Abstract
Extensive work over the past few decades has shown that certain genetic variations interact with life events to confer increased susceptibility for the development of psychological disorders. The deletion variant of the ADRA2B gene, which has been associated with enhanced emotional memory and heightened amygdala responses to emotional stimuli, might confer increased susceptibility for the development of post-traumatic stress disorder (PTSD) or related phenotypes by increasing the likelihood of traumatic memory formation. Thus, we examined whether this genetic variant would predict stress effects on learning and memory in a non-clinical sample. Two hundred and thirty-five individuals were exposed to the socially evaluated cold pressor test or a control condition immediately or 30 min prior to learning a list of words that varied in emotional valence and arousal level. Participants’ memory for the words was tested immediately (recall) and 24 h after learning (recall and recognition), and saliva samples were collected to genotype participants for the ADRA2B deletion variant. Results showed that stress administered immediately before learning selectively enhanced long-term recall in deletion carriers. Stress administered 30 min before learning impaired recognition memory in male deletion carriers, while enhancing recognition memory in female deletion carriers. These findings provide additional evidence to support the idea that ADRA2B deletion variant carriers retain a sensitized stress response system, which results in amplified effects of stress on learning and memory. The accumulating evidence regarding this genetic variant implicates it as a susceptibility factor for traumatic memory formation and PTSD-related phenotypes.
Keywords: stress, learning, memory, ADRA2B, polymorphism, Cortisol
1. Introduction
Not everyone exposed to trauma develops post-traumatic stress disorder (PTSD), suggesting that genetic variation, coupled with differential peri-traumatic physiological and behavioral responses to the trauma, might influence one’s susceptibility to develop PTSD. One possibility is that a particular genotype predisposes an individual to have an attentional bias for threat and to form a stronger, potentially intrusive, memory of the traumatic experience (Amstadter, Nugent, and Koenen, 2009; Skelton, Ressler, Norrholm, Jovanovic, and Bradley-Davino, 2012; Wilker, Elbert, and Kolassa, 2014). A candidate gene variant that has been associated with emotional memory formation and PTSD phenotypes is the Glu301-Glu303 deletion variant of the ADRA2B gene, which codes for the α2b-adrenergic receptor. Although this variant is associated with both agonistic and antagonistic effects in vitro (Small, Brown, Forbes, and Liggett, 2001), researchers have speculated that it results in greater norepinephrine availability during emotional events (Rasch, Spalek, Buholzer, Luechinger, Boesiger, Papassotiropoulos, and de Quervain, 2009), a physiological condition associated with enhanced learning and memory (McGaugh, 2004). In healthy individuals, carriers of the ADRA2B deletion variant exhibit enhanced perception and memory of emotional stimuli (de Quervain, Kolassa, Ertl, Onyut, Neuner, Elbert, and Papassotiropoulos, 2007; Mammarella, Fairfield, Di Domenico, D’Onofrio, Stuppia, and Gatta, 2016; Todd, Muller, Lee, Robertson, Eaton, Freeman, Palombo, Levine, and Anderson, 2013), as well as greater amygdala activity during the encoding of emotionally arousing information (Cousijn, Rijpkema, Qin, van Marle, Franke, Hermans, van Wingen, and Fernandez, 2010; Rasch et al., 2009), which, in some cases, has been selectively reported following stress (Li, Weerda, Milde, Wolf, and Thiel, 2015). Although there are no studies showing a greater incidence of PTSD in deletion carriers, research has shown that the deletion variant is associated with greater intrusiveness of traumatic memories in Rwandan Civil War survivors (de Quervain et al., 2007), and the significant amount of work revealing exaggerated noradrenergic activity in PTSD (Skelton et al., 2012; Strawn and Geracioti, 2008; Zoladz and Diamond, 2013) presents the possibility of a connection between the ADRA2B deletion variant and PTSD-like phenotypes that warrants further investigation.
Our laboratory has been examining pre-learning stress effects on long-term memory to better understand factors, such as the ADRA2B deletion variant, that might influence susceptibility for emotional memory formation (e.g., Zoladz, Kalchik, Hoffman, Aufdenkampe, Lyle, Peters, Brown, Cadle, Scharf, Dailey, Wolters, Talbot, and Rorabaugh, 2014b). The effects of pre-learning stress on long-term memory are complex and depend on multiple factors. For instance, several researchers have shown that pre-learning stress enhances memory for emotional information, while impairing or having no effect on memory for neutral information(Jelicic, Geraerts, Merckelbach, and Guerrieri, 2004; Payne, Jackson, Hoscheidt, Ryan, Jacobs, and Nadel, 2007; Payne, Jackson, Ryan, Hoscheidt, Jacobs, and Nadel, 2006). Additionally, Wolf (2012) revealed that pre-learning stress effects on long-term memory for emotional information are abolished when an immediate recall test is used. Our focus has been on work showing that pre-learning stress effects on long-term memory depend on the temporal relationship between stress and learning (Diamond, Campbell, Park, Halonen, and Zoladz, 2007; Joels, Fernandez, and Roozendaal, 2011; Schwabe, Joels, Roozendaal, Wolf, and Oitzl, 2012; Zoladz, Park, and Diamond, 2011b). When a brief stressor is administered immediately before learning, long-term memory is generally enhanced (e.g., Diamond et al., 2007; Quaedflieg, Schwabe, Meyer, and Smeets, 2013; Vogel and Schwabe, 2016; Zoladz, Clark, Warnecke, Smith, Tabar, and Talbot, 2011a; Zoladz et al., 2014b). However, when the same stressor is temporally separated from learning (e.g., by 30 min), long-term memory is generally impaired (e.g., Quaedflieg et al., 2013; Zoladz et al., 2011a; Zoladz, Warnecke, Woelke, Burke, Frigo, Pisansky, Lyle, and Talbot, 2013). Investigators have contended that these time-dependent effects of pre-learning stress are attributable to a biphasic influence of stress-induced amygdala activation on hippocampal synaptic plasticity, as well as the temporal profiles of stress-induced noradrenergic and corticosteroid activity (Akirav and Richter-Levin, 1999; 2002; Diamond et al., 2007; Joels et al., 2011; Schwabe et al., 2012). Specifically, brief stress experienced immediately before learning enhances long-term memory via the rapid increase in norepinephrine and non-genomic effects of slowly rising corticosteroids exerting excitatory influences on hippocampal synaptic plasticity. In contrast, stress that is temporally separated from learning results in long-term memory impairment due to rising corticosteroid levels exerting gene-dependent, inhibitory influences on hippocampal function. It is our hypothesis that genetic factors might influence this temporal relationship between stress and learning, thus making some individuals more susceptible to stress-induced enhancements or impairments of long-term memory.
Given the association between noradrenergic neurotransmission and stress effects on learning and memory, genetic variants that alter noradrenergic activity could influence stress-memory interactions. Thus, in previous work, we examined the influence of the ADRA2B deletion variant on the effects of immediate pre-learning stress on long-term memory, with the prediction that deletion carriers would be more susceptible to stress-induced enhancements of long-term memory (Zoladz et al., 2014b). We found that the influence of stress on long-term memory was dependent on both genotype and sex. Stressed female, but not male, deletion carriers exhibited enhanced long-term recognition memory, relative to all other groups, and this effect was most pronounced in stressed female deletion carriers exhibiting a robust heart rate response to the stressor. These findings provided evidence that the ADRA2B deletion variant influences susceptibility to stress-induced enhancements of long-term memory and that such an influence might be related to autonomic function. The purpose of the present study was to replicate and extend this work by examining the influence of the ADRA2B deletion variant on the time-dependent effects of pre-learning stress on long-term memory. Specifically, participants were stressed immediately or 30 min before learning a list of words and tested for their memory 24 h later. Based on our previous findings, we predicted that ADRA2B deletion carriers would be more sensitive to stress-induced enhancements, and potentially impairments, of long-term memory and that these effects might be, at least partly, dependent on sex.
2. Material and Methods
2.1. Participants
Two hundred and thirty-five healthy undergraduate students (97 males, 138 females; age: M = 19.84, SD = 1.57), predominantly Caucasian (88.51%), from Ohio Northern University volunteered to participate in the experiments. The overall sample size for each experiment was based on previous work reporting behavioral effects associated with the ADRA2B deletion variant (e.g., Gibbs, Naudts, Azevedo, and David, 2010; Li, Weerda, Guenzel, Wolf, and Thiel, 2013; Naudts, Azevedo, David, van Heeringen, and Gibbs, 2011; Zoladz et al., 2014b) and an a priori power analysis (G*Power 3.1.9.2; University of Kiel, Germany) indicating that in order to attain adequate power (i.e., 1 − β = 0.80) to detect small-to-moderate effect sizes (i.e., partial eta squared = 0.09) for the stress × genotype interactions, we would need a total sample of approximately 130–140 participants (or 260–280 participants across both experiments). Individuals were excluded from participating if they met any of the following conditions: diagnosis of Raynaud’s or peripheral vascular disease; presence of skin diseases, such as psoriasis, eczema or scleroderma; history of syncope or vasovagal response to stress; history of any heart condition or cardiovascular issues (e.g., high blood pressure); history of severe head injury; current treatment with psychotropic medications, narcotics, beta-blockers, steroids or any other medication that was deemed to significantly affect central nervous or endocrine system function; mental or substance use disorder; regular use of recreational drugs; regular nightshift work. Participants were asked to refrain from drinking alcohol or exercising extensively for 24 h prior to the experimental sessions; and, to refrain from eating or drinking anything but water for 2 h prior to the experimental sessions. All experimental procedures were approved by the Institutional Review Board at Ohio Northern University, carried out in accordance with the Declaration of Helsinki, and undertaken with the understanding and written consent of each participant. Participants were awarded class credit and $20 cash upon completion of the study.
2.2. Experimental procedures
All experimental procedures took place between 1000 and 1700 hours in an attempt to control for diurnal fluctuations in cortisol, and all participants were run through the experimental sessions individually. A timeline of all procedures can be found in Figure 1.
Figure 1.
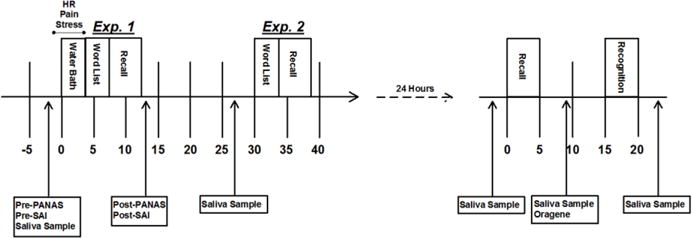
Timeline of the experimental procedures. On Day 1, participants placed their non-dominant hand in ice cold (stress) or warm (no stress) water for 3 min. Stressed participants were also led to believe that they were being videotaped throughout the water bath. Heart rate was continuously recorded throughout the water bath and commenced approximately 1 min prior to participants placing their hand in the water. Subjective pain and stress ratings of the water bath were collected at 1-min intervals. Immediately (Exp. 1) or 30 min (Exp. 2) following the water bath, participants were given a word list to learn, followed immediately by a free recall assessment. Measures of affect (PANAS) and anxiety (SAI) levels were administered before and after the water bath. Saliva samples were also collected before and after the water bath to assess changes in salivary cortisol levels. The next day, participants returned to the laboratory to complete free recall and recognition assessments, which were separated by 15 min. Saliva samples were collected before and after these assessments to assess changes in salivary cortisol levels. Between the two assessments, we collected a saliva sample via the Oragene kit in order to genotype participants for the ADRA2B deletion variant.
2.2.1. Socially Evaluated Cold Pressor Test (SECPT)
Following completion of a short demographics survey and the collection of baseline physiological and self-report measures (see below), participants were asked to submerge their non-dominant hand in a bath of water for 3 min. Participants who had been randomly assigned to the stress condition (Experiment 1: N = 59; 22 males, 37 females; Experiment 2: N = 61; 25 males, 36 females) placed their hand in a bath of ice cold (0–2°C) water, while participants who had been randomly assigned to the control condition (Experiment 1: N = 57; 24 males, 33 females; Experiment 2: N = 58; 26 males, 32 females) placed their hand in a bath of warm (35–37°C) water. The water was maintained at the appropriate temperature by a circulating water bath (Cole-Parmer; Vernon Hills, IL). If a participant found the water bath too painful, he or she was allowed to remove his or her hand from the water and continue with the experiment. Based on previous work (Schwabe, Haddad, and Schachinger, 2008), a social evaluative component was added to the cold pressor manipulation. Participants in the stress condition were misleadingly informed that they were being videotaped during the procedure for subsequent evaluation of their facial expressions, and throughout the water bath manipulation, they were asked to keep their eyes on a camera that was located on the wall of the laboratory.
2.2.2. Subjective and Objective Stress Response Measures
2.2.2.1. The Positive and Negative Affect Schedule (PANAS) and State Anxiety Inventory (SAI)
Immediately before and approximately 10 min after the water bath manipulation, participants completed the PANAS (Watson, Clark, and Tellegen, 1988) and the SAI (state portion of the State-Trait Anxiety Inventory) (Spielberger, Gorsuch, Lushene, Vagg, and Jacobs, 1983). This allowed for a pre-post analysis of stress-induced changes in affect and anxiety, respectively.
2.2.2.2. Subjective Pain and Stress Ratings
Participants rated the painfulness and stressfulness of the water bath at 1-min intervals on 11-point scales ranging from 0–10, with 0 indicating a complete lack of pain or stress and 10 indicating unbearable pain or stress.
2.2.2.3. Cardiovascular Analysis
Heart rate (HR) was measured continuously from approximately 1 min before the water bath until its completion via a BioNomadix pulse transducer (Biopac Systems, Inc.; Goleta, CA) placed on the ring finger of participants’ dominant hand. The pulse transducer was connected to the PPG module of the MP150 Biopac hardware. Average baseline HR (average of 1 min before water bath) and water bath HR (average of water bath) were calculated for statistical analyses.
2.2.2.4. Cortisol Analysis
On Day 1, saliva samples were collected from participants immediately before and 25 min after the water bath to analyze salivary cortisol levels. On Day 2, saliva samples were collected from participants immediately before and 25 min after the free recall assessment to analyze salivary cortisol levels. Saliva samples were collected in a Salivette saliva collection device (Sarstedt, Inc., Newton, NC). The samples were stored at −20°C until being thawed and extracted by low-speed centrifugation. Salivary cortisol levels were then determined by an investigator blind to the conditions of the participants via enzyme immunoassay (Cayman Chemical Co., Ann Arbor, MI; Product #500360) according to the manufacturer’s protocol. Cortisol concentrations were determined in duplicate from a standard curve (6.6, 16.4, 41, 102.4, 256, 640, 1600, and 4000 pg cortisol per ml), and the sensitivity of the assay was 35 pg/ml.
2.2.3. Learning and Memory Task
Immediately (Experiment 1) or 30 min (Experiment 2) following exposure to the water bath, participants were presented with a list of 42 words, which were selected from the Affective Norms for English Words (Bradley and Lang, 1999). Based on standardized valence and arousal ratings, we chose 14 neutral, 14 positive and 14 negative words (7 arousing and 7 non-arousing per category), which, across emotional valence and arousal categories, were balanced for word length and word frequency. As per previous methodology (Schwabe, Bohringer, Chatterjee, and Schachinger, 2008; Zoladz et al., 2011a; Zoladz, Kalchik, Hoffman, Aufdenkampe, Burke, Woelke, Pisansky, and Talbot, 2014a; Zoladz et al., 2014b; Zoladz et al., 2013), participants were instructed to read each word aloud and rate its emotional valence on a scale from −4 (very negative) to +4 (very positive) and its arousal level on a scale of 0 (not arousing) to 8 (very highly arousing), with the aid of self-assessment manikins, on a sheet of paper containing the list of words.
Immediately following word list encoding, participants were given 5 min to write down as many words as they could remember from the list of words they just studied (immediate recall). The next day, participants returned to the laboratory to have their memory for the list of words assessed. Participants were again given 5 min to write down as many words as they could remember from the list of words that they studied on the previous day (delayed recall). Fifteen minutes later, participants were given a recognition test. They were presented with a list of words containing 42 “old” words (i.e., words presented on the previous day) and 42 “new” words (i.e., words not presented on the previous day) and were instructed to label each word as “old” or “new.” The “new” words were matched to the “old” words on emotional valence, arousal level, word length and word frequency. To assess participants’ ability to discriminate between “old” and “new” words, we calculated a sensitivity index (d’ = z[p(hit)–p(false alarm)]) for each category of word to be used for statistical analysis (Wickens, 2002).
2.2.4. Genotyping
On Day 2, during the 15-min delay between free recall and recognition testing, a saliva sample was collected from participants via the OGR-500 Oragene (DNA Genotek, Inc.; Ottawa, ON, Canada). The sample was stored at room temperature, until shipped to DNA Genotek, Inc. for genotyping of the deletion variant in the ADRA2B gene. DNA was extracted from 700 μL of saliva, and quantity and quality control procedures were performed before undergoing TaqMan® assay with PCR amplification for genotype. Primers and probes were obtained through Life Technologies, Inc. (Foster City, CA), and the call rate for the polymorphism was 100%.
2.3. Statistical Analyses
Based on previous work (Cousijn et al., 2010; de Quervain et al., 2007; Li et al., 2013; Li et al., 2015; Rasch et al., 2009; Zoladz et al., 2014b), participants were divided into ADRA2B deletion carriers (homozygous or heterozygous for deletion variant) and non-carriers (wild type) for statistical analyses. The cell sample sizes for the Stress × Sex × Genotype interaction can be found in Table 1. In Experiment 1, 39 of the 70 female participants reported using some form of oral contraceptive, and in Experiment 2, 28 of the 68 female participants reported using some form of oral contraceptive. Preliminary analyses revealed that females who reported taking oral contraceptives were not significantly different from naturally cycling females on any physiological or memory measure, nor did stress or genotype significantly interact with oral contraceptive use in these analyses. Therefore, we treated female participants as a single group in our analyses. All data were analyzed with mixed-model ANOVAs; the between-subjects factors utilized in these analyses were stress, genotype, and sex, and the within-subjects factors were valence and arousal (for recall and recognition data) or time point [for physiological (heart rate, cortisol) and self-report (affect, anxiety, pain/stress ratings) data]. Alpha was set at .05 for all analyses, and Bonferroni-corrected post hoc tests were employed when the omnibus F indicated the presence of a significant effect. For simplicity, we primarily report significant or borderline significant effects and mention non-significant effects only when relevant to the major variables of interest (e.g., stress, genotype).
Table 1.
Sample Sizes for the Stress × Sex × Genotype Interactions
| Stress | No Stress | Total | |
|---|---|---|---|
| Experiment 1 | |||
| Deletion carriers | |||
| Male | 10 | 15 | 25 |
| Female | 20 | 17 | 37 |
|
| |||
| Total | 30 | 32 | 62 |
| Deletion non-carriers | |||
| Male | 12 | 9 | 21 |
| Female | 17 | 16 | 33 |
|
| |||
| Total | 29 | 25 | 54 |
| Total | |||
| Male | 22 | 24 | 46 |
| Female | 37 | 33 | 70 |
|
| |||
| Total | 59 | 57 | 116 |
| Experiment 2 | |||
| Deletion carriers | |||
| Male | 17 | 17 | 34 |
| Female | 21 | 16 | 37 |
|
| |||
| Total | 38 | 33 | 71 |
| Deletion non-carriers | |||
| Male | 8 | 9 | 17 |
| Female | 15 | 16 | 31 |
|
| |||
| Total | 23 | 25 | 48 |
| Total | |||
| Male | 25 | 26 | 51 |
| Female | 36 | 32 | 68 |
|
| |||
| Total | 61 | 58 | 119 |
3. Results
3.1. Experiment 1: Stress Immediately before Learning
3.1.1. Genotype Characteristics
Chi-square goodness-of-fit analyses revealed that there was no significant deviation from the Hardy-Weinberg equilibrium for the ADRA2B deletion variant genotype (χ2(1, N = 116) = 2.82, p = 0.09).
3.1.2. Subjective and Objective Stress Response Measures
3.1.2.1. PANAS and SAI
Neither stress nor genotype had any significant influence on positive or negative affect or self-reported anxiety levels (Table 2).
Table 2.
Pre-Post Changes (± SEM) in Day 1 Heart Rate, Affect, and Anxiety and Day 2 Cortisol
| Measure/Condition | Experiment 1 | Experiment 2 | ||
|---|---|---|---|---|
| Pre | Post/During (HR) | Pre | Post/During (HR) | |
| Day 1 Heart Rate (bpm) | ||||
| Stress | ||||
| Carriers | 76.17 (3.38) | 92.20 (3.89)* | 75.13 (2.90) | 99.69 (2.74)* |
| Non-carriers | 72.75 (3.26) | 96.84 (3.76)* | 72.34 (3.89) | 92.78 (3.68)* |
| No Stress | ||||
| Carriers | 77.64 (3.07) | 85.43 (3.53) | 73.85 (3.10) | 81.30 (2.93) |
| Non-carriers | 78.86 (3.61) | 83.76 (4.15) | 74.37 (3.70) | 81.62 (3.50) |
| Day 1 Negative Affect (PANAS) | ||||
| Stress | ||||
| Carriers | 14.23 (0.78) | 14.88 (0.83) | 13.83 (0.55) | 17.15 (0.67)* |
| Non-carriers | 13.25 (0.76) | 12.99 (0.81) | 13.38 (0.74) | 18.10 (0.90)* |
| No Stress | ||||
| Carriers | 14.65 (0.71) | 14.40 (0.76) | 13.74 (0.59) | 11.83 (0.72) |
| Non-carriers | 13.39 (0.83) | 13.72 (0.90) | 13.98 (0.70) | 11.17 (0.86) |
| Day 1 Anxiety (SAI) | ||||
| Stress | ||||
| Carriers | 35.60 (1.92) | 40.30 (2.01) | 34.85 (1.14) | 46.86 (1.40)* |
| Non-carriers | 34.78 (1.87) | 37.90 (1.96) | 34.50 (1.53) | 47.15 (1.86)* |
| No Stress | ||||
| Carriers | 36.73 (1.75) | 37.08 (1.84) | 35.45 (1.22) | 32.57 (1.49) |
| Non-carriers | 32.21 (2.06) | 37.08 (2.17) | 33.76 (1.45) | 30.26 (1.79) |
| Day 2 Salivary Cortisol (nmol/l) | ||||
| Stress | ||||
| Carriers | 5.11 (0.57) | 5.24 (0.49) | 5.35 (0.49) | 5.74 (0.45) |
| Non-carriers | 5.79 (0.56) | 6.02 (0.48) | 5.13 (0.64) | 5.29 (0.58) |
| No Stress | ||||
| Carriers | 5.52 (0.53) | 5.59 (0.45) | 6.05 (0.51) | 6.29 (0.46) |
| Non-carriers | 4.66 (0.62) | 5.80 (0.53) | 4.78 (0.64) | 4.58 (0.58) |
p < 0.05 relative to no stress
3.1.2.2. Subjective Pain and Stress Ratings
Stressed participants rated the water bath as more painful (effect of stress: F(1,108) = 350.36) and more stressful (effect of stress: F(1,108) = 230.79) than controls (p’s < 0.001). Stressed females also rated than water bath as more painful (Stress × Sex interaction: F(1,108) = 5.18) and more stressful (Stress × Sex interaction: F(1,108) = 6.27) than stressed males (p’s < 0.05).
3.1.2.3. Heart Rate
Stressed participants exhibited significantly greater HR following the water bath, relative to controls (effect of stress: F(1,107) = 4.02; Stress × Time Point interaction: F(1,107) = 9.41; p’s < 0.05; Table 2).
3.1.2.4. Cortisol
On Day 1, stressed participants exhibited significantly greater salivary cortisol levels following the water bath, relative to controls (effect of stress: F(1,107) = 23.58; effect of Time Point: F(1,107) = 36.76; Stress × Time Point interaction: F(1,107) = 43.03; p’s < 0.001) (Figure 2a). No significant differences emerged for Day 2 salivary cortisol levels (Table 2)
Figure 2.
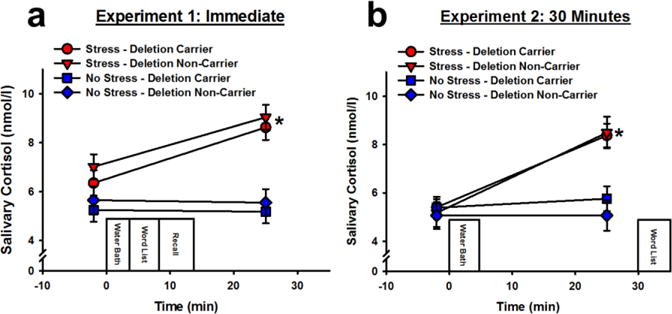
Salivary cortisol concentrations before and after stress exposure in Experiment 1 (a) and Experiment 2 (b). Stressed participants, independent of ADRA2B genotype, exhibited significantly greater salivary cortisol levels than controls following the water bath manipulation. Data are presented as means ± SEM. * p < 0.001 relative to no stress.
3.1.3. Valence and Arousal Ratings of Learned Words
3.1.3.1. Valence Ratings
As expected, participants rated negative words more negatively than neutral words, which were rated more negatively than positive words (effect of valence: F(2,216) = 1804.63, p < 0.001; Table 3). Stressed deletion carriers rated positive and neutral words more negatively than stressed non-carriers, while non-stressed deletion carriers rated negative words more negatively than non-stressed non-carriers (Stress × Genotype × Valence interaction: F(2,216) = 5.66,p < 0.01). Participants also rated arousing words more negatively than non-arousing words (effect of arousal: F(1,108) = 60.62, p < 0.001), and females rated arousing words more negatively than males (Sex × Arousal interaction: F(1,108) = 16.15, p < 0.001). Interestingly, ADRA2B deletion carriers rated words more negatively than non-carriers (effect of genotype: F(1,108) = 5.19, p < 0.05). This effect appeared to be driven by stressed deletion carriers rating the words more negatively than all others group, despite the Stress × Genotype interaction not being significant, F(1,108) = 2.98, p = 0.087.
Table 3.
Valence and Arousal Ratings (± SEM) for Each Word Category
| Word Category | Experiment 1 | Experiment 2 | ||
|---|---|---|---|---|
| Valence | Arousal | Valence | Arousal | |
| Positive, arousing words Ex: intercourse |
2.53 (0.07) | 4.78 (0.13) | 2.46 (0.08) | 4.44 (0.14) |
| Positive, non-arousing words Ex: carefree |
2.42 (0.07) | 3.69 (0.15) | 2.33 (0.08) | 3.32 (0.14) |
| Negative, arousing words Ex: murderer |
−3.00 (0.07) | 3.42 (0.22) | −2.81 (0.08) | 3.16 (0.20) |
| Negative, non-arousing words Ex: useless |
−2.62 (0.07) | 2.43 (0.18) | −2.55 (0.08) | 2.05 (0.15) |
| Neutral, arousing words Ex: lightning |
−1.05 (0.07) | 2.67 (0.15) | −0.80 (0.08) | 2.34 (0.13) |
| Neutral, non-arousing words Ex: icebox |
−0.04 (0.04) | 1.09 (0.11) | 0.07 (0.04) | 0.87 (0.08) |
3.1.3.2. Arousal Ratings
As expected, arousing words were given higher arousal ratings than non-arousing words (effect of arousal: F(1,108) = 199.19, p < 0.001; Table 3). Participants rated positive words as more arousing than negative words, which were rated as more arousing than neutral words (effect of valence: F(2,216) = 107.12, p < 0.001). Females rated words as more arousing than males (effect of sex: F(1,108) = 5.14, p < 0.05).
3.1.4. Memory Testing
3.1.4.1. Immediate Recall
Participants recalled more positive and negative words than neutral words (effect of valence: F(2,216) = 34.23, p < 0.001). They also recalled more arousing words than non-arousing words, particularly male participants and when the words were positive (effect of arousal: F(1,108) = 66.60; Sex × Arousal interaction: F(1,108) = 8.84; Valence × Arousal interaction: F(2,216) = 61.29; p’s < 0.05). There was no significant effect of stress or genotype on immediate recall (Figure 3a).
Figure 3.
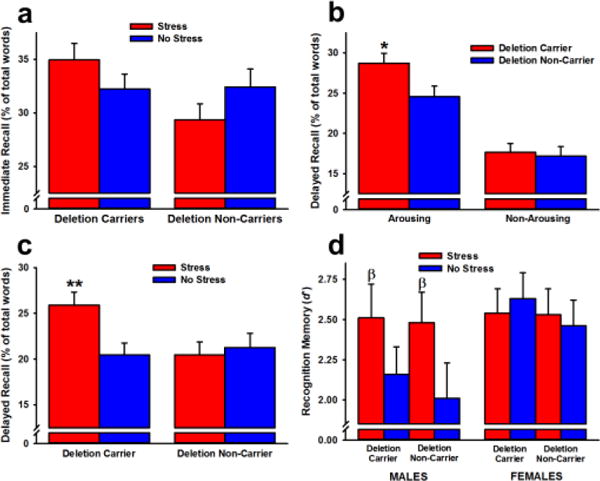
Immediate recall (a), delayed recall (b, c), and recognition memory (d) performance in Experiment 1. No significant effects of stress or genotype were observed for immediate recall (a). ADRA2B deletion carriers recalled more arousing words than non-carriers 24 h following learning (b). Stress immediately before learning selectively enhanced delayed recall in deletion carriers (c). No significant effects of stress or genotype were observed for recognition memory (d). Data are expressed as means ± SEM. * p < 0.05 relative to arousing words recalled by deletion non-carriers; ** p < 0.05 relative to all other groups; βp < 0.10 relative to non-stressed males.
3.1.4.2. Delayed Recall
Twenty-four hours following learning, participants recalled more positive and negative words than neutral words (effect of valence: F(2,216) = 12.27, p < 0.001). They also recalled more arousing words than non-arousing words, particularly when the words were positive (effect of arousal: F(1,108) = 91.09; Valence × Arousal interaction: F(2,216) = 63.38; p’s < 0.001). There was a trend suggesting that deletion carriers recalled more arousing words than non-carriers (Genotype × Arousal interaction: F(1,108) = 3.62, p = 0.06). Because of an a priori prediction consistent with this finding and based on previous work (de Quervain et al., 2007), we performed a planned comparison to compare the recall of arousing words in deletion carriers and non-carriers, which revealed that carriers recalled more arousing words than non-carriers (t(57) = 2.57, p = 0.013) (Figure 3b). The effect of stress depended on genotype (Stress × Genotype interaction: F(1,108) = 4.80, p < 0.05). Specifically, stress enhanced long-term recall in deletion carriers, but had no effect on non-carriers (Figure 3c).
3.1.4.3. Recognition Memory
Participants recognized more positive and neutral words than negative words, and females recognized more positive words than males (effect of valence: F(2,216) = 43.36; Sex × Valence interaction: F(2,216) = 3.95; p’s < 0.05). They also recognized more arousing words than non-arousing words, particularly when they were negative or neutral in valence (effect of arousal: F(1,108) = 34.00; Valence × Arousal interaction: F(2,216) = 4.97, p’s < 0.01). Females recognized more words than males (effect of sex: F(1,108) = 3.92, p = 0.05). There was also a trend for the Stress × Sex interaction, F(1,108) = 2.83, p = 0.096, suggesting that stress tended to enhance recognition memory in males but not females (Figure 3d).
3.2. Experiment 2: Stress 30 Minutes before Learning
3.2.1. Genotype Characteristics
Chi-square goodness-of-fit analyses revealed that there was no significant deviation from the Hardy-Weinberg equilibrium for ADRA2B deletion variant genotype [χ2(1, N = 119) = 0.54, p = 0.46].
3.2.2. Subjective and Objective Stress Response Measures
3.2.2.1. PANAS and SAI
Stressed participants reported greater negative affect (effect of stress: F(1,111) = 20.23; Stress × Time Point interaction: F(1,111) = 102.99) and anxiety (effect of stress: F(1,111) = 35.05; Stress × Time Point interaction: F(1,111) = 117.12) following the water bath, relative to controls (p’s < 0.001; Table 2). Stress had no significant influence on positive affect.
3.2.2.2. Subjective Pain and Stress Ratings
Stressed participants rated the water bath as more painful (effect of stress: F(1,111) = 555.24) and more stressful (effect of stress: F(1,111) = 387.96) than controls (p’s < 0.001).
3.2.2.3. Heart Rate
Stressed participants exhibited significantly greater HR following the water bath, relative to controls (effect of stress: F(1,111) = 7.71; Stress × Time Point interaction: F(1,111) = 29.66; p’s < 0.01; Table 2).
3.2.2.4. Cortisol
On Day 1, stressed participants exhibited significantly greater salivary cortisol levels following the water bath, relative to controls (effect of stress: F(1,110) = 9.54; effect of Time Point: F(1,110) = 61.78; Stress × Time Point interaction: F(1,110) = 48.66; p’s < 0.001; Figure 2b). No significant differences emerged for Day 2 salivary cortisol levels (Table 2).
3.2.3. Valence and Arousal Ratings of Learned Words
3.2.3.1. Valence Ratings
As expected, negative words were rated more negatively than neutral words, which were rated more negatively than positive words (effect of valence: F(2, 222) = 1348.98, p < 0.001; Table 3). The significant Stress × Genotype × Valence interaction observed in Experiment 1 was only borderline significant in Experiment 2, this time suggesting stressed deletion carriers rated negative words more negatively than stressed non-carriers, F(2,222) = 2.89, p = 0.058. Arousing words were rated more negatively than non-arousing words (effect of arousal: F(1,111) = 47.34, p < 0.001), and females rated arousing words more negatively than males (Sex × Arousal interaction: F(1,111) = 13.75, p < 0.001). Again, ADRA2B deletion carriers rated words more negatively than non-carriers, but in Experiment 2, this effect was not significant, F(1,111) = 2.13, p = 0.15.
3.2.3.2. Arousal Ratings
As expected, arousing words were given higher arousal ratings than non-arousing words (effect of arousal: F(1,111) = 259.85, p < 0.001; Table 3). Similar to Experiment 1, positive words were rated as more arousing than negative words, which were rated as more arousing than neutral words (effect of valence: F(2,222) = 106.85, p < 0.001). Females once again rated words as more arousing than males, but the effect was not significant in this experiment (effect of sex: F(1,111) = 0.22, p = 0.64).
3.2.4. Memory Testing
3.2.4.1. Immediate Recall
Participants recalled more positive and negative words than neutral words (effect of valence: F(2,222) = 39.59,p < 0.001). They also recalled more arousing words than non-arousing words, particularly when the words were positive (effect of arousal: F(1,111) = 97.05; Valence × Arousal interaction: F(2,222) = 52.52; p’s < 0.001). Under non-stressed conditions, deletion carriers exhibited poorer immediate recall than non-carriers (Stress × Genotype interaction: F(1,111) = 4.18) (Figure 4a), and stressed males exhibited impaired immediate recall relative to non-stressed males and stressed females (Stress × Sex interaction: F(1,111) = 5.42; p’s < 0.05) (Figure 4b).
Figure 4.
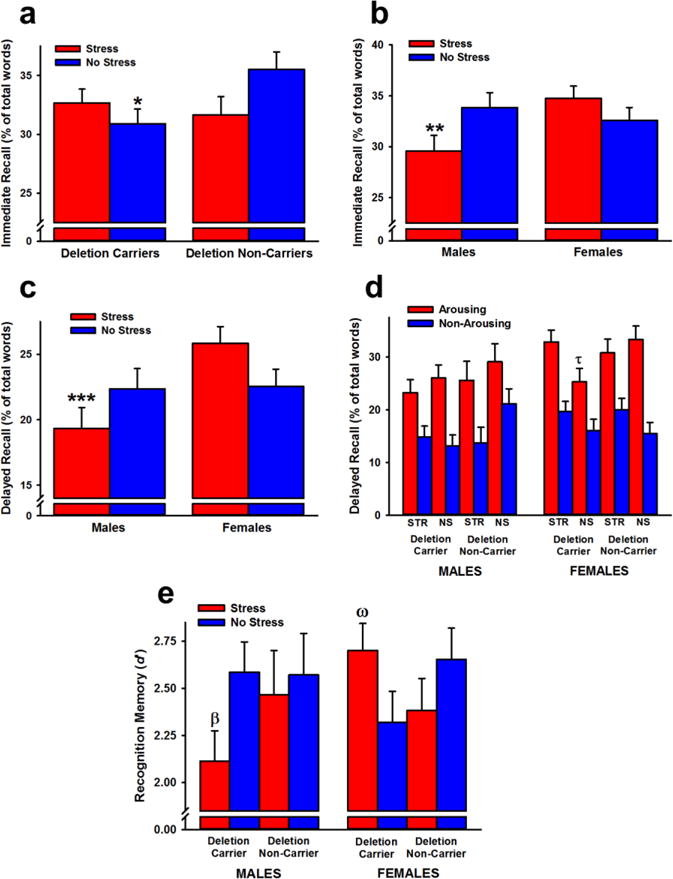
Immediate recall (a, b), delayed recall (c, d), and recognition memory (e) performance in Experiment 2. ADRA2B deletion carriers exhibited poorer immediate recall than non-carriers (a), and stress 30 min before learning selectively impaired immediate recall in males (b). Stress also impaired delayed recall in males (c) and led to superior recall of arousing words in female deletion carriers (d). Stress impaired recognition memory in male deletion carriers and resulted in greater recognition memory in female deletion carriers (e). Data are expressed as means ± SEM. *p < 0.05 relative to non-stressed deletion non-carriers; ** p < 0.05 relative to stressed females and non-stressed males; *** p < 0.05 relative to stressed females; τp < 0.05 relative to stressed female deletion carriers and non-stressed female deletion non-carriers; β p < 0.05 relative to non-stressed male deletion carriers and stressed female deletion carriers; ω p < 0.05 relative to non-stressed female deletion carriers and stressed male deletion carriers.
3.2.4.2. Delayed Recall
Twenty-four hours following learning, participants recalled more positive and negative words than neutral words (effect of valence: F(2,222) = 35.99, p < 0.001). They also recalled more arousing words than non-arousing words, particularly when the words were positive (effect of arousal: F(1,111) = 108.64; Valence × Arousal interaction: F(2,222) = 58.91; p’s < 0.001). Stressed males recalled fewer words than stressed females (Stress × Sex interaction: F(1,111) = 4.86, p < 0.05) (Figure 4c). Stress enhanced recall of arousing words selectively in female deletion carriers (Stress × Sex × Genotype × Arousal interaction: F(1,111) = 4.83,p < 0.05) (Figure 4d).
3.2.4.3. Recognition Memory
Participants recognized more positive and neutral words than negative words (effect of valence: F(2,222) = 20.88, p < 0.001). They also recognized more arousing words than non-arousing words, particularly when they were negative or neutral in valence (effect of arousal: F(1,111) = 30.72; Valence × Arousal interaction: F(2,222) = 5.17, p’s < 0.01). Stress impaired recognition memory in male deletion carriers, while enhancing recognition in female deletion carriers (Stress × Sex × Genotype interaction: F(1,111) = 4.04, p < 0.05; Figure 4e).
4. Discussion
Previous work has shown that the ADRA2B deletion variant is associated with enhanced emotional memory, heightened amygdala responses to emotional stimuli and greater intrusiveness of traumatic memories (Cousijn et al., 2010; de Quervain et al., 2007; Li et al., 2015; Mammarella et al., 2016; Rasch et al., 2009; Todd et al., 2013). We have extended this area of research by demonstrating that the ADRA2B deletion variant influences pre-learning stress effects on long-term memory in a time- and sex-dependent manner. Consistent with our hypothesis, stress administered immediately before learning enhanced long-term recall in deletion carriers only. Interestingly, when stress was temporally separated from learning, the effects were again genotype-dependent. Specifically, stress impaired recognition memory in male deletion carriers, while producing superior recognition memory in female deletion carriers. These findings provide important insight into genetic factors influencing the association between stress and emotional memory formation, which may aid our understanding of susceptibility to the development of traumatic memories.
4.1. Effects of Immediate Pre-Learning Stress
Previous work examining the time-dependent effects of stress on learning and memory has shown that brief stressors administered immediately before learning enhance long-term memory. For recognition memory, we observed a trend for the Stress × Sex interaction, suggesting that stress led to better performance in males, but not females. This trend appeared to be driven largely by poorer recognition memory in non-stressed males, as compared to non-stressed females. Most importantly, stress administered immediately before learning selectively enhanced long-term recall in ADRA2B deletion carriers. This finding is consistent with the working hypothesis that deletion carriers respond to stress with greater noradrenergic and amygdala activity, thus resulting in a stronger excitatory influence on cognitive processes (Cousijn et al., 2010; Rasch et al., 2009). Extensive work has shown that the interaction between norepinephrine and other neurochemicals in the amygdala and hippocampus underlies stress-induced enhancements of long-term memory (McGaugh and Roozendaal, 2009). However, our finding in Experiment 1 is somewhat inconsistent with our previously published work (Zoladz et al., 2014b). In our previous study, we found that the selective effects of immediate pre-learning stress in deletion carriers were limited to long-term recognition memory in females. Here, we observed a selective enhancement of stress in deletion carriers for recall in males and females combined. It is important to note that we used a slightly different stressor in our previous study (cold pressor without a social evaluative component) and included females taking oral contraceptives in the present study, which could have contributed to our different results. It is also important to point out that our finding in Experiment 2 showing that stress administered 30 min prior to learning led to better long-term recognition memory in female deletion carriers is consistent with our previous work. Thus, the notion that this deletion variant exerts sex-dependent influences on stress-memory interactions is still supported by our work overall.
We also observed greater recall of arousing words in ADRA2B deletion carriers in Experiment 1, which is consistent with the original research on this genetic variant’s association with emotional memory (de Quervain et al., 2007). However, we did not observe this effect in our previous study or in Experiment 2. Importantly, the inconsistency in observing this effect is not uncommon (Gibbs et al., 2010; Li et al., 2013; Naudts et al., 2011) and may have to do with the different types of learning stimuli (e.g., words vs. faces vs. photographs) being used or limited statistical power to detect such an effect.
The overall effects observed in Experiment 1, combined with previous work from our laboratory and that of others, support the notion that carriers of the ADRA2B deletion variant have a sensitized stress response system, resulting in more pronounced effects on cognitive processes. In theory, these individuals respond to stress with greater noradrenergic and amygdala activity than normal, which would result in greater stress-induced enhancements of long-term memory. This is consistent with previous work showing that noradrenergic neurotransmission and autonomic nervous system activity are important components to stress-induced enhancements of cognitive processing and suggests that deletion carriers may be more susceptible to emotional memory formation.
4.2. Effects of Delayed Pre-Learning Stress
Consistent with our previous work (Zoladz et al., 2013), stress administered 30 min before learning impaired long-term recall in males, but not females. Interestingly, stress exerted differential effects on long-term recognition memory in male and female deletion carriers. Specifically, the stress-induced impairment of recognition memory was selective to male deletion carriers, a finding that is consistent with previous work (Li et al., 2013), despite differences in methodology. In sharp contrast, stress led to superior recall and recognition memory in female deletion carriers. The finding that stress temporally separated from learning selectively influenced long-term memory in deletion carriers, without influencing non-carriers, is novel and reveals that the sensitized stress response system supposedly existent in deletion carriers can influence encoding processes relatively long after the onset of a stressor.
Our laboratory (e.g., Zoladz et al., 2011a; Zoladz et al., 2014a; Zoladz et al., 2014b; Zoladz, Peters, Kalchik, Hoffman, Aufdenkampe, Woelke, Wolters, and Talbot, 2014c; Zoladz et al., 2013), as well as that of others (e.g., Diamond et al., 2007; Joels et al., 2011; Schwabe et al., 2012), has speculated that, under normal circumstances, stress that is temporally separated from learning results in long-term memory impairment. Interestingly, we did not observe such an effect in females previously (Zoladz et al., 2013), and in the present study, females with the ADRA2B deletion variant exhibited superior memory following stress. A significant amount of work has shown that stress sex-dependently influences learning and memory. For instance, multiple studies have reported significant effects of stress on learning and memory in males, while observing no effects or opposite effects in females (Andreano and Cahill, 2006; Jackson, Payne, Nadel, and Jacobs, 2006; Payne et al., 2006; Wolf, Schommer, Hellhammer, McEwen, and Kirschbaum, 2001; Zorawski, Blanding, Kuhn, and LaBar, 2006). In addition, females have been reported to be more sensitive to stress- and arousal-induced enhancements of memory- or attention-related processes (Felmingham, Tran, Fong, and Bryant, 2012; Schwabe, Hoffken, Tegenthoff, and Wolf, 2013). Some of these studies have reported effects that mirror those reported here — that is, stress resulted in greater memory or attentional processes in females, while impairing them in males (Payne et al., 2006; Schwabe et al., 2013). Our results differ in one important way; they suggest that the differential effects of stress on learning and memory in males versus females also depend on ADRA2B genotype.
According to our results, the ADRA2B deletion variant might amplify the delayed effects of stress that is temporally removed from learning. In males, this led to a selective impairment of recognition memory; whereas in females, it resulted in superior recognition memory. It is important to note, however, that the superior recognition memory observed in female deletion carriers appeared to be driven largely by lower performance in non-stressed females. Still, the sex differences observed in the present study support our previous speculation that the temporal dynamics of stress effects on learning and memory may be sex-dependent (Zoladz et al., 2014c; Zoladz et al., 2013). That is to say, instead of pre-learning stress that is temporally separated from the learning experience exerting deleterious influences on long-term memory, we have now reported on two separate occasions that females exposed to such stress are either unaffected or exhibit superior memory. Thus, females, especially those carrying the ADRA2B deletion variant, may be more likely to exhibit enhancements of learning and memory consolidation long after the initiation of stress. The female immunity to pre-learning stress-induced impairments of long-term memory, along with the finding of superior long-term recognition memory in female deletion carriers reported here, could be related to differential encoding of the learning material. Indeed, in the present work, females in each study rated the studied words as more negative and/or more arousing than males. Because emotionally arousing material is better remembered than non-emotional material, a greater perception of emotional arousal in the learned material could result in greater stress effects on memory in females. Ultimately, our findings could help explain why females are at significantly greater risk for traumatic memory formation and PTSD development (Tolin and Foa, 2006).
4.3. Limitations
The present experiments did have some limitations worth noting. A priori power analyses indicated that we required 130–140 participants per experiment to have adequate statistical power (1−β = 0.80) to detect effect sizes of η2p = 0.09 for the Stress × Genotype interaction. However, our sample size in each experiment was slightly less than the required number of participants, thus resulting in reduced statistical power to detect such effects. Additionally, given our sample size, it is possible that we were unable to detect smaller effects. Although such issues certainly suggest that our findings are preliminary in nature and should be interpreted cautiously, it is worth pointing out that the present study is not the only one reporting significant Stress × Genotype interactions for the ADRA2B deletion variant (e.g., Li et al., 2013; Zoladz et al., 2014b) and further supports previous work in this area.
In the present experiments, we treated female participants as a single entity for data analysis, despite many of them reporting that they took some form of oral contraceptive. It is well-documented in the literature that oral contraceptive use significantly influences stress response systems, such as blunting the cortisol response to stress (Kajantie and Phillips, 2006; Kirschbaum, Kudielka, Gaab, Schommer, and Hellhammer, 1999; Kirschbaum, Pirke, and Hellhammer, 1995; Mordecai, Rubin, Eatough, Sundermann, Drogos, Savarese, and Maki, 2017; Nielsen, Segal, Worden, Yim, and Cahill, 2013; Roche, King, Cohoon, and Lovallo, 2013). There is also increasing evidence that oral contraceptives can influence emotional memory formation (Merz and Wolf, 2017; Nielsen, Ertman, Lakhani, and Cahill, 2011; Nielsen et al., 2013). Thus, even though we observed no significant differences between females reporting oral contraceptive use and naturally cycling females on physiological and memory measures, it is possible that oral contraceptives could have influenced our results.
As expected, stressed participants in Experiment 2 exhibited significant increases in negative affect and anxiety following the water bath manipulation; however, stressed participants in Experiment 1 did not exhibit these changes. This inconsistency across the two experiments was unexpected and complicates comparisons being made between the two studies. Nevertheless, stressed participants in Experiment 1 did exhibit significant stress-induced increases in salivary cortisol levels, heart rate, and subjective pain/stress ratings, which were comparable to those observed in stressed participants from Experiment 2.
Finally, because ethnicity is a well-defined confound in genotyping results, we should emphasize that our sample was largely (>88%) comprised of Caucasian individuals. This bias in participant ethnicity is attributable to the limited ethnic diversity of our population. Thus, readers should consider that the external validity of our findings is limited.
4.4. Conclusions
We have shown that carriers of the ADRA2B deletion variant are more sensitive to pre-learning stress-induced enhancements and impairments of long-term memory. We have also reported that female deletion carriers exhibit stress-induced enhancements of long-term memory independent of when the stressor is administered prior to learning. Our findings may provide insight into how this genetic variant increases one’s susceptibility to traumatic memory formation and, perhaps, the development of PTSD.
Highlights.
Stress immediately before learning enhanced memory in ADRA2B deletion carriers.
Stress 30 min before learning impaired memory in male ADRA2B deletion carriers.
Stress 30 min before learning enhanced memory in female ADRA2B deletion carriers.
ADRA2B deletion variant might predict susceptibility to traumatic memory formation.
Acknowledgments
Funding Source
The research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under award number R15MH104836. The National Institutes of Health had no further role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akirav I, Richter-Levin G. Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. J Neurosci. 1999;19:10530–10535. doi: 10.1523/JNEUROSCI.19-23-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Richter-Levin G. Mechanisms of amygdala modulation of hippocampal plasticity. J Neurosci. 2002;22:9912–9921. doi: 10.1523/JNEUROSCI.22-22-09912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Koenen KC. Genetics of PTSD: Fear Conditioning as a Model for Future Research. Psychiatr Ann. 2009;39:358–367. doi: 10.3928/00485713-20090526-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol Sci. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Technical Report C-1. The Center for Research in Psychophysiology, University of Florida; 1999. Affective norms for English words (ANEW): instruction manual and affective ratings. [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Marle HJ, Franke B, Hermans EJ, van Wingen G, Fernandez G. Acute stress modulates genotype effects on amygdala processing in humans. Proc Natl Acad Sci U S A. 2010;107:9867–9872. doi: 10.1073/pnas.1003514107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Kolassa IT, Ertl V, Onyut PL, Neuner F, Elbert T, Papassotiropoulos A. A deletion variant of the alpha2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat Neurosci. 2007;10:1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham KL, Tran TP, Fong WC, Bryant RA. Sex differences in emotional memory consolidation: The effect of stress-induced salivary alpha-amylase and cortisol. Biol Psychol. 2012;89:539–544. doi: 10.1016/j.biopsycho.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Gibbs AA, Naudts KH, Azevedo RT, David AS. Deletion variant of alpha2b-adrenergic receptor gene moderates the effect of COMT val(158)met polymorphism on episodic memory performance. Eur Neuropsychopharmacol. 2010;20:272–275. doi: 10.1016/j.euroneuro.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress differentially modulates fear conditioning in healthy men and women. Biol Psychiatry. 2006;59:516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Jelicic M, Geraerts E, Merckelbach H, Guerrieri R. Acute stress enhances memory for emotional words, but impairs memory for neutral words. Int J Neurosci. 2004;114:1343–1351. doi: 10.1080/00207450490476101. [DOI] [PubMed] [Google Scholar]
- Joels M, Fernandez G, Roozendaal B. Stress and emotional memory: a matter of timing. Trends Cogn Sci. 2011;15:280–288. doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology. 1995;20:509–514. doi: 10.1016/0306-4530(94)00078-o. [DOI] [PubMed] [Google Scholar]
- Li S, Weerda R, Guenzel F, Wolf OT, Thiel CM. ADRA2B genotype modulates effects of acute psychosocial stress on emotional memory retrieval in healthy young men. Neurobiol Learn Mem. 2013;103:11–18. doi: 10.1016/j.nlm.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Li S, Weerda R, Milde C, Wolf OT, Thiel CM. ADRA2B genotype differentially modulates stress-induced neural activity in the amygdala and hippocampus during emotional memory retrieval. Psychopharmacology (Berl) 2015;232:755–764. doi: 10.1007/s00213-014-3710-3. [DOI] [PubMed] [Google Scholar]
- Mammarella N, Fairfield B, Di Domenico A, D’Onofrio L, Stuppia L, Gatta V. The modulating role of ADRA2B in emotional working memory: Attending the negative but remembering the positive. Neurobiol Learn Mem. 2016;130:129–134. doi: 10.1016/j.nlm.2016.02.009. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Wolf OT. Sex differences in stress effects on emotional learning. J Neurosci Res. 2017;95:93–105. doi: 10.1002/jnr.23811. [DOI] [PubMed] [Google Scholar]
- Mordecai KL, Rubin LH, Eatough E, Sundermann E, Drogos L, Savarese A, Maki PM. Cortisol reactivity and emotional memory after psychosocial stress in oral contraceptive users. J Neurosci Res. 2017;95:126–135. doi: 10.1002/jnr.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudts KH, Azevedo RT, David AS, van Heeringen C, Gibbs AA. Influence of COMT val158met and ADRA2B deletion polymorphisms on recollection and familiarity components of human emotional memory. J Psychopharmacol. 2011;26:819–829. doi: 10.1177/0269881111416688. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Ertman N, Lakhani YS, Cahill L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiol Learn Mem. 2011;96:378–384. doi: 10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biol Psychol. 2013;92:257–266. doi: 10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn Mem. 2007;14:861–868. doi: 10.1101/lm.743507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Ryan L, Hoscheidt S, Jacobs JW, Nadel L. The impact of stress on neutral and emotional aspects of episodic memory. Memory. 2006;14:1–16. doi: 10.1080/09658210500139176. [DOI] [PubMed] [Google Scholar]
- Quaedflieg CW, Schwabe L, Meyer T, Smeets T. Time dependent effects of stress prior to encoding on event-related potentials and 24h delayed retrieval. Psychoneuroendocrinology. 2013;38:3057–3069. doi: 10.1016/j.psyneuen.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain DJ. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci U S A. 2009;106:19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, King AC, Cohoon AJ, Lovallo WR. Hormonal contraceptive use diminishes salivary cortisol response to psychosocial stress and naltrexone in healthy women. Pharmacol Biochem Behav. 2013;109:84–90. doi: 10.1016/j.pbb.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Bohringer A, Chatterjee M, Schachinger H. Effects of pre-learning stress on memory for neutral, positive and negative words: Different roles of cortisol and autonomic arousal. Neurobiol Learn Mem. 2008;90:44–53. doi: 10.1016/j.nlm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008;33:890–895. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Hoffken O, Tegenthoff M, Wolf OT. Opposite effects of noradrenergic arousal on amygdala processing of fearful faces in men and women. Neuroimage. 2013;73:1–7. doi: 10.1016/j.neuroimage.2013.01.057. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joels M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: An update and integration. Neurosci Biobehav Rev. 2012;36:1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B. PTSD and gene variants: new pathways and new thinking. Neuropharmacology. 2012;62:628–637. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KM, Brown KM, Forbes SL, Liggett SB. Polymorphic deletion of three intracellular acidic residues of the alpha 2B-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J Biol Chem. 2001;276:4917–4922. doi: 10.1074/jbc.M008118200. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RD, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25:260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- Todd RM, Muller DJ, Lee DH, Robertson A, Eaton T, Freeman N, Palombo DJ, Levine B, Anderson AK. Genes for emotion-enhanced remembering are linked to enhanced perceiving. Psychol Sci. 2013;24:2244–2253. doi: 10.1177/0956797613492423. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132:959–992. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- Vogel S, Schwabe L. Stress in the zoo: Tracking the impact of stress on memory formation over time. Psychoneuroendocrinology. 2016;71:64–72. doi: 10.1016/j.psyneuen.2016.04.027. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wickens TD. Elementary signal detection theory. Oxford: University Press; 2002. [Google Scholar]
- Wilker S, Elbert T, Kolassa IT. The downside of strong emotional memories: How human memory-related genes influence the risk for posttraumatic stress disorder — A selective review. Neurobiol Learn Mem. 2014;112:75–86. doi: 10.1016/j.nlm.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Wolf OT. Immediate recall influences the effects of pre-encoding stress on emotional episodic long-term memory consolidation in healthy young men. Stress. 2012;15:272–280. doi: 10.3109/10253890.2011.622012. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Clark B, Warnecke A, Smith L, Tabar J, Talbot JN. Pre-learning stress differentially affects long-term memory for emotional words, depending on temporal proximity to the learning experience. Physiol Behav. 2011a;103:467–476. doi: 10.1016/j.physbeh.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Diamond DM. Current status on behavioral and biological markers of PTSD: a search for clarity in a conflicting literature. Neurosci Biobehav Rev. 2013;37:860–895. doi: 10.1016/j.neubiorev.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Kalchik AE, Hoffman MM, Aufdenkampe RL, Burke HM, Woelke SA, Pisansky JM, Talbot JN. Brief, pre-retrieval stress differentially influences long-term memory depending on sex and corticosteroid response. Brain Cogn. 2014a;55:277–285. doi: 10.1016/j.bandc.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Kalchik AE, Hoffman MM, Aufdenkampe RL, Lyle SM, Peters DM, Brown CM, Cadle CE, Scharf AR, Dailey AM, Wolters NE, Talbot JN, Rorabaugh BR. ADRA2B deletion variant selectively predicts stress-induced enhancement of long-term memory in females. Psychoneuroendocrinology. 2014b;48:111–122. doi: 10.1016/j.psyneuen.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Park CR, Diamond DM. Neurobiological basis of the complex effects of stress on memory and synaptic plasticity. In: Conrad CD, editor. The Handbook of Stress: Neuropsychological Effects on the Brain. Oxford, UK: Wiley-Blackwell; 2011b. pp. 157–178. [Google Scholar]
- Zoladz PR, Peters DM, Kalchik AE, Hoffman MM, Aufdenkampe RL, Woelke SA, Wolters NE, Talbot JN. Brief, pre-learning stress reduces false memory production and enhances true memory selectively in females. Physiol Behav. 2014c;128:270–276. doi: 10.1016/j.physbeh.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Warnecke AJ, Woelke SA, Burke HM, Frigo RM, Pisansky JM, Lyle SM, Talbot JN. Pre-learning stress that is temporally removed from acquisition exerts sex-specific effects on long-term memory. Neurobiol Learn Mem. 2013;100:77–87. doi: 10.1016/j.nlm.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Zorawski M, Blanding NQ, Kuhn CM, LaBar KS. Effects of stress and sex on acquisition and consolidation of human fear conditioning. Learn Mem. 2006;13:441–450. doi: 10.1101/lm.189106. [DOI] [PMC free article] [PubMed] [Google Scholar]


