Abstract
Background & objectives:
Millets are rich source of dietary fibre and non-starchy polysaccharides with low glycaemic index (GI), hence can be used as a therapeutic diet. This study was conducted to estimate the effects of a millet-based dosa (foxtail dosa) compared to a rice dosa for breakfast on postprandial glucose levels in patients with type 2 diabetes mellitus (T2DM).
Methods:
The GI of rice dosa and foxtail millet dosa was estimated. A total of 105 T2DM participants were randomly selected for the study. The participants were on oral hypoglycaemic agents (OHA) and not on insulin. In this study, each individual served as their own control and experimental group. The postprandial increase in blood glucose was compared after a breakfast of rice dosa and millet dosa. Single and paired t test was used to note the change in blood glucose levels and the level of the significance.
Results:
The GI of foxtail millet dosa was 59.25 and rice dosa was 77.96. There was a significant reduction (P<0.001) in the postprandial glucose level of patients who consumed a millet-based dosa when compared to those who consumed a rice-based dosa. No significant reduction was observed in the fasting glucose levels.
Interpretation & conclusions:
The results suggested that replacing a rice-based breakfast item with a millet-based breakfast item lowers the postprandial blood glucose levels in T2DM patients. Thus, millets may have a protective role in the management of hyperglycaemia. Further studies need to be done in a systematic manner to confirm these findings.
Keywords: Foxtail millet, glycaemic index, postprandial glucose levels, rice, type 2 diabetes
The global prevalence of diabetes was estimated to be nine per cent in 2014 among adults aged 18 years or more1. Diabetes has reached at an epidemic level, with around 366 million people with diabetes globally in 2011 and it is expected to increase to 552 million by 20302. A WHO-ICMR study based on non-communicable diseases’ risk factor surveillance showed that the prevalence of self-reported diabetes was 7.3 per cent in urban, 3.2 per cent in peri-urban and 3.1 per cent in rural areas3. The prevalence levels of diabetes and prediabetes were 10.4 and 8.3 per cent, respectively4. In 2012, an estimated 1.5 million deaths were directly caused by diabetes5.
More than 80 per cent of diabetes deaths occur in low- and middle-income countries5. The need for implementation of effective dietary strategies in diabetes prevention and management has been emphasized by the success of diet and lifestyle changes in preventing diabetes in high-risk patients6. Another study confirmed postprandial hyperglycaemia as an independent risk factor for cardiovascular disease (CVD) in type 2 diabetes7. Postprandial hyperglycaemia produces oxidative stress, which, in turn, induces endothelial dysfunction and inflammation8, all well-recognized risk factors for CVD. One dietary strategy aimed at improving both diabetes control and cardiovascular risk factors is the use of low glycaemic index (GI) diets9.
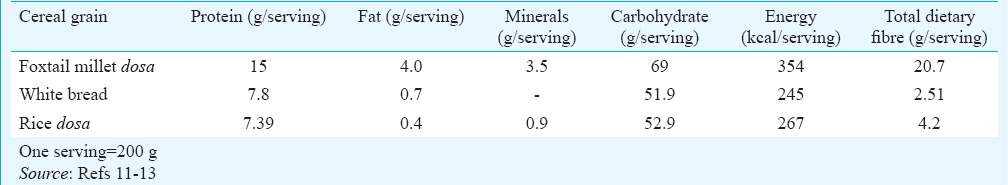
Millets are small seeded grasses that grow well in dry zones as rain-fed crops, under marginal conditions of soil fertility and moisture in the semi-arid tropic region. These are highly nutritious, non-glutinous and non-acid-forming foods. Compared to polished rice, millets release a lesser percentage of glucose and over a longer period. This lowers the risk of diabetes10. Millet grains are considered superior among the major cereals with respect to protein, energy, vitamins and minerals. Besides, these are a rich source of dietary fibre, phytochemicals and non-starchy polysaccharides and reputed to have a low GI, and hence can be used as a therapeutic diet. This study was undertaken to assess the GI of a common Indian recipe (rice dosa) and a millet-based recipe (foxtail dosa). The aim was also to estimate the effect of a single change in the diet in any one meal with a low-GI food on the postprandial level of glucose in patients with type 2 diabetes mellitus (T2DM). The proximate principles of rice and foxtail millet dosa are given in Table I.
Table I.
Proximate principles of rice dosa, foxtail millet dosa and white bread
Material & Methods
Preparation of the rice- and millet-based dosa: For the preparation of the millet dosa, 90 g of unpolished foxtail millet was purchased from the local market along with 20 g of black gram pulses dal), soaked for four hours and were ground with 90 ml of water. The batter was kept overnight for fermentation. The same recipe was followed for preparing the rice-based dosa with rice substituting the foxtail millet.
The final quantity of batter was weighed accurately. This weight (430g) of foxtail millet batter provided 36.4 g of available carbohydrate (CHO) while rice batter provided 77.0 g of available carbohydrate13. Hence, 290 g of foxtail batter and 140 g of rice batter were used to make two dosas. The participants were asked to consume both dosas. This quantity provided 50 g of available carbohydrate. The available carbohydrate was calculated by subtracting the total dietary fibre (TDF) which includes the soluble and insoluble fibre from the total carbohydrate present in 100 g of the test food. The available carbohydrate from black gram was constant in the two varieties of dosa as 20 g was used for both the recipes.
Reference food: White bread was chosen as the reference food14 (purchased from the local bakery). The available carbohydrate was calculated by subtracting the TDF from total CHO. Therefore, 101 g of bread contained 50 g of available CHO.
Estimation of glycaemic index: Ten normal volunteers were selected and the reference sample and the test items were given with a two-day wash out period14. The average fasting blood glucose was 82 mg/dl (average of three different readings on three different days.) The participants were all females between the age range of 22-44 yr and body mass index (BMI) range of 23-28 kg/m2. The purpose of the study was explained to each participant and written informed consent to participate in the study was obtained. They consumed two dosas such that these provided 50 g of carbohydrate and four slices of bread calculated for available carbohydrate13.
After an overnight fast, finger prick blood samples were investigated at 0 (before test meal), 15, 30, 45, 60, 90 and 120 min15. The blood glucose levels at the specified time intervals were measured by glucometer15,16 (Accu-Check meter, Roche, Switzerland).
The blood glucose concentrations after the test meal and reference food were used to draw a blood glucose response curve for the two hours period. The values at 75 and 105 min were obtained by extrapolation15. The incremental area under the curve (AUC) for each meal and reference food was calculated for each volunteer separately to reflect the total rise in the glucose concentration after eating the test and reference food15. The AUC was calculated using the general formula14. GI (%) was calculated as follows15:
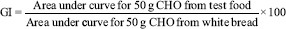
Research design: The study was carried out at M.V. Hospital for Diabetes, Chennai, and the participants were enrolled over a period of eight months in 2014 and 2015 in the department of Diet and Nutrition of the Hospital. Ethical clearance was obtained from the Institutional Ethics Committee. The study protocol was explained to the participants and those who were willing to participate were enrolled in the study and written informed consent was obtained from each participant. A total of 105 participants with T2DM (M69:F36) visiting the M.V. Hospital for Diabetes, Chennai, India, who were never on insulin therapy but were on the same oral hypoglycaemic agents (OHA) for one year and whose blood glucose levels were above normal, were randomly selected for the study of an age group between 35 and 55 yr. The 105 eligible participants were randomly allocated to either rice or millet group on the first day and random sequences were generated using WinPepi software (New York, USA). The Figure represents the study design, the number of eligible participants and the number included for analysis.
Figure.
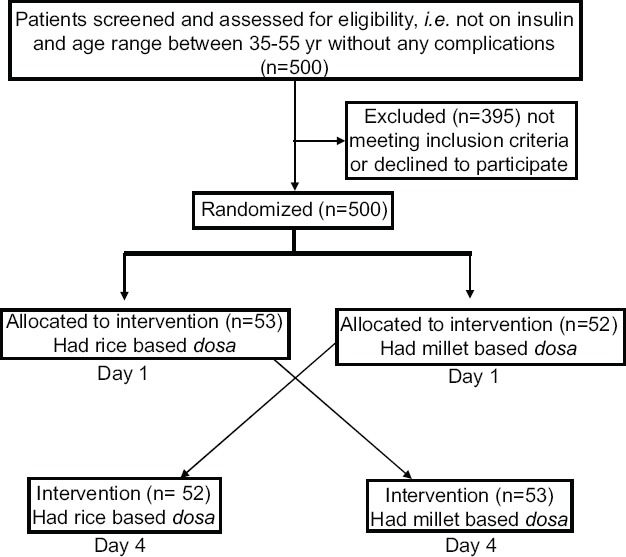
Flow chart showing the design of the study.
Body weight and height were measured before the study and BMI was calculated. Waist and hip circumference of the participants were also measured. Duration of diabetes and information on educational status of the participants were collected using an open-ended questionnaire.
Each individual served as their own control and experimental group. At baseline, all participants were advised to report to the hospital in the fasting state and fasting blood glucose levels were estimated and then 53 participants consumed rice dosa and the rest consumed millet dosa for breakfast, and after one and half hours, the postprandial blood glucose levels were estimated. The blood glucose levels were estimated using the glucose oxidase-peroxidase method in the venous blood using BS 400 Mindray autoanalyzer (Diays Company, Germany). After two days of washout period, millet dosa was given to the first group and rice dosa to the second group and their fasting and postprandial glucose levels were estimated. Acceptability of the millet-based dosa was analyzed using organoleptic rating scale17 with a score of 1-9. The organoleptic scale was given to every individual who participated in the study and they were asked to score the quality of the product based on the colour, flavour and taste of the product. Since the rice-based dosa is common recipe in everyday breakfast, the organoleptic score was not carried out to estimate the acceptability.
Statistical analysis: Statistical analysis was performed for the collected data using SPSS software version 19. (SPSS Inc. IBM, USA) The independent sample and paired t test were used to determine the changes in blood glucose levels.
Results
Of the 105 T2DM patients, 69 were male. The mean age of the participants was 49.3 ± 9.9 yr, mean height was 162.3 ± 8.6 cm, mean weight was 72.2 ± 10.4 kg and mean BMI was 27.4 ± 3.7 kg/m2. The mean waist circumference of the female participants was 90.6 ± 9.2 cm and of the male was 96.6 ± 9.2 cm. The mean duration of diabetes was 4.2 ± 1.9 yr (Table II).
Table II.
Baseline characteristics of the patients
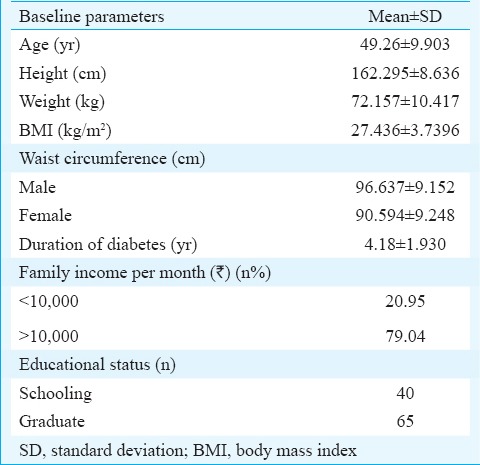
Demographic details revealed that 21 per cent of participants were in the lowest income group category and 79 per cent were in the middle- and high-income groups. Concerning the educational status, 40 per cent of participants had completed schooling and 65 per cent had done their graduation. The details are shown in Table II.
The acceptability of foxtail millet was evaluated using the organoleptic rating scale. Eighty nine per cent of the participants liked the product (score ≥6), <1 per cent neither liked nor disliked the product (score = 5) and about 9.5 per cent disliked the product (score ≤ 4).
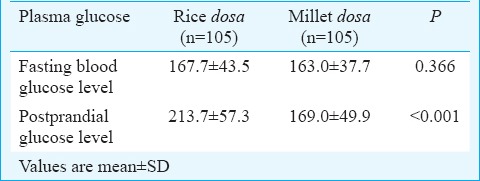
Glycaemic index: The GI of millet-based dosa was 59.25 and that of rice-based dosa was 77.86. The study participants who consumed a millet-based dosa showed a significant reduction in their postprandial glucose levels when compared to the postprandial levels of those who consumed rice-based dosa. No significant differences were observed in the fasting blood glucose levels in both groups (Table III). The mean fasting blood glucose was similar in both the groups, and the postprandial values were significantly (P<0.001) higher in the rice group (Table III).
Table III.
Fasting and postprandial blood glucose levels (mg/dl) in the study participants
Discussion
The GI of the dosa prepared from foxtail millet was low. This may be due to the high levels of soluble dietary fibre in the millet. The TDF was reported to be 27.02 per cent, soluble dietary fibre 11.56 per cent and the insoluble dietary fibre 15.44 per cent11. It has been reported that the high viscosity of the soluble fibre delays digestion and absorption18.
The glucose levels can be maintained to near normal with the help of dietary modification. It was observed in the present study that replacing rice-based dosa with millet-based dosa showed a significant reduction in the postprandial blood glucose levels. The results were in agreement with the previous study19 which showed a 29 per cent decrease in the serum glucose level after consumption of millet-based burfi.
The incremental improvement in glycaemic control afforded by a low-GI diet can be compared with that achieved with other medical interventions such as OHA or insulin20. One of the potential effects of low-GI diets is to reduce insulin secretion in patients with type 2 diabetes and to reduce daily insulin requirements in patients with type 1 diabetes21,22. Wolever et al21 observed a 30 per cent reduction in urinary C peptide levels in patients with type 2 diabetes on a low-GI diet, indicating reduced endogenous insulin demand. After supplementing only one meal with a lower GI food in the meal, namely breakfast in the present study, the participants showed a reduction in their glycaemic profile. A similar result in the use of low-GI foxtail millet biscuits has been reported23.
The high-soluble dietary fibre content has been found to reduce gastric emptying and absorption of glucose after a meal, resulting in improved glucose tolerance. The soluble dietary fibre component has been reported to decrease the activity of digestive enzymes, thus resulting in incomplete hydrolysis of carbohydrates, protein and fats and delaying absorption18. There was a weak but significant correlation between the amount of TDF per 50 g carbohydrate portion of food and GI according to Wolever24. Cellulose content of the food was the best predictor of the GI of the food24. Foxtail millet contains 8.0 g/100 g of crude fibre and rice has 0.2 g/100 g of crude fibre13. The TDF in foxtail millet has been reported to be 27 per cent11 while rice contains only 4.1 per cent13. Low-GI diets favourably influence postprandial metabolism, lowering insulin resistance and lipid and clotting parameters. Together these metabolic effects may explain the long-term benefits of low-GI diets on CVD observed in large cohort follow up studies25.
The metabolic effects relate to the rate at which glucose is absorbed from the small intestine. A reduced rate of glucose absorption after the consumption of low-GI carbohydrate foods will reduce the postprandial rise in gut hormones (e.g., incretins) and insulin. The prolonged absorption of carbohydrate seen over time will maintain suppression of the free fatty acids (FFAs) and the counter regulatory responses, while at the same time achieving lower blood glucose concentrations. Over time, with the reduction in FFA concentrations and the rise in the respiratory quotient with tissue insulinization, glucose is withdrawn from the circulation at a faster rate. Consequently, blood glucose concentrations return towards baseline despite continued glucose absorption from the small intestine. The rise in peak postprandial blood glucose is therefore, reduced together with the incremental blood glucose area above baseline22.
It has been suggested that obesity is related to GI or glycaemic load26,27,28. Studies on altering GI and load have indicated that if the GI and load of the first meal is lower, less food is consumed in the subsequent meal28,29. The use of GI is encouraged as a way to improve the quality of carbohydrates consumed30 and use of GI is of particular benefit for controlling glycaemia, lipids and weight9.
Our study had several limitations. The sample for the present study comprised only 105 patients with T2DM visiting a hospital for diabetes. This sample was only a small portion of all patients with diabetes limiting the generalization of findings. The study was limited to a one-time individual postprandial response to a single meal consisting of low-GI food. Hence, a long-term effect of consumption of millet dosa on blood lipid profile and glycosylated haemoglobin (HbA1c) in T2DM patients would be beneficial. Another limitation of the study was that the available carbohydrate was estimated using the difference method.
In conclusion, our study indicated the potential benefits of foxtail millet in the diet therapy for patients with diabetes. However, there is a scope to explore the potential benefits of millets in the management of metabolic disorders through long-term feeding interventions. If used on a long-term basis, it could be beneficial in controlling lipid profile as well as the HbA1c levels in such patients.
Acknowledgment
The authors thank Prof. M. Viswanathan Diabetes Research Centre for the funding to carry out this work.
Footnotes
Conflicts of Interest: None.
References
- 1.World Health Organization. Global status report on noncommunicable diseases. Geneva: World Health Organization; 2014. [DOI] [PubMed] [Google Scholar]
- 2.IDF diabetes atlas. 5th ed. Brussels, Belgium: International Diabetes Federation; 2011. International Diabetes Federation. [PubMed] [Google Scholar]
- 3.Mohan V, Mathur P, Deepa R, Deepa M, Shukla DK, Menon GR, et al. Urban rural differences in prevalence of self-reported diabetes in India – The WHO-ICMR Indian NCD risk factor surveillance. Diabetes Res Clin Pract. 2008;80:159–68. doi: 10.1016/j.diabres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Anjana RM, Pradeepa M, Deepa M, Datta V, Sudha R, Unnikrishnan A, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Global health estimates: deaths by cause, age, sex and country, 2000-2012. Geneva: WHO; 2014. [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91:813–9. doi: 10.1210/jc.2005-1005. [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A. The possible role of postprandial hyperglycaemia in the pathogenesis of diabetic complications. Diabetologia. 2003;46(Suppl 1):M9–16. doi: 10.1007/s00125-002-0931-5. [DOI] [PubMed] [Google Scholar]
- 9.Kirpitch AR, Maryniuk MD. The 3 R's of glycemic index: recommendations research, and the real world. Clin Diabetes. 2011;29:155–9. [Google Scholar]
- 10.Jali MV, Kamatar MY, Jali SM, Hiremath MB, Naik RK. Efficacy of value added foxtail millet therapeutic food in the management of diabetes and dyslipidamea in type 2 diabetic patients. Rec Res Sci Technol. 2012;4:3–4. [Google Scholar]
- 11.Bangoura ML, Ming ZH, Atindana JN, Xue ZK, Tolno MB, Wei P. Extraction and fractionation of insoluble fibers from foxtail millet (Setaria italica (L.) P. Beauv) Am J Food Technol. 2011;6:1034–44. [Google Scholar]
- 12.Ranhotra G, Gelroth J. Soluble and total dietary fiber in white bread. Am Assoc Cereal Chem. 1988;65:155. [Google Scholar]
- 13.Gopalan C, Ramasastri BV, Balasubramaniam SC. Nutritive value of Indian foods. Hyderabad, India: National Institute of Nutrition; 1991. [Google Scholar]
- 14.Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, et al. Glycaemic index methodology. Nutr Res Rev. 2005;18:145–71. doi: 10.1079/NRR2005100. [DOI] [PubMed] [Google Scholar]
- 15.Chlup R, Bartek J, Reznícková M, Zapletalová J, Doubravová B, Chlupová L, et al. Determination of the glycaemic index of selected foods (white bread and cereal bars) in healthy persons. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148:17–25. doi: 10.5507/bp.2004.003. [DOI] [PubMed] [Google Scholar]
- 16.Hettiaratchi UP, Ekanayake S, Welihinda J. How accurate is glucometer in determining glycemic index? Int Food Res J. 2012;19:1511–6. [Google Scholar]
- 17.Rana S, Sharma S, Katare C, Shrivatava V, Prasad GB. Glycemic response and glycemic index of common sweeteners and honey incorporated products. IOSR J Nurs Health Sci. 2012;1:40–4. [Google Scholar]
- 18.Bisoi PC, Sahoo G, Mishra SK, Das C, Das KL. Hypoglycemic effects of insoluble fiber rich fraction of different cereals and millets. J Food Process Technol. 2012;3:11. [Google Scholar]
- 19.Anju T, Sarita S, Gurmukh S. Effect of foxtail millet (Setaria italica) supplementation on serum glucose, serum lipids and glycosylated hemoglobin in type 2 diabetics. Diabetol Croat. 2011;40:23–8. [Google Scholar]
- 20.Garg A. High-monounsaturated-fat diets for patients with diabetes mellitus: a meta-analysis. Am J Clin Nutr. 1998;67(3 Suppl):577S–82S. doi: 10.1093/ajcn/67.3.577S. [DOI] [PubMed] [Google Scholar]
- 21.Wolever TM, Nguyen PM, Chiasson JL, Hunt JA, Josse RG, Palmason C, et al. Determinants of diet glycemic index calculated retrospectively from diet records of 342 individuals with non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1994;59:1265–9. doi: 10.1093/ajcn/59.6.1265. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins DJ, Kendall CW, Augustin LS, Franceschi S, Hamidi M, Marchie A, et al. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76:266S–73S. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- 23.Anju T, Jr, Sarita S. Suitability of foxtail millet (Setaria italica) and barnyard millet (Echinochloa frumentacea) for development of low glycemic index biscuits. Malays J Nutr. 2010;16:361–8. [PubMed] [Google Scholar]
- 24.Wolever TM. Relationship between dietary fiber content and composition in foods and the glycemic index. Am J Clin Nutr. 1990;51:72–5. doi: 10.1093/ajcn/51.1.72. [DOI] [PubMed] [Google Scholar]
- 25.Brynes AE, Adamson J, Dornhorst A, Frost GS. The beneficial effect of a diet with low glycaemic index on 24 h glucose profiles in healthy young people as assessed by continuous glucose monitoring. Br J Nutr. 2005;93:179–82. doi: 10.1079/bjn20041318. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103:E26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 27.Roberts SB. High-glycemic index foods, hunger, and obesity: is there a connection? Nutr Rev. 2000;58:163–9. doi: 10.1111/j.1753-4887.2000.tb01855.x. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–23. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins DJ, Kendall CW, McKeown-Eyssen G, Josse RG, Silverberg J, Booth GL, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–53. doi: 10.1001/jama.2008.808. [DOI] [PubMed] [Google Scholar]
- 30.Joslin Diabetes Center & Joslin Clinic, Clinical Nutrition Guideline For Overweight And Obese Adults With Type 2 Diabetes, Prediabetes Or Those At High Risk For Developing Type 2 Diabetes. Boston, MA, USA: Joslin Diabetes Center, Publications Department, 617-226-5815; 2011. [accessed on April 9, 2015]. Available from: www.joslin.org/Nutrition_Guidelines-8.22.11(1).pdf . [Google Scholar]


