Abstract
Sleep disturbances impair cognitive functioning in typically developing populations. Children with attention-deficit/hyperactivity disorder (ADHD), a disorder characterized by impaired inhibitory control and attention, commonly experience sleep disturbances. Whether inhibitory impairments are related to sleep deficits in children with ADHD is unknown. Children with ADHD (n = 18; Mage = 6.70 years) and typically developing controls (n = 15; Mage = 6.73 years) completed a Go/No-Go task to measure inhibitory control and sustained attention before and after polysomnography-monitored overnight sleep. Inhibitory control and sustained attention were improved following overnight sleep in typically developing children. Moreover, morning inhibitory control was positively correlated with rapid eye movement (REM) theta activity in this group. Although REM theta activity was greater in children with ADHD compared to typically developing children, it was functionally insignificant. Neither inhibitory control nor sustained attention were improved following overnight sleep in children with ADHD symptoms, and neither of these behaviors was associated with REM theta activity in this group. Taken together, these results indicate that elevated REM theta activity may be functionally related to ADHD symptomology, possibly reflecting delayed cortical maturation.
Keywords: ADHD, inhibitory control, sustained attention, sleep, REM, theta activity
Inhibitory control, the ability to suppress prepotent responses, is compromised by sleep deficits (Chuah et al. 2006; Drummond et al. 2006; Goel et al. 2009). Individuals with attention-deficit/hyperactivity disorder (ADHD) have impaired inhibitory control (Schachar et al. 1995; Oosterlaan et al. 1998; Castellanos et al. 2000; Yong-Liang et al. 2000; Durston et al. 2003) and commonly experience sleep disturbances (Cohen-Zion and Ancoli-Israel 2004; Owens 2005; Yoon et al. 2012). However, it is unknown whether sleep disturbances are related to cognitive impairments in this population. If so, sleep may be a target for early diagnosis and treatment of ADHD.
Individuals with ADHD have longer sleep latency and reduced sleep duration and efficiency relative to typically developing (TD) controls (Yoon et al. 2012; Weiss et al. 2015). Studies utilizing polysomnography indicate no consistent differences in sleep macrostructure (i.e., sleep stages; Cohen-Zion and Ancoli-Israel 2004; Sadeh et al. 2006; Herman 2015). However, sleep microstructure differs between children with ADHD and TD children: slow wave activity (SWA; the spectral power of the delta frequency band) is reported to be greater in children with ADHD (Ringli et al. 2013). Preliminary evidence indicates that theta activity (the spectral power of the theta frequency band) is marginally greater during non-rapid eye movement sleep (nREM) in ADHD children 10–12 years of age (Saletin et al. 2016). During wakefulness, theta activity is likewise elevated in young adults with ADHD compared to TD young adults (Barry et al. 2003; Hermens et al. 2005; Snyder and Hall 2006). Whether theta activity differs in early childhood when most ADHD symptoms emerge (Applegate et al. 1997; American Academy of Pediatrics 2011) is unknown.
Slow wave and theta activity decline across childhood into adolescence (Campbell and Feinberg 2009). Developmental changes in SWA and theta activity reflect changes in cortical plasticity and brain maturation (Cajochen et al. 1999; Kurth et al. 2010; Leemburg et al. 2010; Ringli et al. 2013). Supporting this pattern, the rates of decline for SWA and theta activity across development parallel the rate of cortical thinning (Shaw et al. 2008; Campbell and Feinberg 2009). Slow wave and theta activity are both associated with cognitive functioning. For example, consolidation of memories over an interval of sleep correlates with SWA (Benedict et al. 2009; Walker 2009) and theta activity (Nishida et al. 2009; Prehn-Kristensen et al. 2013; Hutchinson and Rathore 2015; Schreiner et al. 2015) in the sleep bout. Prefrontal rapid eye movement (REM) sleep theta activity is also positively correlated with decision-making in young adults (Seeley et al. 2016). Likewise, waking theta activity is linked to inhibitory control in TD populations (Cavanagh and Frank 2014).
These studies pose the hypothesis that differences in sleep microstructure may contribute to reduced inhibitory control, a core deficit in individuals with ADHD. To test this hypothesis, children completed a Go/No-Go task (see Figure 1) to gauge inhibitory control and sustained attention before (baseline session) and after (morning session) overnight sleep. High-density polysomnography was used to measure sleep macro- and microstructure. We hypothesized that TD children would exhibit sleep-dependent enhancement of inhibitory control and sustained attention whereas children with ADHD symptoms would not. Moreover, we hypothesized that group differences in inhibitory control and sustained attention, observed after sleep, would be associated with sleep microstructure, specifically SWA and theta activity.
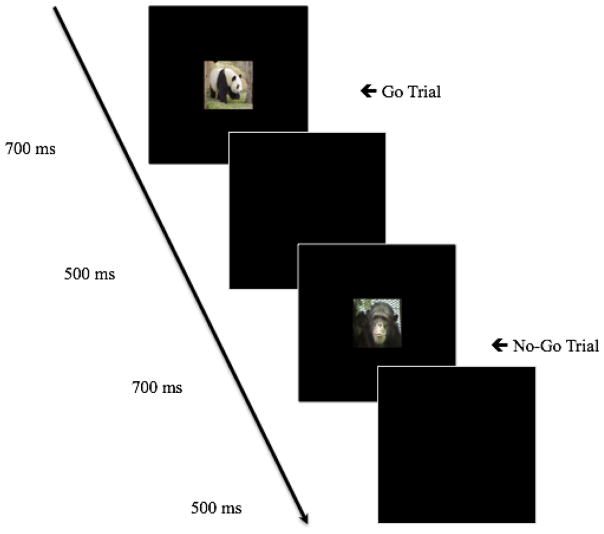
Fig. 1.
Order of stimulus presentation during the Go/No-Go task. Go trials were those in which images of animals such as a panda (shown above) were presented. No-Go trials were those in which an image of a chimpanzee (shown above) was presented
Methods
Participants
Children, 4–8 years of age, were recruited through community advertisements and the Child Studies Database at the University of Massachusetts Amherst. Caregivers completed a pre-screening phone interview to determine their child’s eligibility and group placement (ADHD or TD control) using the ADHD section of the Diagnostic Interview Schedule for Children IV (DISC-IV; Shaffer et al. 2000). The DISC-IV is a structured, diagnostic interview used to assess pediatric psychiatric disorders in children 4 years of age and older (Shaffer et al. 2000; Rolon-Arroyo et al. 2016). The ADHD section of the DISC-IV has adequate test-retest reliability (Kappa = 0.79). As Oppositional Defiant Disorder (ODD) is highly comorbid with childhood ADHD (Waschbusch 2002), the ODD scale of the DISC-IV was used to determine whether symptoms of ODD contributed to behavioral outcomes in our sample. All interviews were conducted by a masters-level graduate student (C.I. Lugo-Candelas), supervised by a licensed clinician (E.A. Harvey).
Exclusion criteria included a current diagnosis or history of intellectual disabilities, hearing or visual disabilities, receptive language delay, cerebral palsy, epilepsy, autism, or psychosis. Children (both ADHD and TD) with a current diagnosis or history of sleep disorders (i.e., sleep apnea, sleep disordered breathing, or restless leg syndrome) were not included in this study as these disorders may confound results. The ADHD group was composed of children who had at least six symptoms of hyperactivity/impulsivity, at least three of which were present in two settings, listed in the ADHD section of the DISC-IV. Hyperactive/impulsive symptoms and not inattentive symptoms were used to determine ADHD status because the presentation of predominately inattentive symptoms typically has later age of onset and is thought to be distinct from presentations involving hyperactivity/impulsivity (Applegate et al. 1997). As ADHD is not typically diagnosed until children enroll in formal schooling, children in this sample were not required to have a physician’s formal diagnosis of the disorder (Applegate et al. 1997; American Academy of Pediatrics 2011). Importantly, accumulating evidence indicates that an ADHD diagnosis can be reliably assigned during the preschool years (Rolon-Arroyo et al. 2016). Typically developing controls were defined as having three or fewer symptoms on the ADHD section of DISC-IV.
Thirty-three children (9 F; Mage = 6.71, SD = 0.91 years) were tested. Eighteen children (5 F; Mage = 6.70, SD = 1.07 years) were placed in the ADHD group. Fifteen children (4 F; Mage = 6.73, SD = 0.71 years) were classified as TD controls.
Seven children in the ADHD group (0 F; Mage = 6.79, SD = 1 year) had a prior diagnosis of ADHD whereas 10 (5 F; Mage = 6.61, SD = 1.23 years) did not (diagnosis data missing from 1 child). Only two enrolled children were taking medication for ADHD (1 Tenex, 1 Adderall). As these medications may alter sleep physiology, participants were asked to abstain from using them 48 hours prior to the overnight visit (Konofal et al. 2010). Statistical outcomes (i.e., behavior and sleep physiology) did not differ when the two children with a history of medication use were excluded from analyses.
According to caregiver report, 72.7% of the children tested were white/Caucasian, 6.1% were Latino/Hispanic, 3.0% were black/African American, 3.0% were Asian, and 15.2% were biracial/mixed race. Of the caregivers for enrolled children, 12.1% earned a high school diploma, 6.1% earned an Associate’s Degree, 27.3% earned a Bachelor’s Degree, 48.5% earned a Master’s Degree, and 6.1% earned a Doctorate.
Sleep Physiology
Polysomnography recordings of overnight sleep were obtained using customized high-density polysomnography electrode caps (EasyCap). These caps had 24 EEG electrodes assigned to O1, O2, C3, C4, CP1, CP2, CP5, CP6, F3, F4, Fz, FCz, FC1, FC2, FC5, FC6, F7, F8, P3, P4, P7, P8, Pz, and POz. The montage also included two electrooculogram leads and two electromyogram leads (affixed to the chin). Data were recorded relative to mid-forehead ground placed at FPz. EEG data were recorded referenced to Cz and contralateral mastoids (A1 and A2).
Polysomnography was scored according to the revised American Academy of Sleep Medicine manual (American Academy of Sleep Medicine 2007) by a trained researcher. Scoring was confirmed against a second trained researcher, who was unaware of the participant’s group status (ADHD versus TD). On average, 84% of the sleep stages scored were the same between the two scorers (ranging from 80% to 93%). Importantly, inter-rater reliability did not differ for groups. As such, results are based on staging from the initial scorer.
Spectral analysis was conducted using Brain Analyzer 2 software (Version 2.4; Brain Products). Previous studies have identified links between frontal theta activity and inhibitory control (Cavanagh and Frank 2014). Consistent with these studies and others, spectral power was drawn from F4 (Mann et al. 1992). Spectral power is reported in power density (μV2/Hz). Slow wave activity was characterized as activity between 0.5 and 4 Hz (delta) recorded during slow wave sleep (SWS) and nREM stage 2 and SWS combined (Benedict et al. 2009; Prehn-Kristensen et al. 2013). Theta activity is defined as activity between 4 and 7 Hz recorded during REM and nREM sleep (Nishida et al. 2009; Prehn-Kristensen et al. 2013). Analysis of sleep stages and spectral power was averaged across all participants within each group.
Behavioral Measures
To assess inhibitory control and sustained attention, children completed a Go/No-Go task. The Go/No-Go task is a valid and reliable measure of inhibition and attention in young children (Kindlon et al. 1995; Bezdjian et al. 2009). Stimuli used in the Go/No-Go task were 10 images of animals. Go trials (75% of trials) featured images of various animals (e.g., giraffe, elephant, panda). In remaining trials, No-Go trials (25% of trials), a chimpanzee was presented (see Figure 1). The order of No-Go and Go trials varied with the exception that No-Go trials were separated by 0, 2, or 4 Go trials (to prevent children from learning this pattern of trial presentation). Displayed images were 3 inches in height and 4 inches in length; each centered on a 14-inch computer screen positioned approximately 15 inches from the child.
Each trial began with the presentation of an animal image for 700 ms. Children were instructed to respond, via a button press on a mouse, for all of the animals (Go trials), except for the chimpanzee for which they were to inhibit their response (No-Go trials). A blank screen was presented for 500 ms between trials. Two pseudo-random trial orders were used for all participants (for baseline and morning sessions, trial order counterbalanced across participants).
Procedure
Procedures were approved by the Institutional Review Board at the University of Massachusetts Amherst. Caregivers consented to their child’s participation and child verbal assent was obtained before commencing with experimental procedures. Children followed a self-selected sleep schedule prior to the experimental procedures performed in the lab.
Caregivers and children were scheduled to arrive at the sleep lab approximately 1 hour before the child’s typical bedtime. After acclimating to the sleep lab, children completed the Go/No-Go task (baseline session). To begin, children were given 12 practice trials to ensure that they understood task instructions. Subsequently, children were presented with test trials in 2 blocks of 60 trials each (total of 120 test trials). The task took approximately 10 minutes to complete.
Following completion of the task and prior to bedtime, children were fitted with a polysomnography cap. Children and caregivers slept in separate beds within the same room overnight. The following morning, the cap was removed. Approximately 30 minutes after wake onset (to mitigate sleep inertia), children completed the Go/No-Go task once more (morning session). Caregivers were provided monetary compensation and children were given an age-appropriate prize for their participation.
Results
Demographic information is presented in Table 1. Child age (t(31) = −0.08, p = 0.937), gender (X2 (1, N = 33) = 0.01, p = 0.943), average sleep duration (from caregiver report; t(30) = 0.91, p = 0.372; data missing from 1 child), and ethnicity (X2 (4, N = 33) = 6.25, p = 0.182) were not significantly different between groups.
Table 1.
Group differences in participant demographics and behaviors.
| ADHD Mean (SD) |
TD Mean (SD) |
p-value | |
|---|---|---|---|
| Participant Demographics | |||
| Age (years) | 6.70 (1.07) | 6.73 (0.71) | 0.937 |
| Gender (Females: Males) | 5:13 | 4:11 | 0.943 |
| Hyperactive Symptoms | 7.28 (1.02) | 0.27 (0.80) | < 0.001 a |
| Inattentive Symptoms | 6.06 (2.13) | 0.67 (1.59) | < 0.001 a |
| ODD Symptoms | 4.33 (2.09) | 1.47 (1.96) | < 0.001 a |
| Average Sleep Duration (Hours) | 10.59 (0.81) | 10.28 (1.09) | 0.372 |
| Average Bedtime | 8:46 PM (38.24 minutes) | 8:18 PM (43.35 minutes) | 0.254 |
| Behaviors | |||
| Baseline Inhibitory Control b | 70.74 (16.39) | 74.44 (10.44) | 0.456 |
| Morning Inhibitory Control b | 74.63 (15.04) | 84.44 (8.79) | 0.033 a |
| Baseline Sustained Attention b | 77.84 (16.88) | 70.74 (21.15) | 0.292 |
| Morning Sustained Attention b | 76.67 (17.05) | 80.52 (14.42) | 0.494 |
Note: ADHD: n = 18; TD: n = 15
95% CIs = [6.35, 7.67], [4.03, 6.75], [1.42, 4.31], and [−0.19, −0.01], respectively
% Correct
REM theta activity is greater in children with ADHD symptoms
Four children in the ADHD group were omitted from sleep physiology analyses due to recording error (n = 3) and noncompliance (n = 1).1 Thus, results pertaining to sleep physiology are presented for 14 children in the ADHD group (4 F; Mage = 6.77, SD = 1.05 years), with 7.29 symptoms of hyperactivity (SD = 0.91) and 6.29 symptoms of inattention (SD = 1.73) on average, and 15 TD controls (4 F; Mage = 6.73, SD = 0.71 years).
Independent samples t-tests were used compare sleep microstructure between groups. Theta activity recorded during REM was significantly greater in the ADHD group compared to the TD group (Table 2). Theta activity recorded during nREM sleep did not differ between groups, supporting REM-specific elevation of theta activity in the ADHD group. To determine the specificity of REM theta elevation in this sample, full power curves were evaluated (see Supplemental Figure 1). In addition to REM theta activity, SWA recorded during REM sleep was elevated in ADHD children. However, nREM SWA and SWS-specific SWA did not differ between groups. Collectively, these findings indicate that low frequency spectral activity (SWA and theta activity) was significantly elevated in ADHD children during REM but not nREM sleep.
Table 2.
Group differences in sleep macrostructure and microstructure (F4).
| ADHD Mean (SD) |
TD Mean (SD) |
p-value | |
|---|---|---|---|
| Macrostructure | |||
| TST (minutes) | 554.71 (70.28) | 552.62 (53.82) | 0.929 |
| SOL (minutes) | 52.93 (44.89) | 49.33 (22.32) | 0.785 |
| WASO (minutes) | 13.59 (10.52) | 21.45 (20.44) | 0.209 |
| Sleep efficiency (%) | 97.36 (1.88) | 94.80 (5.20) | 0.094 |
| nREM stage 1 (%) | 9.06 (3.47) | 10.66 (3.59) | 0.233 |
| nREM stage 2 (%) | 52.58 (10.67) | 49.06 (8.64) | 0.336 |
| SWS (%) | 22.03 (6.80) | 22.92 (4.14) | 0.671 |
| REM (%) | 16.33 (7.13) | 17.32 (7.56) | 0.720 |
| Microstructure (μV2/Hz) | |||
| SWA (SWS) | 503.70 (110.38) | 447.76 (146.35) | 0.258 |
| SWA (nREM) | 277.21 (77.51) | 239.13 (89.01) | 0.231 |
| Theta (REM) | 24.75 (9.06) | 17.44 (5.55) | 0.014 a |
| Theta (nREM) | 28.49 (9.08) | 29.27 (11.54) | 0.842 |
Note: TST = total sleep time; SOL = sleep onset latency; WASO = wake after sleep onset; nREM = non-rapid eye movement sleep; SWS = slow wave sleep; REM = rapid eye movement; SWA = slow wave activity; SWA and theta activity recorded from frontal electrode (F4).
95% CI [1.63, 12.99]
Exploratory independent samples t-tests were used to confirm that sleep macrostructure (sleep stages) did not differ between groups. Consistent with prior studies (Cohen-Zion and Ancoli-Israel 2004; Sadeh et al. 2006; Herman 2015), there were no group differences in sleep macrostructure (Table 2).2 Sleep physiology did not differ between children with or without a prior diagnosis of ADHD (ps > 0.133), with the exception that children with a prior diagnosis had less nREM stage 1 (M = 7.13, SD = 2.15) than those who were not diagnosed (M = 11.32, SD = 3.80; t(11) = −2.50, p = 0.030, 95% CI [−7.88, −0.50]).
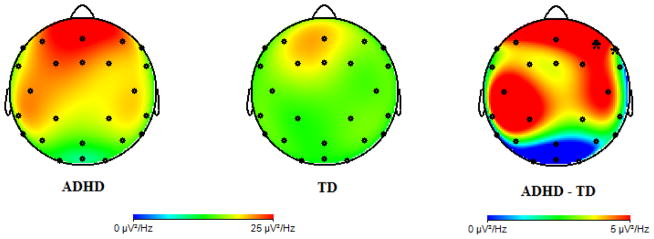
Given the significant difference in REM theta activity at the a priori chosen frontal electrode site (F4; Table 2), we examined whether there were region-specific differences in theta activity between the ADHD and TD groups. In addition to F4, theta activity was greater in the ADHD group at F8 (t(25) = 2.19, p = 0.038) and marginally greater at central electrodes C3 (t(26) = 2.02, p = 0.054) and C4 (t(26) = 1.82, p = 0.081; Figure 2), indicating region-specific enhancement.
Fig. 2.
Topographic distributions of REM theta activity for ADHD (left) and TD children (middle). Group difference in theta activity (ADHD minus TD) plotted on the right. Note: Electrodes where group differences are statically significant are marked; *p < 0.05
Inhibitory control and sustained attention are improved following sleep in TD children
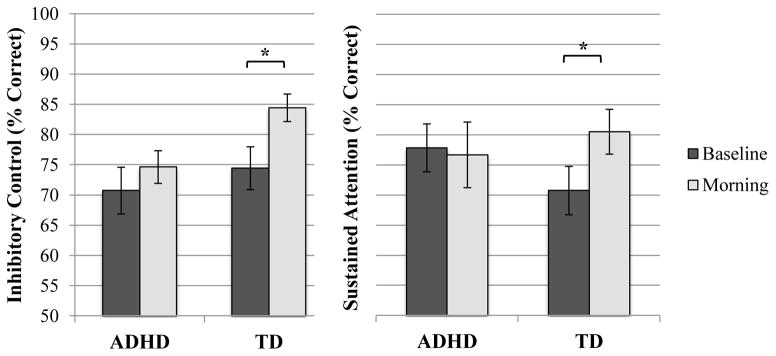
Whether inhibitory control and sustained attention are modified by sleep in TD children is unknown. To assess the effect of sleep on these measures, we computed accuracy (% correct) for No-Go and Go trials. Greater accuracy on No-Go trials reflects greater inhibitory control whereas greater accuracy on Go trials corresponds to greater sustained attention (O’Connell et al. 2009; McDermott et al. 2012). Paired samples t-tests were used to assess within-group changes in inhibitory control and sustained attention between the baseline (before sleep) and morning (after overnight sleep) sessions. Inhibitory control improved in the morning relative to baseline (t(14) = −3.57, p = 0.003, 95% CI [ −0.16, −0.04]), such that morning performance was significantly greater than baseline performance (Figure 3). Similarly, sustained attention was significantly greater in the morning, relative to baseline (t(14) = −3.25, p = 0.026, 95% CI [ −0.18, −0.01]).3
Fig. 3.
Group differences in inhibitory control and sustained attention during the baseline and morning testing sessions. Note: Means represent those from paired samples t-tests; Error bars represent standard error; *p ≤ 0.05
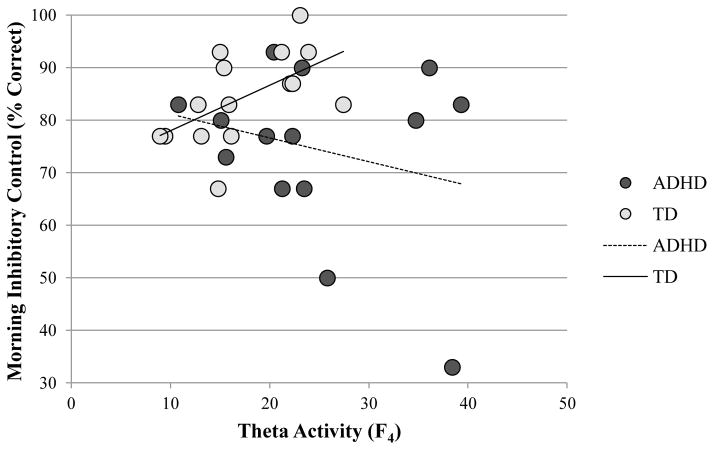
Improved inhibitory control and sustained attention following sleep could reflect circadian variation in performance or practice effects that are independent of sleep per se. Alternatively, changes in performance may reflect sleep-specific mechanisms. Partial correlations (controlling for baseline scores) between morning inhibitory control and total sleep time (r = 0.33, p = 0.255) and morning sustained attention and total sleep time (r = −0.13, p = 0.664) were not significant. Morning inhibitory control was significantly positively associated with REM theta activity at frontal electrode site F4 (r = 0.61, p = 0.021; Figure 4), whereas sustained attention was not (r = −0.10, p = 0.727). Consistent with this finding, morning inhibitory control was significantly positively correlated with average frontal REM theta activity recorded at F3, F4, and FZ combined (r = 0.65, p = 0.013), indicating that these relations are bilateral. Moreover, baseline inhibitory control was not associated with REM theta activity (r = −0.01, p = 0.971), supporting this sleep-dependent effect. Neither morning inhibitory control nor sustained attention were associated with nREM SWA (rs between −0.29 and −0.09, ps ≥ 0.322). Although SWA recorded during REM sleep was elevated in ADHD children (see Supplemental Figure 1), it was functionally insignificant; unlike REM theta activity, REM SWA was not correlated with morning inhibitory control in TD children (r = 0.03, p = 0.909). Moreover, morning inhibitory control was not associated with the percentage of time spent in nREM stage 2, SWS, or REM sleep (rs between −0.01 and 0.18, ps ≥ 0.534), supporting a theta-specific enhancement of inhibitory control for the TD children.
Fig. 4.
Correlations between frontal theta activity (F4; in μV2/Hz) and inhibitory control measured during the morning testing session
To determine whether variables other than REM theta activity contributed to morning inhibitory control, a linear regression model was used. Baseline inhibitory control, child age and gender, hyperactive and inattentive symptoms, total sleep time, and REM theta activity (F4) were simultaneously entered as predictor variables in a model evaluating morning inhibitory control in TD children. Consistent with the results of the correlation, theta activity significantly predicted morning inhibitory control in TD children (β = 0.01, p = 0.034). All other variables were not significant (ps ≥ 0.124).
Inhibitory control and sustained attention are unchanged following sleep in children with ADHD symptoms
In contrast to results in TD children, neither inhibitory control (t(17) = −0.89, p = 0.386) nor sustained attention (t(17) = 0.71, p = 0.488) changed in the morning compared to baseline in the ADHD group (Figure 3).4 These null findings are unlikely due to low power in the ADHD group given the high power observed in the TD group (achieved power = 0.905). Moreover, inhibitory control and sustained attention did not differ for ADHD children with or without a prior diagnosis of the disorder during the baseline or morning testing sessions (ps ≥ 0.655).
Partial correlations indicated that morning inhibitory control (r = −0.15, p = 0.617) and sustained attention (r = −0.32, p = 0.282) were not associated with total sleep time. Interestingly, although children with ADHD symptoms had greater theta activity, neither morning inhibitory control (r = −0.21, p = 0.489; Figure 4) nor sustained attention (r = −0.31, p = 0.310) were associated with REM theta activity in this group. Similarly, the correlation between morning inhibitory control and average REM theta activity recorded at F3, F4, and FZ (combined) was not significant (r = −0.40, p = 0.182). Baseline inhibitory control was not associated with REM theta activity in this group (r = −0.22, p = 0.457). Relations between these behaviors and nREM SWA were also not significant (rs between −0.07 and 0.05, ps ≥ 0.828). REM SWA was also not associated with morning inhibitory control in the ADHD group (r = −0.29, p = 0.362). Likewise, morning inhibitory control was not associated with the percentage of time spent in nREM stage 2, SWS, or REM sleep (rs between −0.25 and 0.15, ps ≥ 0.409).
Theta activity did not significantly predict morning inhibitory control (β = −0.01, p = 0.456) in a linear regression model, suggesting that the mechanism underlying enhanced morning inhibitory control in TD children is absent in ADHD children. Baseline inhibitory control, child age and gender, hyperactive and inattentive symptoms, and total sleep time did not predict morning inhibitory control (ps ≥ 0.294), consistent with findings in TD children.
A Fisher r-to-z-transformation was used to compare the difference between correlation coefficients (morning inhibitory control and REM theta activity) in the TD and ADHD groups. The results of this analysis indicate that the correlation between morning inhibitory control and REM theta activity in the TD group (r = 0.61) was marginally greater than that of the ADHD group (r = −0.21; z = −1.89, p = 0.058).
Discussion
We report evidence that differences in REM sleep microstructure contribute to impairments in daytime inhibition in children with symptoms of ADHD. Typically developing children had overnight enhancement of inhibitory control and sustained attention. Moreover, REM theta activity was positively associated with morning inhibitory control in TD children but not in children with ADHD in spite of overall greater REM theta activity in the ADHD group.
Inhibitory control was improved following overnight sleep in TD children. Although circadian processes influence inhibitory control (Sagaspe et al. 2012), our data support an active role of sleep in improving inhibition. Morning inhibitory control was specifically associated with REM theta activity during the overnight sleep bout, suggesting overnight improvement is likely a REM theta-dependent process. The non-significant associations between baseline inhibitory control and REM theta activity in the TD and ADHD groups further qualified this sleep-dependent effect. Additionally, the results of linear regression analyses suggest that REM theta activity predicts morning inhibitory control in TD children, even when accounting for child age, gender, symptomology, and total sleep time.
Not surprisingly, inhibitory control was lower overall in ADHD children (Schacher et al. 1995; Barkley 1997; Oosterlaan et al. 1998; Castellanos et al. 2000; Yong-Liang et al. 2000; Durston et al. 2003). Strikingly, however, inhibitory control was unchanged following overnight sleep in the ADHD group. Here too, a circadian explanation is unlikely. ADHD is associated with a shortening of the circadian cycle (Baird et al. 2012), which would predict performance improvements in the morning relative to the evening. To the contrary, performance was unchanged. Rather, we posit that the REM theta-dependent process that supports improvements in inhibitory control in TD children is altered in ADHD. Even in the presence of elevated REM theta activity, a significant correlation between REM theta and behavior, which was observed in TD children, was not present in the ADHD group. As the difference between correlation coefficients in the TD and ADHD groups was only marginally significant, this interpretation should be taken with caution. We speculate that differential associations between REM theta and behavior may reflect impairments in theta modulation in individuals with ADHD (Hermens et al. 2005). To a certain point, theta activity may increase inhibitory control; however, past this point, elevated theta activity may impair inhibitory control. This concept is consistent with work in young adults where both low and high levels of cortical activity are indicative of performance difficulties (see Haier et al, 1988). Similarly, having low or high levels of REM theta activity may be detrimental to subsequent inhibitory processes.
Work in primates suggests that waking theta activity coordinates neural interactions between structures responsible for cognitive control (for review, see Womelsdorf et al. 2011). Specifically, theta oscillations in the anterior cingulate cortex modulate excitation of post-synaptic neuronal groups in other structures in the cognitive control network (e.g., hippocampus, frontal and sensory cortices). These interactions are phase-locked to task-related events that require cognitive control, including inhibition. We posit that this same mechanism may underlie REM theta-dependent enhancement of inhibitory control: REM theta activity may enhance communication between neural structures that support inhibitory control. Provided that children with ADHD have increased REM theta activity, these structures may be over stimulated and, consequently, less efficient during subsequent assessments of inhibition. Additional studies utilizing neuroimaging techniques are needed to test this hypothesis directly.
Elevated REM theta activity in the ADHD group may also reflect a maturational lag in this population compared to the TD group. Topographic assessment of theta activity supports this hypothesis: the greatest difference in theta activity between groups was found in frontal and central regions, areas that lag in the posterior-anterior trajectory of cortical development (Shaw et al. 2008). Theta activity during REM may be a particularly important marker for identifying developmental delays, as REM sleep processes direct brain maturation throughout early development (Marks et al. 1995). As such, although individuals with ADHD have more theta activity than TD controls, these children may require additional theta activity to facilitate sleep-dependent enhancement of inhibitory processes. Alternatively, elevated REM theta may reflect an increased sleep need for ADHD children compared to TD controls. Theta activity is known to increase with sleep deprivation as has been shown in both animal and human paradigms (Borbely et al. 1984; Cajochen et al. 1999). Thus, elevated REM theta activity in the ADHD group, in the absence of a difference in total sleep time on the experimental night (see Table 2) or average sleep duration (assessed via caregiver report), may suggest a greater sleep need for children with ADHD. Additional studies targeting theta activity in children with ADHD are needed to explore both hypotheses further. Given that children with ADHD commonly experience sleep disruptions (Cohen-Zion and Ancoli-Israel 2004; Owens 2005; Yoon et al. 2012), future studies take into account the prior sleep history of TD and ADHD children and assess sleep physiology following an optimized or stabilized sleep schedule.
Counter to Ringli and colleagues (2013), we did not find group differences in nREM SWA. In a cross-sectional study, Campbell and Feinberg (2009) reported that SWA decline is not evident until late childhood (9–12 years of age). Theta decline, on the other hand, is evident earlier in development (6–9 years of age). As such, the lack of group differences in SWA in the present study may reflect the fact that children were 4–8 years of age, younger than those tested in previous studies (Ringli et al. 2013). Longitudinal assessments of sleep EEG trajectories are needed to better understand developmental differences in the trajectories of SWA and theta decline in children with ADHD. Alternatively, differences between EEG measures in Ringli’s study and our own may have contributed to differences in SWA findings. Ringli and colleagues (2013) normalized spectral power in order to compare topographical differences in SWA in TD and ADHD children. As the primary aim of this study was to assess group differences in spectral power in frontal regions associated with inhibitory control, non-normalized power density was compared between the TD and ADHD groups.
Notably, sustained attention was also improved following overnight sleep in TD children but not children with ADHD symptoms. However, morning sustained attention in TD children was not associated with increases in REM theta activity or any other aspect of sleep physiology. Sustained attention did not correlate with inhibitory control during the baseline or morning assessments, suggesting these processes are independent (ps ≥ 0.292; Schachar et al. 1995). Importantly, consistent with our baseline measures, sustained attention is not a core deficit in ADHD (Castellanos et al. 2006). In fact, the ADHD group tended to do better than the TD group at baseline leaving less room for overnight change in performance in the ADHD group compared to the TD group. As hyperactive/impulsive children were sampled in the current study, additional research assessing the role of sleep on behavior in children with the predominantly inattentive symptoms are needed.
In summary, these results suggest that increased REM theta activity may be functionally related to ADHD symptomology, providing a target for intervention. Identifying and treating symptoms in early childhood is particularly important given that symptoms typically persist throughout development and are related to maladaptive outcomes such as poor academic performance and interpersonal skills (Ingram et al. 1999). Regarding treatment, sleep extension and sleep hygiene interventions could be implemented as a means of enhancing sleep quality and, in turn, alleviating symptoms (Hiscock et al. 2015).
Supplementary Material
Full power curves for REM and nREM sleep at frontal electrode F4. Raw values were used in statistical analyses; however, log-transformed values are displayed in this figure to aid in interpretation of group differences in activity across REM and nREM sleep
Acknowledgments
This study was supported in part by R01 HL111695 from the National Institutes of Health (R.M.C. Spencer). The authors would like to thank Helen Root and Dr. Laura B. F. Kurdziel for their assistance with data collection.
Footnotes
Results were unchanged when the children without usable sleep physiology data (n = 4) were omitted from analyses.
Group differences in sleep physiology were not different when ODD symptoms were controlled for.
Behavioral findings were unchanged when controlling for ODD symptoms.
Behavioral findings were unchanged when controlling for ODD symptoms.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- American Academy of Pediatrics: Management steering committee on quality improvement and subcommittee on attention-deficit/hyperactivity disorder. ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–1022. doi: 10.1542/peds.2011-2654. https://doi.org/10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C. American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: Rules, terminology, and technical specifications. American Academy of Sleep Medicine; 2007. [Google Scholar]
- Applegate B, Lahey B, Biederman J, Hynd G, Barkley R, Ollendick T, Frick PJ, Greenhill L, McBurnett K, Newcorn JH, Kerdyk L, Garfinkel B, Waldman I, Shaffer D. Validity of the age-of-onset criterion for ADHD: A report from the DSM-IV field trials. J Am Acad of Child Adoles Psychiatry. 1997;36:1211–1221. [PubMed] [Google Scholar]
- Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry. 2012;17:988–995. doi: 10.1038/mp.2011.149. [DOI] [PubMed] [Google Scholar]
- Barkley R. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol. 2003;114:171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Benedict C, Scheller J, Rose-John S, Born J, Marshall L. Enhancing influence of intranasal interleukin-6 on slow-wave activity and memory consolidation during sleep. FASEB J. 2009;23:3629–3636. doi: 10.1096/fj.08-122853. https://doi.org/10.1096/fj.08-122853. [DOI] [PubMed] [Google Scholar]
- Bezdjian S, Baker LA, Lozano DI, Raine A. Assessing inattention and impulsivity in children during the Go/NoGo task. Brit J Dev Psychol. 2009;27:365–383. doi: 10.1348/026151008X314919. https://doi.org/10.1348/026151008X314919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely A, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984;14:171–182. doi: 10.1016/0166-4328(84)90186-4. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online. 1999;2:65–69. [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci. 2009;106:5177–5180. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F, Marvasti F, Ducharme J, Walter J, Israel M, Krain A, Pavlovsky C, Hommer D. Executive function oculomotor tasks in girls with ADHD. J Am Acad Child Adolesc Psychiatry. 2000;39:644–650. doi: 10.1097/00004583-200005000-00019. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. https://doi.org/10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. https://doi.org/10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah YML, Venkatraman V, Dinges DF, Chee MWL. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26:7156–7162. doi: 10.1523/JNEUROSCI.0906-06.2006. https://doi.org/10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Zion M, Ancoli-Israel S. Sleep in children with attention-deficit hyperactivity disorder (ADHD): A review of naturalistic and stimulant intervention studies. Sleep Med Rev. 2004;8:379–402. doi: 10.1016/j.smrv.2004.06.002. https://doi.org/10.1016/j.smrv.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Drummond S, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006;15:261–265. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. https://doi.org/10.1016/S0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Goel N, Rao H, Durmer J, Dinges D. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–339. doi: 10.1055/s-0029-1237117. https://doi.org/10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Nuechterlein KH, Hazlett E, Wu JC, Paek J, Browning HL, Buchsbaum MS. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12:199–217. https://doi.org/10.1016/0160-2896(88)90016-5. [Google Scholar]
- Herman JH. Attention deficit/hyperactivity disorder and sleep in children. Sleep Med Clin. 2015;10:143–149. doi: 10.1016/j.jsmc.2015.02.003. https://doi.org/10.1016/j.jsmc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Soei EXC, Clarke SD, Kohn MR, Gordon E, Williams LM. Resting EEG theta activity predicts cognitive performance in attention-deficit hyperactivity disorder. Pediatr Neurol. 2005;32:248–256. doi: 10.1016/j.pediatrneurol.2004.11.009. https://doi.org/10.1016/j.pediatrneurol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Hiscock H, Sciberras E, Mensah F, Gerner B, Efron D, Khano S, Oberklaid F. Impact of a behavioural sleep intervention on symptoms and sleep in children with attention deficit hyperactivity disorder, and parental mental health: randomised controlled trial. BMJ. 2015;350:h68–h68. doi: 10.1136/bmj.h68. https://doi.org/10.1136/bmj.h68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison IC, Rathore S. The role of REM sleep theta activity in emotional memory. Front Psychol. 2015;6 doi: 10.3389/fpsyg.2015.01439. https://doi.org/10.3389/fpsyg.2015.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram S, Hechtman L, Morgenstern G. Outcome issues in ADHD: Adolescent and adults long-term outcome. Ment Retard Dev Disabil Res Rev. 1999;5:243–250. [Google Scholar]
- Kindlon D, Mezzacappa E, Earls F. Psychometric properties of impulsivity measures: Temporal stability, validity and factor structure. J Chil Psychol Psychiatry. 1995;36:645–661. doi: 10.1111/j.1469-7610.1995.tb02319.x. [DOI] [PubMed] [Google Scholar]
- Konofal E, Lecendreux M, Cortese S. Sleep and ADHD. Sleep Med. 2010;11:652–658. doi: 10.1016/j.sleep.2010.02.012. https://doi.org/10.1016/j.sleep.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: A high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. https://doi.org/10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemburg S, Vyazovskiy VV, Olcese U, Bassetti CL, Tononi G, Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci. 2010;107:15939–15944. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C, Lubar J, Zimmerman A, Miller C, Muenchen R. Quantitative analysis of EEG in boys with attention-deficit-hyperactivity disorder: Controlled study with clinical implications. Pediatr Neurol. 1992;8:30–36. doi: 10.1016/0887-8994(92)90049-5. [DOI] [PubMed] [Google Scholar]
- Marks G, Shaffery J, Oksenberg A, Speciale S, Roffwarg H. A functional role of REM sleep in brain maturation. Behav Brain Res. 1995;69:1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Westerlund A, Zeanah CH, Nelson CA, Fox NA. Early adversity and neural correlates of executive function: Implications for academic adjustment. Dev Cogn Neurosci. 2012;2:S59–S66. doi: 10.1016/j.dcn.2011.09.008. https://doi.org/10.1016/j.dcn.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. https://doi.org/10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Bellgrove MA, Turin A, Ward S, Foxe JJ, Robertson IH. Two types of action error: Electrophysiological evidence for separable inhibitory and sustained attention neural mechanisms producing error on go/no-go tasks. J Cogn Neurosci. 2009;21:93–104. doi: 10.1162/jocn.2009.21008. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD+ CD, anxious, and control children: A meta-analysis of studies with the stop task. J Child Psychol Psychiat. 1998;39:411–425. [PubMed] [Google Scholar]
- Owens J. The ADHD and sleep conundrum: A review. J Dev Beh Pediatr. 2005;26:312–322. doi: 10.1097/00004703-200508000-00011. [DOI] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Munz M, Molzow I, Wilhelm I, Wiesner CD, Baving L. Sleep promotes consolidation of emotional memory in healthy children but not in children with attention-deficit hyperactivity disorder. PLoS One. 2013;8:e65098. doi: 10.1371/journal.pone.0065098. https://doi.org/10.1371/journal.pone.0065098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli M, Souissi S, Kurth S, Brandeis D, Jenni OG, Huber R. Topography of sleep slow wave activity in children with attention-deficit/hyperactivity disorder. Cortex. 2013;49:340–347. doi: 10.1016/j.cortex.2012.07.007. https://doi.org/10.1016/j.cortex.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Rolon-Arroyo B, Arnold DH, Harvey EA, Marshall N. Assessing attention and disruptive behavior symptoms in preschool-age children: The utility of the Diagnostic Interview Schedule for Children. J Child Fam Stud. 2016;25:65–76. doi: 10.1007/s10826-015-0203-x. https://doi.org/10.1007/s10826-015-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Pergamin L, Bar-Haim Y. Sleep in children with attention-deficit hyperactivity disorder: A meta-analysis of polysomnographic studies. Sleep Med Rev. 2006;10:381–398. doi: 10.1016/j.smrv.2006.03.004. https://doi.org/10.1016/j.smrv.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Sagaspe P, Taillard J, Amiéva H, Beck A, Rascol O, Dartigues JF, Capelli A, Philip P. Influence of age, circadian and homeostatic processes on inhibitory motor control: A Go/Nogo task study. PLoS One. 2012;7:e39410. doi: 10.1371/journal.pone.0039410. https://doi.org/10.1371/journal.pone.0039410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletin JM, Coon WG, Carskadon MA. Stage 2 sleep EEG sigma activity and motor learning in childhood ADHD: A pilot study. J Clin Child Adolesc Psychol. 2016:1–10. doi: 10.1080/15374416.2016.1157756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar R, Tannock R, Marriott M, Logan G. Deficient inhibitory control in attention deficit hyperactivity disorder. J Abnor Child Psychol. 1995;23:411–437. doi: 10.1007/BF01447206. [DOI] [PubMed] [Google Scholar]
- Schreiner T, Lehmann M, Rasch B. Auditory feedback blocks memory benefits of cueing during sleep. Nature Comm. 2015;6:8729. doi: 10.1038/ncomms9729. https://doi.org/10.1038/ncomms9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley CJ, Smith CT, MacDonald KJ, Beninger RJ. Ventromedial prefrontal theta activity during rapid eye movement sleep is associated with improved decision-making on the Iowa Gambling task. Behav Neurosci. 2016 doi: 10.1037/bne0000123. https://doi.org/10.1037/bne0000123. [DOI] [PubMed]
- Shaffer D, Fisher P, Lucas C, Dulcan M, Schwab-Stone M. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from pervious versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. https://doi.org/10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. https://doi.org/10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurophysiol. 2006;23:441–456. doi: 10.1097/01.wnp.0000221363.12503.78. https://doi.org/10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- Walker M. The role of slow wave sleep in memory processing. J Clin Sleep Med. 2009;5:S20–S26. [PMC free article] [PubMed] [Google Scholar]
- Waschbusch DA. A meta-analytic examination of comorbid hyperactive-impulsive-attention problems and conduct problems. Psychol Bull. 2002;128:118–150. doi: 10.1037/0033-2909.128.1.118. https://doi.org/10.1037//0033-2909.128.1.118. [DOI] [PubMed] [Google Scholar]
- Weiss MD, Craig SG, Davies G, Schibuk L, Stein M. New research on the complex interaction of sleep and ADHD. Curr Sleep Med Rep. 2015;1:114–121. https://doi.org/10.1007/s40675-015-0018-8. [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci U S A. 2010;107:5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong-Liang G, Robaey P, Karayanidis F, Bourassa M, Pelletier G, Geoffroy G. ERPs and behavioral inhibition in a Go/No-Go task in children with attention-deficit/hyperactivity disorder. Brain and Cogn. 2000:43. [PubMed] [Google Scholar]
- Yoon SYR, Jain U, Shapiro C. Sleep in attention-deficit/hyperactivity disorder in children and adults: Past, present, and future. Sleep Med Rev. 2012;16:371–388. doi: 10.1016/j.smrv.2011.07.001. https://doi.org/10.1016/j.smrv.2011.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full power curves for REM and nREM sleep at frontal electrode F4. Raw values were used in statistical analyses; however, log-transformed values are displayed in this figure to aid in interpretation of group differences in activity across REM and nREM sleep