratory at UNT Health Science Center and Labhasetwar laboratory at Cleveland Clinic for their help during the writing of the manuscript. National Institute of Neurological Disorders and Stroke (NINDS) awards R01 NS048837 to AG and R01 NS070896 to VL supported the presented work.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- Aβ
Amyloid-β
- AADC
Amino acid decarboxylase
- AAV
Adeno-associated virus
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- ApoE
Apolipoprotein E
- AQP
Aquaporin
- AV
Adenoviral vector
- BBB
Blood-brain barrier
- Bcl-w
B-cell lymphoma-w
- BDNF
Brain-derived neurotrophic factor
- BMVEC
Brain microvascular endothelial cell
- Cas9
CRISPR-associated system 9
- CMV
Cytomegalovirus
- CNS
Central nervous system
- CNTF
Ciliary neurotrophic factor
- CPF
Chondroitin polymerizing factor
- CRISPR
Clustered regulatory interspaced short palindromic repeats
- CS-1
Chondroitin synthase-1
- EAAT
Excitatory amino acid transporter
- EPO
Erythropoietin
- GALC
Galactocerebrosidase
- GDNF
Glial cell-derived neurotrophic factor
- GFAP
Glial fibrillary acidic protein
- gfa2
GFAP essential promoter segment
- GFP
Green fluorescent protein
- GLAST
Glutamate transporter
- GS
Glutamine synthase
- h
Human
- HAND
HIV-associated neurocognitive disorders
- HD
Huntington’s disease
- HIV
Human immunodeficiency virus
- htt
Huntingtin
- ICV
Intracerebroventricular
- IGF
Insulin-like growth factor
- IL
Interleukin
- kb
Kilobase
- LCMV
Lymphocytic choriomeningitis virus
- LV
Lentiviral vector
- MBP
Myelin basic protein
- MS
Multiple sclerosis
- MCAO
Middle cerebral artery occlusion
- MHC
Major histocompatibility complex
- miRNA/miR/mi
microRNA
- muLV
Murine leukemia virus
- NAb
Neutralizing antibody
- NDDs
Neurological disease and disorders
- Nef
Negative regulatory factor
- NGF
Nerve growth factor
- NHP
Non-human primate
- NMDA
N-methyl-D-aspartate
- NP
Nanoparticle
- NSE
Neuron-specific enolase
- NTRN
neurturin
- PAMAM
Polyamidoamine
- PD
Parkinson’s disease
- pDNA
Plasmid DNA
- PDGF
Platelet-derived growth factor
- PEG
Polyethylene glycol
- PEI
Polyethylenimine
- PLGA
Poly(lactic-co-glycolic) acid
- RNAi
RNA interference
- scAAV
Self-complementary AAV
- ssAAV
Single-stranded AAV
- SCI
Spinal cord injury
- SD
Sprague-Dawley
- shRNA
Short hairpin RNA
- siRNA
Small interfering RNA
- SN
Substantia nigra
- SVZ
Subventricular zone
- Syn
Synapsin
- TH
Tyrosine hydroxylase
- TNF
Tumor necrosis factor
- VEGF
Vascular endothelial growth factor
- VSV-G
Vesicular stomatitis virus-G
Footnotes
Conflicts of Interest
The authors declare no conflict of interest associated to the presented work.
Bibliography
- Aartsen WM, Van Cleef KW, Pellissier LP, Hoek RM, Vos RM, Blits B, Ehlert EM, Balaggan KS, Ali RR, Verhaagen J. GFAP-driven GFP expression in activated mouse Müller glial cells aligning retinal blood vessels following intravitreal injection of AAV2/6 vectors. PLoS One. 2010;5:e12387. doi: 10.1371/journal.pone.0012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte endothelial interactions at the blood brain barrier. Nature Reviews Neuroscience. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abordo-Adesida E, Follenzi A, Barcia C, Sciascia S, Castro MG, Naldini L, Lowenstein PR. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum Gene Ther. 2005;16:741–751. doi: 10.1089/hum.2005.16.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam SA, Schnell O, Pöschl J, Eigenbrod S, Kretzschmar HA, Tonn JC, Schüller U. ALDH1A1 is a marker of astrocytic differentiation during brain development and correlates with better survival in glioblastoma patients. Brain Pathol. 2012;22:788–797. doi: 10.1111/j.1750-3639.2012.00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita H, Nakatani T, Kuroki K, Maenaka K, Tange K, Nakai Y, Harashima H. Effect of hydrophobic scaffold on the cellular uptake and gene transfection activities of DNA-encapsulating liposomal nanoparticles via intracerebroventricular administration. International journal of pharmaceutics. 2015;490:142–145. doi: 10.1016/j.ijpharm.2015.05.043. [DOI] [PubMed] [Google Scholar]
- Alberdi E, Wyssenbach A, Alberdi M, Sanchez-Gomez MV, Cavaliere F, Rodriguez JJ, Verkhratsky A, Matute C. Ca(2+) -dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid beta-treated astrocytes and in a model of Alzheimer’s disease. Aging Cell. 2013;12:292–302. doi: 10.1111/acel.12054. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Barres BA. Neuroscience: glia more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacology & therapeutics. 2013;138:155–175. doi: 10.1016/j.pharmthera.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Ambrosi G, Cerri S, Blandini F. A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. Journal of Neural Transmission. 2014;121:849–859. doi: 10.1007/s00702-013-1149-z. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Ceccherini-Silberstein F, Erfle V, Aloisi F, Levi G. Gene transfer in astrocytes: comparison between different delivering methods and expression of the HIV-1 protein Nef. Journal of neuroscience research. 1999;55:569–577. doi: 10.1002/(SICI)1097-4547(19990301)55:5<569::AID-JNR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- An H, Cho D-W, Lee SE, Yang Y-S, Han S-C, Lee CJ. Differential Cellular Tropism of Lentivirus and Adeno-Associated Virus in the Brain of Cynomolgus Monkey. Experimental neurobiology. 2016;25:48–54. doi: 10.5607/en.2016.25.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E, da Silva CG, Guimarães DM, Szczupak D, Parente-Bruno DR, Carvalho LR, Polichiso L, Gomes BV. Cell number changes in Alzheimer’s disease relate to dementia, not to plaques and tangles. Brain. 2013;136:3738–3752. doi: 10.1093/brain/awt273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JL, Kadan MJ, Gorziglia MI, Kaleko M, Connelly S. Generation and characterization of E1/E2a/E3/E4-deficient adenoviral vectors encoding human factor VIII. Molecular Therapy. 2001;3:329. doi: 10.1006/mthe.2001.0264. [DOI] [PubMed] [Google Scholar]
- Anglade P, Vyas S, Javoy-Agid F, Herrero M, Michel P, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch E, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histology and histopathology. 1997;12:25–32. [PubMed] [Google Scholar]
- Aronin N, DiFiglia M. Huntingtin-lowering strategies in Huntington’s disease: Antisense oligonucleotides, small RNAs, and gene editing. Movement Disorders. 2014;29:1455–1461. doi: 10.1002/mds.26020. [DOI] [PubMed] [Google Scholar]
- Arregui L, Benítez JA, Razgado LF, Vergara P, Segovia J. Adenoviral astrocyte-specific expression of BDNF in the striata of mice transgenic for Huntington’s disease delays the onset of the motor phenotype. Cell Mol Neurobiol. 2011;31:1229–1243. doi: 10.1007/s10571-011-9725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PloS one. 2013;8:e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashutosh, Chao C, Borgmann K, Brew K, Ghorpade A. Tissue inhibitor of metalloproteinases-1 protects human neurons from staurosporine and HIV-1-induced apoptosis: mechanisms and relevance to HIV-1-associated dementia. Cell Death Dis. 2012;3:e332. doi: 10.1038/cddis.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AveXis I. AveXis Reports Data from Ongoing Phase 1 Trial of AVXS-101 in Spinal Muscular Atrophy Type 1. AxeVis Inc; 2016. [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Bartlett JS, Samulski RJ, McCown TJ. Selective and rapid uptake of adeno-associated virus type 2 in brain. Human gene therapy. 1998;9:1181–1186. doi: 10.1089/hum.1998.9.8-1181. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Weinberg MS, Samulski RJ. Parkinson’s disease gene therapy: success by design meets failure by efficacy. Molecular Therapy. 2014;22:487–497. doi: 10.1038/mt.2013.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Zhao W, Liao B, Kano O, Wang J, Huang A, Appel SH, Henkel JS. Neuroinflammation modulates distinct regional and temporal clinical responses in ALS mice. Brain, behavior, and immunity. 2011;25:1025–1035. doi: 10.1016/j.bbi.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. Journal of Pharmacology and Experimental Therapeutics. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini MJ, Hereñú CB, Goya RG, Garcia-Segura LM. Insulin-like growth factor-I gene delivery to astrocytes reduces their inflammatory response to lipopolysaccharide. Journal of neuroinflammation. 2011;8:1. doi: 10.1186/1742-2094-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemelmans A-P, Horellou P, Pradier L, Brunet I, Colin P, Mallet J. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transfer. Human gene therapy. 1999;10:2987–2997. doi: 10.1089/10430349950016393. [DOI] [PubMed] [Google Scholar]
- Benkhelifa-Ziyyat S, Besse A, Roda M, Duque S, Astord S, Carcenac R, Marais T, Barkats M. Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Molecular Therapy. 2013;21:282–290. doi: 10.1038/mt.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenisti-Zarom L, Regan RF. Astrocyte-specific heme oxygenase-1 hyperexpression attenuates heme-mediated oxidative injury. Neurobiology of disease. 2007;26:688–695. doi: 10.1016/j.nbd.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, Chan CM, McCrate M, Chicoine LG, Coley BD. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Molecular Therapy. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature Reviews Neuroscience. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Blömer U, Naldini L, Verma IM, Trono D, Gage FH. Applications of gene therapy to the CNS. Human molecular genetics. 1996;5:1397–1404. doi: 10.1093/hmg/5.supplement_1.1397. [DOI] [PubMed] [Google Scholar]
- Blömer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. Journal of virology. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillée S, Velde CV, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Human gene therapy. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta neuropathologica. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. The Journal of Neuroscience. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochemical pharmacology. 2010;79:77–89. doi: 10.1016/j.bcp.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, MacLaren RE, Charlton HM. Immunological instability of persistent adenovirus vectors in the brain: peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination. The Journal of neuroscience. 1996;16:3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas R, Avila-Rodriguez M, Vega-Vela EN, Echeverria V, González J, Hidalgo AO, Santos BA, Aliev G, Barreto EG. Growth factors and astrocytes metabolism: Possible roles for platelet derived growth factor. Medicinal Chemistry. 2016;12:204–210. doi: 10.2174/1573406411666151019120444. [DOI] [PubMed] [Google Scholar]
- Callaway EM. A molecular and genetic arsenal for systems neuroscience. Trends Neurosci. 2005;28:196–201. doi: 10.1016/j.tins.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Sew T, Montero L, Burton EA, Greenamyre JT. Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. Exp Neurol. 2011;228:41–52. doi: 10.1016/j.expneurol.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Thrash JC, Walter B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin Neurosci Res. 2006;6:237–245. doi: 10.1016/j.cnr.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Vandenberghe LH, Parente MK, Carnish ER, Wilson JM, Wolfe JH. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Molecular Therapy. 2008 doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M, Nokisalmi P, Raki M, Laasonen L, Särkioja M. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer research. 2010;70:4297–4309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- Ceulemans A-G, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y. The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. Journal of neuroinflammation. 2010;7:1. doi: 10.1186/1742-2094-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi M, Molino Y, Sreedhar B, Khrestchatisky M, Kaczmarek L. Tissue inhibitor of matrix metalloproteinases-1 loaded poly (lactic-co-glycolic acid) nanoparticles for delivery across the blood brain barrier. International journal of nanomedicine. 2014;9:575. doi: 10.2147/IJN.S54750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, McCarty D, Bruce A, Suzuki K. Gene transfer and expression in oligodendrocytes under the control of myelin basic protein transcriptional control region mediated by adeno-associated virus. Gene therapy. 1998:5. doi: 10.1038/sj.gt.3300547. [DOI] [PubMed] [Google Scholar]
- Chen X, Lin W, Lin J, Wu C, Zhang L, Huang Z, Lai J. The protective effects of the lentivirus-mediated neuroglobin gene transfer on spinal cord injury in rabbits. Spinal cord. 2012;50:467–471. doi: 10.1038/sc.2011.138. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Chang Y-N, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Ciesielska A, Hadaczek P, Mittermeyer G, Zhou S, Wright JF, Bankiewicz KS, Forsayeth J. Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses. Molecular Therapy. 2013;21:158–166. doi: 10.1038/mt.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr HIV Res. 2012;10:392–406. doi: 10.2174/157016212802138832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros IE, Ghorpade A. Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes. Neuropharmacology. 2014;85:499–507. doi: 10.1016/j.neuropharm.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo AM, Alberghina L, Papa M. Astrogliosis as a therapeutic target for neurodegenerative diseases. Neuroscience letters. 2014;565:59–64. doi: 10.1016/j.neulet.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Colin A, Faideau M, Dufour N, Auregan G, Hassig R, Andrieu T, Brouillet E, Hantraye P, Bonvento G, Déglon N. Engineered lentiviral vector targeting astrocytes in vivo. Glia. 2009;57:667–679. doi: 10.1002/glia.20795. [DOI] [PubMed] [Google Scholar]
- Cucchiarini M, Ren X, Perides G, Terwilliger E. Selective gene expression in brain microglia mediated via adeno-associated virus type 2 and type 5 vectors. Gene therapy. 2003;10:657–667. doi: 10.1038/sj.gt.3301925. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Guidi L, Stromillo M, Bartolozzi M, Federico A. Imaging neuronal and axonal degeneration in multiple sclerosis. Neurological Sciences. 2003;24:s283–s286. doi: 10.1007/s10072-003-0175-2. [DOI] [PubMed] [Google Scholar]
- Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert review of neurotherapeutics. 2009;9:1519–1527. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclaux M, Teigell M, Amar L, Vogel R, y Ribotta MG, Privat A, Mallet J. A novel and efficient gene transfer strategy reduces glial reactivity and improves neuronal survival and axonal growth in vitro. PLoS One. 2009;4:e6227. doi: 10.1371/journal.pone.0006227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar A, Gardner J, Borgmann K, Wu L, Ghorpade A. Novel role of TGF-beta in differential astrocyte-TIMP-1 regulation: implications for HIV-1-dementia and neuroinflammation. J Neurosci Res. 2006;83:1271–1280. doi: 10.1002/jnr.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel J, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Dittgen T, Nimmerjahn A, Komai S, Licznerski P, Waters J, Margrie TW, Helmchen F, Denk W, Brecht M, Osten P. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proceedings of the National Academy of Sciences. 2004;101:18206–18211. doi: 10.1073/pnas.0407976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Thi N, Saillour P, Ferrero L, Dedieu J, Mallet J, Paunio T. Delivery of GDNF by an E1, E3/E4 deleted adenoviral vector and driven by a GFAP promoter prevents dopaminergic neuron degeneration in a rat model of Parkinson’s disease. Gene therapy. 2004;11:746–756. doi: 10.1038/sj.gt.3302222. [DOI] [PubMed] [Google Scholar]
- Dong J-Y, Fan P-D, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Human gene therapy. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- Donsante A, McEachin Z, Riley J, Leung C, Kanz L, O’Connor D, Boulis N. Intracerebroventricular delivery of self-complementary adeno-associated virus serotype 9 to the adult rat brain. Gene therapy. 2016;23:401–407. doi: 10.1038/gt.2016.6. [DOI] [PubMed] [Google Scholar]
- Dormond E, Perrier M, Kamen A. From the first to the third generation adenoviral vector: what parameters are governing the production yield? Biotechnology advances. 2009;27:133–144. doi: 10.1016/j.biotechadv.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Drinkut A, Tereshchenko Y, Schulz JB, Bähr M, Kügler S. Efficient gene therapy for Parkinson’s disease using astrocytes as hosts for localized neurotrophic factor delivery. Molecular Therapy. 2012;20:534–543. doi: 10.1038/mt.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling J, Jagust W, Christine C, Starr P, Larson P, Bankiewicz K, Aminoff M. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Escartin C, Brouillet E, Gubellini P, Trioulier Y, Jacquard C, Smadja C, Knott GW, Kerkerian-Le Goff L, Déglon N, Hantraye P. Ciliary neurotrophic factor activates astrocytes, redistributes their glutamate transporters GLAST and GLT-1 to raft microdomains, and improves glutamate handling in vivo. The Journal of neuroscience. 2006;26:5978–5989. doi: 10.1523/JNEUROSCI.0302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MC, Couch Y, Sibson N, Turner MR. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Molecular and cellular neurosciences. 2013;53:34–41. doi: 10.1016/j.mcn.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Ezcurra ALDL, Chertoff M, Ferrari C, Graciarena M, Pitossi F. Chronic expression of low levels of tumor necrosis factor-α in the substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiology of disease. 2010;37:630–640. doi: 10.1016/j.nbd.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington’s disease. Progress in neurobiology. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Ferrari CC, Depino AM, Prada F, Muraro N, Campbell S, Podhajcer O, Perry VH, Anthony DC, Pitossi FJ. Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. The American journal of pathology. 2004;165:1827–1837. doi: 10.1016/S0002-9440(10)63438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nature biotechnology. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Kotter MR. The biology of CNS remyelination. Journal of neurology. 2008;255:19–25. doi: 10.1007/s00415-008-1004-6. [DOI] [PubMed] [Google Scholar]
- Fuller S, Munch G, Steele M. Activated astrocytes: a therapeutic target in Alzheimer’s disease? Expert review of neurotherapeutics. 2009;9:1585–1594. doi: 10.1586/ern.09.111. [DOI] [PubMed] [Google Scholar]
- Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Van Eldik LJ, Norris CM. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. The Journal of Neuroscience. 2012;32:16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T, Gersbach CA, Barbas CF. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends in biotechnology. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galou M, Pournin S, Ensergueix D, Ridet JL, Tchélingérian JL, Lossouran L, Privat A, Babinet C, Dupouey P. Normal and pathological expression of GFAP promoter elements in transgenic mice. Glia. 1994;12:281–293. doi: 10.1002/glia.440120405. [DOI] [PubMed] [Google Scholar]
- Garden GA, Möller T. Microglia biology in health and disease. Journal of Neuroimmune Pharmacology. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Gardner J, Ghorpade A. Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. J Neurosci Res. 2003;74:801–806. doi: 10.1002/jnr.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldenhuys W, Mbimba T, Bui T, Harrison K, Sutariya V. Brain-targeted delivery of paclitaxel using glutathione-coated nanoparticles for brain cancers. Journal of drug targeting. 2011;19:837–845. doi: 10.3109/1061186X.2011.589435. [DOI] [PubMed] [Google Scholar]
- Geppert M, Hohnholt MC, Thiel K, Nürnberger S, Grunwald I, Rezwan K, Dringen R. Uptake of dimercaptosuccinate-coated magnetic iron oxide nanoparticles by cultured brain astrocytes. Nanotechnology. 2011;22:145101. doi: 10.1088/0957-4484/22/14/145101. [DOI] [PubMed] [Google Scholar]
- Gerdes CA, Castro MG, Löwenstein PR. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Molecular Therapy. 2000;2:330. doi: 10.1006/mthe.2000.0140. [DOI] [PubMed] [Google Scholar]
- Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholizadeh S, Tharmalingam S, MacAldaz ME, Hampson DR. Transduction of the central nervous system after intracerebroventricular injection of adeno-associated viral vectors in neonatal and juvenile mice. Human gene therapy methods. 2013;24:205–213. doi: 10.1089/hgtb.2013.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 an update. The journal of gene medicine. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- Giralt A, Friedman HC, Caneda-Ferron B, Urban N, Moreno E, Rubio N, Blanco J, Peterson A, Canals JM, Alberch J. BDNF regulation under GFAP promoter provides engineered astrocytes as a new approach for long-term protection in Huntington’s disease. Gene Ther. 2010;17:1294–1308. doi: 10.1038/gt.2010.71. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Poluektova L, Gendelman HE. Rodent models for HIV-associated neurocognitive disorders. Trends Neurosci. 2012;35:197–208. doi: 10.1016/j.tins.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goula D, Remy J, Erbacher P, Wasowicz M, Levi G, Abdallah B, Demeneix B. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene therapy. 1998;5:712–717. doi: 10.1038/sj.gt.3300635. [DOI] [PubMed] [Google Scholar]
- Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Molecular Therapy. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskreutz J, Van Den Bosch L, Keller BU. Calcium dysregulation in amyotrophic lateral sclerosis. Cell calcium. 2010;47:165–174. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Guerrero-Cázares H, Tzeng SY, Young NP, Abutaleb AO, Quiñones-Hinojosa A, Green JJ. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS nano. 2014;8:5141–5153. doi: 10.1021/nn501197v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak S-J, Yun Y, Kim KN, Ha Y. Therapeutic Use of 3β-[N-(N′, N′-Dimethylaminoethane) Carbamoyl] Cholesterol-Modified PLGA Nanospheres as Gene Delivery Vehicles for Spinal Cord Injury. PloS one. 2016;11:e0147389. doi: 10.1371/journal.pone.0147389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends in molecular medicine. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Health USNIo. Neurodegeneration clinical trials. NIH; 2016. [Google Scholar]
- Hendriks W, Eggers R, Verhaagen J, Boer G. Gene transfer to the spinal cord neural scar with lentiviral vectors: predominant transgene expression in astrocytes but not in meningeal cells. Journal of neuroscience research. 2007;85:3041–3052. doi: 10.1002/jnr.21432. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Kilic E, Kügler S, Isenmann S, Bähr M. Adenovirus-mediated glial cell line-derived neurotrophic factor (GDNF) expression protects against subsequent cortical cold injury in rats. Neurobiology of disease. 2001a;8:964–973. doi: 10.1006/nbdi.2001.0448. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Kilic E, Kügler S, Isenmann S, Bähr M. Adenovirus-mediated GDNF and CNTF pretreatment protects against striatal injury following transient middle cerebral artery occlusion in mice. Neurobiology of disease. 2001b;8:655–666. doi: 10.1006/nbdi.2001.0399. [DOI] [PubMed] [Google Scholar]
- Hermens WT, Verhaagen J. Viral vectors, tools for gene transfer in the nervous system. Progress in neurobiology. 1998;55:399–432. doi: 10.1016/s0301-0082(98)00007-0. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? The Lancet Neurology. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Homs J, Ariza L, Pages G, Udina E, Navarro X, Chillón M, Bosch A. Schwann cell targeting via intrasciatic injection of AAV8 as gene therapy strategy for peripheral nerve regeneration. Gene therapy. 2011;18:622–630. doi: 10.1038/gt.2011.7. [DOI] [PubMed] [Google Scholar]
- Hu W, Kaminski R, Yang F, Zhang Y, Cosentino L, Li F, Luo B, Alvarez-Carbonell D, Garcia-Mesa Y, Karn J. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proceedings of the National Academy of Sciences. 2014;111:11461–11466. doi: 10.1073/pnas.1405186111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Zhang D, Pang H, Caudle WM, Li Y, Gao H, Liu Y, Qian L, Wilson B, Di Monte DA. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. The Journal of Immunology. 2008;181:7194–7204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D-Y, Carlezon WA, Jr, Isacson O, Kim K-S. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Human gene therapy. 2001;12:1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- Institut USN, Health et al. Search results on for Ibudilast clinical trials. 2016. Ibudilast clinical trials. [Google Scholar]
- Jaffer H, Morris VB, Stewart D, Labhasetwar V. Advances in Stroke Therapy. Drug Deliv Transl Res. 2011;1:409–419. doi: 10.1007/s13346-011-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson J, Ericson C, Jansson M, Björk E, Lundberg C. Targeted transgene expression in rat brain using lentiviral vectors. Journal of neuroscience research. 2003;73:876–885. doi: 10.1002/jnr.10719. [DOI] [PubMed] [Google Scholar]
- Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Science translational medicine. 2013;5:209ra152–209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez JL, Clemente MI, Weber ND, Sanchez J, Ortega P, de la Mata FJ, Gómez R, García D, López-Fernández LA, Muñoz-Fernández MÁ. Carbosilane dendrimers to transfect human astrocytes with small interfering RNA targeting human immunodeficiency virus. BioDrugs. 2010;24:331–343. doi: 10.2165/11538400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Joris F, Manshian BB, Peynshaert K, De Smedt SC, Braeckmans K, Soenen SJ. Assessing nanoparticle toxicity in cell-based assays: influence of cell culture parameters and optimized models for bridging the in vitro in vivo gap. Chemical Society Reviews. 2013;42:8339–8359. doi: 10.1039/c3cs60145e. [DOI] [PubMed] [Google Scholar]
- Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nature medicine. 2010;16:1210–1214. doi: 10.1038/nm.2224. [DOI] [PubMed] [Google Scholar]
- Kaminski R, Chen Y, Fischer T, Tedaldi E, Napoli A, Zhang Y, Karn J, Hu W, Khalili K. Elimination of HIV-1 genomes from human T-lymphoid cells by CRISPR/Cas9 gene editing. Scientific reports. 2016:6. doi: 10.1038/srep22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. The Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Katz MG, Fargnoli AS, Williams RD, Bridges CR. Gene therapy delivery systems for enhancing viral and nonviral vectors for cardiac diseases: current concepts and future applications. Human gene therapy. 2013;24:914–927. doi: 10.1089/hum.2013.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I-D, Lim C-M, Kim J-B, Nam HY, Nam K, Kim S-W, Park J-S, Lee J-K. Neuroprotection by biodegradable PAMAM ester (e-PAM-R)-mediated HMGB1 siRNA delivery in primary cortical cultures and in the postischemic brain. Journal of Controlled Release. 2010;142:422–430. doi: 10.1016/j.jconrel.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Kim I-D, Shin J-H, Kim S-W, Choi S, Ahn J, Han P-L, Park J-S, Lee J-K. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Molecular Therapy. 2012;20:829–839. doi: 10.1038/mt.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-B, Choi JS, Nam K, Lee M, Park J-S, Lee J-K. Enhanced transfection of primary cortical cultures using arginine-grafted PAMAM dendrimer, PAMAM-Arg. Journal of controlled release. 2006;114:110–117. doi: 10.1016/j.jconrel.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Yamamoto M, Schroder B, Jacobsen MT, Swan RJ, Lambert MP, Klein WL, Gendelman HE, Ransohoff RM, Ikezu T. AAV1/2-mediated CNS gene delivery of dominant-negative CCL2 mutant suppresses gliosis, β-amyloidosis, and learning impairment of APP/PS1 mice. Molecular Therapy. 2009;17:803–809. doi: 10.1038/mt.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klejbor I, Stachowiak E, Bharali D, Roy I, Spodnik I, Morys J, Bergey E, Prasad P, Stachowiak M. ORMOSIL nanoparticles as a non-viral gene delivery vector for modeling polyglutamine induced brain pathology. Journal of neuroscience methods. 2007;165:230–243. doi: 10.1016/j.jneumeth.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Koerber JT, Klimczak R, Jang J-H, Dalkara D, Flannery JG, Schaffer DV. Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Molecular Therapy. 2009;17:2088–2095. doi: 10.1038/mt.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev. 2014;71:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Kügler S, Lingor P, Schöll U, Zolotukhin S, Bähr M. Differential transgene expression in brain cells in vivo and in vitro from AAV-2 vectors with small transcriptional control units. Virology. 2003;311:89–95. doi: 10.1016/s0042-6822(03)00162-4. [DOI] [PubMed] [Google Scholar]
- Kuhn B, Hoogland TM, Wang SS-H. Injection of recombinant adenovirus for delivery of genetically encoded calcium indicators into astrocytes of the cerebellar cortex. Cold Spring Harb Protoc. 2011;2011:1217–1223. doi: 10.1101/pdb.prot065797. [DOI] [PubMed] [Google Scholar]
- Kwon EJ, Lasiene J, Jacobson BE, Park I-K, Horner PJ, Pun SH. Targeted nonviral delivery vehicles to neural progenitor cells in the mouse subventricular zone. Biomaterials. 2010;31:2417–2424. doi: 10.1016/j.biomaterials.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Progress in neurobiology. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Lai Z, Brady RO. Gene transfer into the central nervous system in vivo using a recombinanat lentivirus vector. Journal of neuroscience research. 2002;67:363–371. doi: 10.1002/jnr.10137. [DOI] [PubMed] [Google Scholar]
- Lawlor PA, Bland RJ, Mouravlev A, Young D, During MJ. Efficient gene delivery and selective transduction of glial cells in the mammalian brain by AAV serotypes isolated from nonhuman primates. Molecular Therapy. 2009;17:1692–1702. doi: 10.1038/mt.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56:481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A, Siddiqui MS. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. The Lancet Neurology. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- Lisovoski F, Akli S, Peltekian E, Vigne E, Haase G, Perricaudet M, Dreyfus PA, Kahn A, Peschanski M. Phenotypic alteration of astrocytes induced by ciliary neurotrophic factor in the intact adult brain, as revealed by adenovirus-mediated gene transfer. The Journal of neuroscience. 1997;17:7228–7236. doi: 10.1523/JNEUROSCI.17-19-07228.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Martins I, Chiorini J, Davidson B. Adeno-associated virus type 4 (AAV4) targets ependyma and astrocytes in the subventricular zone and RMS. Gene therapy. 2005;12:1503–1508. doi: 10.1038/sj.gt.3302554. [DOI] [PubMed] [Google Scholar]
- Liu Y, Himes BT, Moul J, Huang W, Chow SY, Tessler A, Fischer I. Application of recombinant adenovirus for in vivo gene delivery to spinal cord. Brain research. 1997;768:19–29. doi: 10.1016/s0006-8993(97)00587-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu Z, Wang Y, Liang Y-R, Wen X, Hu J, Yang X, Liu J, Xiao S, Cheng D. Investigation of the performance of PEG PEI/ROCK-II-siRNA complexes for Alzheimer’s disease in vitro. Brain research. 2013;1490:43–51. doi: 10.1016/j.brainres.2012.10.039. [DOI] [PubMed] [Google Scholar]
- Liu Y, Miao Q, Yuan J, Han Se, Zhang P, Li S, Rao Z, Zhao W, Ye Q, Geng J. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. The Journal of Neuroscience. 2015;35:9336–9355. doi: 10.1523/JNEUROSCI.3975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobello K, Ryan JM, Liu E, Rippon G, Black R. Targeting Beta amyloid: a clinical review of immunotherapeutic approaches in Alzheimer’s disease. International Journal of Alzheimer’s Disease. 2012;2012 doi: 10.1155/2012/628070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Morris VB, Labhasetwar V. Codelivery of DNA and siRNA via arginine-rich PEI-based polyplexes. Molecular pharmaceutics. 2015;12:621–629. doi: 10.1021/mp5006883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S-M, Tremblay M-È, King IL, Qi J, Reynolds HM, Marker DF, Varrone JJ, Majewska AK, Dewhurst S, Gelbard HA. HIV-1 Tat-induced microgliosis and synaptic damage via interactions between peripheral and central myeloid cells. PloS one. 2011;6:e23915. doi: 10.1371/journal.pone.0023915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu-Nguyen NB, Broadstock M, Schliesser MG, Bartholomae CC, von Kalle C, Schmidt M, Yáñez-Muñoz RJ. Transgenic expression of human glial cell line-derived neurotrophic factor from integration-deficient lentiviral vectors is neuroprotective in a rodent model of Parkinson’s disease. Human gene therapy. 2014;25:631–641. doi: 10.1089/hum.2014.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther EM, Koehler Y, Diendorf J, Epple M, Dringen R. Accumulation of silver nanoparticles by cultured primary brain astrocytes. Nanotechnology. 2011;22:375101. doi: 10.1088/0957-4484/22/37/375101. [DOI] [PubMed] [Google Scholar]
- Malhotra M, Tomaro-Duchesneau C, Saha S, Kahouli I, Prakash S. Development and characterization of chitosan-PEG-TAT nanoparticles for the intracellular delivery of siRNA. International journal of nanomedicine. 2013;8:2041–2052. doi: 10.2147/IJN.S43683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi G, Xu Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion. 2005;5:77–87. doi: 10.1016/j.mito.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Mangraviti A, Tzeng SY, Kozielski KL, Wang Y, Jin Y, Gullotti D, Pedone M, Buaron N, Liu A, Wilson DR. Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo. ACS nano. 2015;9:1236–1249. doi: 10.1021/nn504905q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos P, Zhang C, Berry S, Oh Y, Lee S, Eberhart CG, Woodworth GF, Suk JS, Hanes J. Highly PEGylated DNA nanoparticles provide uniform and widespread gene transfer in the brain. Advanced healthcare materials. 2015;4:1023–1033. doi: 10.1002/adhm.201400800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver SR, Lee CS, Lee JM, Green SH, Sands MS, Snider BJ, Goldberg MP. Lentiviral transduction of murine oligodendrocytes in vivo. Journal of neuroscience research. 2005;82:397–403. doi: 10.1002/jnr.20626. [DOI] [PubMed] [Google Scholar]
- McMorris FA, McKinnon RD. Regulation of oligodendrocyte development and CNS myelination by growth factors: prospects for therapy of demyelinating disease. Brain Pathol. 1996;6:313–329. doi: 10.1111/j.1750-3639.1996.tb00858.x. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. Journal of neurochemistry. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Meng X, Yang F, Ouyang T, Liu B, Wu C, Jiang W. Specific gene expression in mouse cortical astrocytes is mediated by a 1740bp-GFAP promoter-driven combined adeno-associated virus 2/5/7/8/9. Neuroscience letters. 2015;593:45–50. doi: 10.1016/j.neulet.2015.03.022. [DOI] [PubMed] [Google Scholar]
- Merienne N, Delzor A, Viret A, Dufour N, Rey M, Hantraye P, Déglon N. Gene transfer engineering for astrocyte-specific silencing in the CNS. Gene therapy. 2015;22:830–839. doi: 10.1038/gt.2015.54. [DOI] [PubMed] [Google Scholar]
- Merrill J, Scolding N. Mechanisms of damage to myelin and oligodendrocytes and their relevance to disease. Neuropathology and applied neurobiology. 1999;25:435–458. doi: 10.1046/j.1365-2990.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- Midoux P, Breuzard G, Gomez JP, Pichon C. Polymer-based gene delivery: a current review on the uptake and intracellular trafficking of polyplexes. Current gene therapy. 2008;8:335–352. doi: 10.2174/156652308786071014. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Soderquist RG, Malone SM, Mahoney JH, Hughes TS, Langer SJ, Sloane EM, Maier SF, Leinwand LA, Watkins LR. Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron glia biology. 2006;2:293–308. doi: 10.1017/S1740925X07000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake N, Miyake K, Yamamoto M, Hirai Y, Shimada T. Global gene transfer into the CNS across the BBB after neonatal systemic delivery of single-stranded AAV vectors. Brain research. 2011;1389:19–26. doi: 10.1016/j.brainres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Morris VB, Labhasetwar V. Arginine-rich polyplexes for gene delivery to neuronal cells. Biomaterials. 2015;60:151–160. doi: 10.1016/j.biomaterials.2015.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Uchida K, Kobayashi S, Inukai T, Horiuchi Y, Yayama T, Sato R, Baba H. Rescue of rat anterior horn neurons after spinal cord injury by retrograde transfection of adenovirus vector carrying brain-derived neurotrophic factor gene. Journal of neurotrauma. 2007;24:703–712. doi: 10.1089/neu.2006.0004. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Navarro V, Millecamps S, Geoffroy M, Robert J, Valin A, Mallet J, Le Gal La Salle G. Efficient gene transfer and long-term expression in neurons using a recombinant adenovirus with a neuron-specific promoter. Gene therapy. 1999;6:1884–1892. doi: 10.1038/sj.gt.3301008. [DOI] [PubMed] [Google Scholar]
- Newland B, Abu-Rub M, Naughton M, Zheng Y, Pinoncely A, Collin E, Dowd E, Wang W, Pandit A. GDNF gene delivery via a 2-(dimethylamino) ethyl methacrylate based cyclized knot polymer for neuronal cell applications. ACS chemical neuroscience. 2013;4:540–546. doi: 10.1021/cn4000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchia GP, Frigeri A, Liuzzi GM, Svelto M. Inhibition of aquaporin-4 expression in astrocytes by RNAi determines alteration in cell morphology, growth, and water transport and induces changes in ischemia-related genes. The FASEB journal. 2003;17:1508–1510. doi: 10.1096/fj.02-1183fje. [DOI] [PubMed] [Google Scholar]
- Niranjan R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: focus on astrocytes. Molecular neurobiology. 2014;49:28–38. doi: 10.1007/s12035-013-8483-x. [DOI] [PubMed] [Google Scholar]
- Nolte C, Matyash M, Pivneva T, Schipke CG, Ohlemeyer C, Hanisch UK, Kirchhoff F, Kettenmann H. GFAP promoter-controlled EGFP-expressing transgenic mice: A tool to visualize astrocytes and astrogliosis in living brain tissue. Glia. 2001;33:72–86. [PubMed] [Google Scholar]
- Nomoto T, Okada T, Shimazaki K, Mizukami H, Matsushita T, Hanazono Y, Kume A, Katsura K-i, Katayama Y, Ozawa K. Distinct patterns of gene transfer to gerbil hippocampus with recombinant adeno-associated virus type 2 and 5. Neuroscience letters. 2003;340:153–157. doi: 10.1016/s0304-3940(03)00095-8. [DOI] [PubMed] [Google Scholar]
- Palfi S, Gurruchaga JM, Ralph GS, Lepetit H, Lavisse S, Buttery PC, Watts C, Miskin J, Kelleher M, Deeley S. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. The Lancet. 2014;383:1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- Park IK, Lasiene J, Chou SH, Horner PJ, Pun SH. Neuron-specific delivery of nucleic acids mediated by Tet1-modified poly (ethylenimine) The journal of gene medicine. 2007;9:691–702. doi: 10.1002/jgm.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel A, Zolotukhin S, Schrimsher G, Muzyczka N, Reier P. Efficient transduction of green fluorescent protein in spinal cord neurons using adeno-associated virus vectors containing cell-type specific promoters. Gene therapy. 1997;4:16–24. doi: 10.1038/sj.gt.3300358. [DOI] [PubMed] [Google Scholar]
- Peel AL, Klein RL. Adeno-associated virus vectors: activity and applications in the CNS. Journal of neuroscience methods. 2000;98:95–104. doi: 10.1016/s0165-0270(00)00183-7. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Zheng J, Miller D, Gendelman HE. Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. J Leukoc Biol. 2000;68:413–422. [PubMed] [Google Scholar]
- Petrosyan H, Alessi V, Singh V, Hunanyan A, Levine J, Arvanian V. Transduction efficiency of neurons and glial cells by AAV-1,-5,-9,-rh10 and-hu11 serotypes in rat spinal cord following contusion injury. Gene therapy. 2014;21:991–1000. doi: 10.1038/gt.2014.74. [DOI] [PubMed] [Google Scholar]
- Peviani M, Kurosaki M, Terao M, Lidonnici D, Gensano F, Battaglia E, Tortarolo M, Piva R, Bendotti C. Lentiviral vectors carrying enhancer elements of Hb9 promoter drive selective transgene expression in mouse spinal cord motor neurons. Journal of neuroscience methods. 2012;205:139–147. doi: 10.1016/j.jneumeth.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Pilakka-Kanthikeel S, Atluri VSR, Sagar V, Saxena SK, Nair M. Targeted brain derived neurotropic factors (BDNF) delivery across the blood-brain barrier for neuro-protection using magnetic nano carriers: an in-vitro study. PLoS One. 2013;8:e62241. doi: 10.1371/journal.pone.0062241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Progress in neurobiology. 2010;92:293–315. doi: 10.1016/j.pneurobio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Popescu BFG, Lucchinetti CF. Pathology of demyelinating diseases. Annual Review of Pathology: Mechanisms of Disease. 2012;7:185–217. doi: 10.1146/annurev-pathol-011811-132443. [DOI] [PubMed] [Google Scholar]
- Rafi MA, Rao HZ, Passini MA, Curtis M, Vanier MT, Zaka M, Luzi P, Wolfe JH, Wenger DA. AAV-mediated expression of galactocerebrosidase in brain results in attenuated symptoms and extended life span in murine models of globoid cell leukodystrophy. Molecular Therapy. 2005;11:734–744. doi: 10.1016/j.ymthe.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Rafii MS, Baumann TL, Bakay RA, Ostrove JM, Siffert J, Fleisher AS, Herzog CD, Barba D, Pay M, Salmon DP. A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer’s disease. Alzheimer’s & Dementia. 2014;10:571–581. doi: 10.1016/j.jalz.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Rahim A, Wong A, Howe S, Buckley S, Acosta-Saltos A, Elston K, Ward N, Philpott N, Cooper J, Anderson P. Efficient gene delivery to the adult and fetal CNS using pseudotyped non-integrating lentiviral vectors. Gene therapy. 2009;16:509–520. doi: 10.1038/gt.2008.186. [DOI] [PubMed] [Google Scholar]
- Rahim AA, Wong AM, Hoefer K, Buckley SM, Mattar CN, Cheng SH, Chan JK, Cooper JD, Waddington SN. Intravenous administration of AAV2/9 to the fetal and neonatal mouse leads to differential targeting of CNS cell types and extensive transduction of the nervous system. The FASEB Journal. 2011;25:3505–3518. doi: 10.1096/fj.11-182311. [DOI] [PubMed] [Google Scholar]
- Rao KS, Reddy MK, Horning JL, Labhasetwar V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29:4429–4438. doi: 10.1016/j.biomaterials.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Morales AA, Pearse DD. The Comparative Utility of Viromer RED and Lipofectamine for Transient Gene Introduction into Glial Cells. BioMed research international. 2015;2015 doi: 10.1155/2015/458624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. The Journal of neuroscience. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock RB, Peterson PK. Microglia as a pharmacological target in infectious and inflammatory diseases of the brain. Journal of Neuroimmune Pharmacology. 2006;1:117–126. doi: 10.1007/s11481-006-9012-8. [DOI] [PubMed] [Google Scholar]
- Rodgers JM, Robinson AP, Miller SD. Strategies for protecting oligodendrocytes and enhancing remyelination in multiple sclerosis. Discovery medicine. 2013;16:53. [PMC free article] [PubMed] [Google Scholar]
- Rogers M-L, Rush RA. Non-viral gene therapy for neurological diseases, with an emphasis on targeted gene delivery. Journal of controlled release. 2012;157:183–189. doi: 10.1016/j.jconrel.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Romero M, Smith G. Adenoviral gene transfer into the normal and injured spinal cord: enhanced transgene stability by combined administration of temperature-sensitive virus and transient immune blockade. Gene therapy. 1998;5:1612–1621. doi: 10.1038/sj.gt.3300774. [DOI] [PubMed] [Google Scholar]
- Rungta RL, Choi HB, Lin PJ, Ko RW, Ashby D, Nair J, Manoharan M, Cullis PR, MacVicar BA. Lipid nanoparticle delivery of siRNA to silence neuronal gene expression in the brain. Molecular Therapy Nucleic Acids. 2013;2:e136. doi: 10.1038/mtna.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch L, San Sebastian W, Kells AP, Salegio EA, Heller G, Bringas JR, Pivirotto P, DeArmond S, Forsayeth J, Bankiewicz KS. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Molecular Therapy. 2014;22:329–337. doi: 10.1038/mt.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Sebastian W, Samaranch L, Heller G, Kells AP, Bringas J, Pivirotto P, Forsayeth J, Bankiewicz KS. Adeno-associated virus type 6 is retrogradely transported in the non-human primate brain. Gene therapy. 2013;20:1178–1183. doi: 10.1038/gt.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, Ribecco-Lutkiewicz M, Tremblay R, Kiuchi K, Sikorska M. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiology of disease. 2009;33:405–414. doi: 10.1016/j.nbd.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Science, medicine, and the future: Parkinson’s disease. BMJ. 1999;318:311–314. doi: 10.1136/bmj.318.7179.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster DJ, Dykstra JA, Riedl MS, Kitto KF, Belur LR, McIvor RS, Elde RP, Fairbanks CA, Vulchanova L. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Neuroanatomy and transgenic technologies. 2015 doi: 10.3389/fnana.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia J, Vergara P, Brenner M. Astrocyte-specific expression of tyrosine hydroxylase after intracerebral gene transfer induces behavioral recovery in experimental Parkinsonism. Gene therapy. 1998;5 doi: 10.1038/sj.gt.3300776. [DOI] [PubMed] [Google Scholar]
- Serramía MJ, Álvarez S, Fuentes-Paniagua E, Clemente MI, Sánchez-Nieves J, Gómez R, de la Mata J, Muñoz-Fernández MÁ. In vivo delivery of siRNA to the brain by carbosilane dendrimer. Journal of Controlled Release. 2015;200:60–70. doi: 10.1016/j.jconrel.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Shah L, Yadav S, Amiji M. Nanotechnology for CNS delivery of bio-therapeutic agents. Drug delivery and translational research. 2013;3:336–351. doi: 10.1007/s13346-013-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Fan Y, Su H, Zhu Y, Chen Y, Liu W, Young WL, Yang G-Y. Adeno-associated viral vector-mediated hypoxia-regulated VEGF gene transfer promotes angiogenesis following focal cerebral ischemia in mice. Gene therapy. 2008;15:30–39. doi: 10.1038/sj.gt.3303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsova Z, Malik JM, Michel U, Bahr M, Kugler S. Promoters and serotypes: targeting of adeno-associated virus vectors for gene transfer in the rat central nervous system in vitro and in vivo. Exp Physiol. 2005;90:53–59. doi: 10.1113/expphysiol.2004.028159. [DOI] [PubMed] [Google Scholar]
- Shi N, Zhang Y, Zhu C, Boado RJ, Pardridge WM. Brain-specific expression of an exogenous gene after iv administration. Proceedings of the National Academy of Sciences. 2001;98:12754–12759. doi: 10.1073/pnas.221450098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestroni A, Faull RL, Strand AD, Möller T. Distinct neuroinflammatory profile in post-mortem human Huntington’s disease. Neuroreport. 2009;20:1098–1103. doi: 10.1097/WNR.0b013e32832e34ee. [DOI] [PubMed] [Google Scholar]
- Sonawane ND, Szoka FC, Verkman A. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. Journal of Biological Chemistry. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- Stangel M, Gold R, Gass A, Haas J, Jung S, Elias W, Zettl UK. Current issues in immunomodulatory treatment of multiple sclerosis--a practical approach. J Neurol. 2006;253(Suppl 1):I32–36. doi: 10.1007/s00415-006-1108-9. [DOI] [PubMed] [Google Scholar]
- Steiner J, Haughey N, Li W, Venkatesan A, Anderson C, Reid R, Malpica T, Pocernich C, Butterfield DA, Nath A. Oxidative stress and therapeutic approaches in HIV dementia. Antioxidants & redox signaling. 2006;8:2089–2100. doi: 10.1089/ars.2006.8.2089. [DOI] [PubMed] [Google Scholar]
- Su X, Kells AP, Huang EJ, Lee HS, Hadaczek P, Beyer J, Bringas J, Pivirotto P, Penticuff J, Eberling J. Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Human gene therapy. 2009;20:1627–1640. doi: 10.1089/hum.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Clark K, Peel A, Mao X, Chang Q, Simon R, Greenberg D. Adeno-associated virus-mediated delivery of BCL-w gene improves outcome after transient focal cerebral ischemia. Gene therapy. 2003;10:115–122. doi: 10.1038/sj.gt.3301868. [DOI] [PubMed] [Google Scholar]
- Tan J-KY, Pham B, Zong Y, Perez C, Maris DO, Hemphill A, Miao CH, Matula TJ, Mourad PD, Wei H. Microbubbles and ultrasound increase intraventricular polyplex gene transfer to the brain. Journal of Controlled Release. 2016;231:86–93. doi: 10.1016/j.jconrel.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. Journal of psychosomatic research. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Teng Z-P, Chen J, Chau L-Y, Galunic N, Regan RF. Adenoviral transfer of the heme oxygenase-1 gene protects striatal astrocytes from heme-mediated oxidative injury. Neurobiology of disease. 2004;17:179–187. doi: 10.1016/j.nbd.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, Kochanek S, Castro MG, Löwenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proceedings of the National Academy of Sciences. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley RB, Vesey MJ, Barati S, Rush RA, Ferguson IA. Improved non-viral transfection of glial and adult neural stem cell lines and of primary astrocytes by combining agents with complementary modes of action. The journal of gene medicine. 2004;6:1023–1032. doi: 10.1002/jgm.584. [DOI] [PubMed] [Google Scholar]
- Tosi G, Bortot B, Ruozi B, Dolcetta D, Vandelli M, Forni F, Severini G. Potential use of polymeric nanoparticles for drug delivery across the blood-brain barrier. Current medicinal chemistry. 2013;20:2212–2225. doi: 10.2174/0929867311320170006. [DOI] [PubMed] [Google Scholar]
- Towne C, Schneider B, Kieran D, Redmond D, Aebischer P. Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene therapy. 2010;17:141–146. doi: 10.1038/gt.2009.119. [DOI] [PubMed] [Google Scholar]
- Tuinstra HM, Ducommun MM, Briley WE, Shea LD. Gene delivery to overcome astrocyte inhibition of axonal growth: An in vitro Model of the glial scar. Biotechnology and bioengineering. 2013;110:947–957. doi: 10.1002/bit.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra HM, Aviles MO, Shin S, Holland SJ, Zelivyanskaya ML, Fast AG, Ko SY, Margul DJ, Bartels AK, Boehler RM. Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials. 2012;33:1618–1626. doi: 10.1016/j.biomaterials.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, De Brabander J. Neuronal changes in normal human aging and Alzheimer’s disease. Brain and cognition. 2002;49:268–276. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- Van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Current neurology and neuroscience reports. 2010;10:207–214. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartak-Sharma N, Gelman BB, Joshi C, Borgamann K, Ghorpade A. Astrocyte elevated gene-1 is a novel modulator of HIV-1-associated neuroinflammation via regulation of nuclear factor-kappaB signaling and excitatory amino acid transporter-2 repression. J Biol Chem. 2014;289:19599–19612. doi: 10.1074/jbc.M114.567644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bernhardi R, Eugenín-von Bernhardi L, Eugenín J. Microglial cell dysregulation in brain aging and neurodegeneration. Frontiers in aging neuroscience. 2015:7. doi: 10.3389/fnagi.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernhardi R, Ramirez G, De Ferrari GV, Inestrosa NC. Acetylcholinesterase induces the expression of the beta-amyloid precursor protein in glia and activates glial cells in culture. Neurobiol Dis. 2003;14:447–457. doi: 10.1016/j.nbd.2003.08.014. [DOI] [PubMed] [Google Scholar]
- von Jonquieres G, Mersmann N, Klugmann CB, Harasta AE, Lutz B, Teahan O, Housley GD, Fröhlich D, Krämer-Albers E-M, Klugmann M. Glial promoter selectivity following AAV-delivery to the immature brain. PLoS One. 2013;8:e65646. doi: 10.1371/journal.pone.0065646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Charlson F, Davis A, Degenhardt L, Dicker D. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Miyamoto A, Nabekura J. Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 2013;36:209–217. doi: 10.1016/j.tins.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang S. Astrocytic expression of transgene in the rat brain mediated by baculovirus vectors containing an astrocyte-specific promoter. Gene therapy. 2006;13:1447–1456. doi: 10.1038/sj.gt.3302771. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang C, Clark K, Sferra T. Recombinant AAV serotype 1 transduction efficiency and tropism in the murine brain. Gene therapy. 2003a;10:1528–1534. doi: 10.1038/sj.gt.3302011. [DOI] [PubMed] [Google Scholar]
- Wang CY, Yang SH, Tzeng SF. MicroRNA-145 as one negative regulator of astrogliosis. Glia. 2015;63:194–205. doi: 10.1002/glia.22743. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhong L, Nahid MA, Gao G. The potential of adeno-associated viral vectors for gene delivery to muscle tissue. Expert opinion on drug delivery. 2014;11:345–364. doi: 10.1517/17425247.2014.871258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Bordey A. The astrocyte odyssey. Progress in neurobiology. 2008;86:342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-Q, Chen Q, Wang X, Wang Q-C, Wang Y, Cheng H-P, Guo C, Sun Q, Chen Q, Tang T-S. Dysregulation of mitochondrial calcium signaling and superoxide flashes cause mitochondrial genomic DNA damage in Huntington disease. Journal of Biological Chemistry. 2013;288:3070–3084. doi: 10.1074/jbc.M112.407726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lin F, Wang J, Wu J, Han R, Zhu L, Zhang G, DiFiglia M, Qin Z. Truncated N-terminal huntingtin fragment with expanded-polyglutamine (htt552-100Q) suppresses brain-derived neurotrophic factor transcription in astrocytes. Acta biochimica et biophysica Sinica. 2012:gmr125. doi: 10.1093/abbs/gmr125. [DOI] [PubMed] [Google Scholar]
- Wang X, Li X, Xu L, Zhan Y, Yaish-Ohad S, Erhardt JA, Barone FC, Feuerstein GZ. Up-regulation of secretory leukocyte protease inhibitor (SLPI) in the brain after ischemic stroke: adenoviral expression of SLPI protects brain from ischemic injury. Molecular pharmacology. 2003b;64:833–840. doi: 10.1124/mol.64.4.833. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Kobinger GP, Passini MA, Wilson JM, Wolfe JH. Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Molecular Therapy. 2002;5:528. doi: 10.1006/mthe.2002.0584. [DOI] [PubMed] [Google Scholar]
- Weinberg M, Blake B, Samulski RJ, Mccown TJ. The influence of epileptic neuropathology and prior peripheral immunity on CNS transduction by rAAV2 and rAAV5. Gene therapy. 2011;18:961–968. doi: 10.1038/gt.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann CM, Ferreira J, Keeler AM, Su Q, Qui L, Shaffer SA, Xu Z, Gao G, Sena-Esteves M. Systemic AAV9 gene transfer in adult GM1 gangliosidosis mice reduces lysosomal storage in CNS and extends lifespan. Human molecular genetics. 2015;24:4353–4364. doi: 10.1093/hmg/ddv168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Advanced drug delivery reviews. 2012;64:686–700. doi: 10.1016/j.addr.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C-F, Yin H, Borlongan CV, Chao J, Chao L. Adrenomedullin gene delivery protects against cerebral ischemic injury by promoting astrocyte migration and survival. Human gene therapy. 2004;15:1243–1254. doi: 10.1089/hum.2004.15.1243. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang T, Sun GY, Ding S. Specific disruption of astrocytic Ca 2+ signaling pathway in vivo by adeno-associated viral transduction. Neuroscience. 2010;170:992–1003. doi: 10.1016/j.neuroscience.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials. 2012;33:8167–8176. doi: 10.1016/j.biomaterials.2012.07.046. [DOI] [PubMed] [Google Scholar]
- Yang B, Li S, Wang H, Guo Y, Gessler DJ, Cao C, Su Q, Kramer J, Zhong L, Ahmed SS. Global CNS transduction of adult mice by intravenously delivered rAAVrh. 8 and rAAVrh. 10 and nonhuman primates by rAAVrh. 10. Molecular Therapy. 2014;22:1299–1309. doi: 10.1038/mt.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Yang W-H, Chen S-S, Ma B-F, Li B, Lu T, Qu T-Y, Klein RL, Zhao L-R, Duan W-M. Pre-immunization with an intramuscular injection of AAV9-human erythropoietin vectors reduces the vector-mediated transduction following re-administration in rat brain. PloS one. 2013;8:e63876. doi: 10.1371/journal.pone.0063876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D, Fong DM, Lawlor PA, Wu A, Mouravlev A, McRae M, Glass M, Dragunow M, During MJ. Adenosine kinase, glutamine synthetase and EAAT2 as gene therapy targets for temporal lobe epilepsy. Gene therapy. 2014;21:1029–1040. doi: 10.1038/gt.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurek D, Hasselrot U, Cass W, Sesenoglu-Laird O, Padegimas L, Cooper M. Age and lesion-induced increases of GDNF transgene expression in brain following intracerebral injections of DNA nanoparticles. Neuroscience. 2015;284:500–512. doi: 10.1016/j.neuroscience.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurek DM, Fletcher AM, Smith GM, Seroogy KB, Ziady AG, Molter J, Kowalczyk TH, Padegimas L, Cooper MJ. Long-term transgene expression in the central nervous system using DNA nanoparticles. Molecular Therapy. 2009;17:641–650. doi: 10.1038/mt.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R, Wang H, Mueller C, Sena-Esteves M, Brown R. Several rAAV vectors efficiently cross the blood brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Molecular Therapy. 2011;19:1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schlachetzki F, Zhang Y-F, Boado RJ, Pardridge WM. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Human gene therapy. 2004;15:339–350. doi: 10.1089/104303404322959498. [DOI] [PubMed] [Google Scholar]
- Zhao C, Strappe PM, Lever AM, Franklin RJ. Lentiviral vectors for gene delivery to normal and demyelinated white matter. Glia. 2003;42:59–67. doi: 10.1002/glia.10195. [DOI] [PubMed] [Google Scholar]
- Zheng JC, Huang Y, Tang K, Cui M, Niemann D, Lopez A, Morgello S, Chen S. HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1β and TNF-α: Involvement of mitogen-activated protein kinases and protein kinase R. Journal of neuroimmunology. 2008;200:100–110. doi: 10.1016/j.jneuroim.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinger A, Barcia C, Herrero MT, Guillemin GJ. The involvement of neuroinflammation and kynurenine pathway in Parkinson’s disease. Parkinson’s disease. 2011;2011 doi: 10.4061/2011/716859. [DOI] [PMC free article] [PubMed] [Google Scholar]