Sir,
The emergence of carbapenem resistance in Enterobacteriaceae is a growing public health problem worldwide. Among the Enterobacteriaceae, Escherichia coli and Klebsiella pose greatest risk to public health, because of their high prevalence, wide range of clinical infections, multidrug resistance and rapid dissemination of resistance to other organisms1,2.
Carbapenems are regularly used as the last choice for the management of multidrug-resistant E. coli and Klebsiella infections3. However, the recent emergence and dissemination of carbapenem resistance raise a question on the effectiveness of empirical therapy with carbapenems4,5. In India, there are no valid data on the prevalence of carbapenem-resistant Enterobacteriaceae (CRE)6; although a few reports have been published from metropolitan cities which are biased in sampling towards the selection of resistant strains7,8. Therefore, this hospital-based study was aimed to assess the occurrence of CRE in a rural part of Tamil Nadu, south India.
A cross-sectional prospective study was conducted in the microbiology department to measure the carbapenem resistance in E. coli and Klebsiella spp. among the patients attending a 900-bedded tertiary care teaching hospital (Government Theni Medical College, Theni, India). The study was conducted from January 2012 to December 2014. All clinical isolates of E. coli and Klebsiella spp. recovered from the clinical specimens such as urine, pus, sputum, blood, body fluids, stool and others, collected from the patients of this hospital were studied. All isolates were tested for ertapenem (ETP) (Merck, India) susceptibility by disc diffusion antimicrobial susceptibility test (AST) method using in-house prepared 10 μg ETP disc by following the Clinical and Laboratory Standards Institute (CLSI) recommendations9 with ATCC 25922 E. coli as susceptible quality control. Isolates showing the zone of inhibition of <22 mm to ETP disc were identified as non-susceptible (NS)10,11, and randomly selected ETP NS isolates were preserved for minimum inhibitory concentration (MIC) testing. Four carbapenems including ETP (Merck), imipenem (IPM), meropenem (MEM) and doripenem (DOR) (Sigma, USA) were tested for MIC by agar dilution AST method12 for the concentration extending from 0.016 to 64 μg/ml. The susceptibility breakpoints (intermediate range) were >0.5 to <2 μg/ml for ETP, and >1 to <4 μg/ml for IPM, MEM and DOR, and results were interpreted as per the CLSI guidelines11. The susceptibility related information was saved and analyzed with WHONET software ver. 5.6 (www.whonet.org); further, the statistical analysis was performed by Chi-square test. The study protocol was approved by the ethics committee of Government Theni Medical College, Theni.
During the study period, a total of 2292 non-repetitive clinical isolates of E. coli (n = 1338) and Klebsiella spp. (n = 954) were isolated. Of these, 444 isolates were identified as ETP NS by disc diffusion method, and these included 207 (15.5%) isolates E. coli and 237 (24.8%) Klebsiella spp. Among the 444 ETP NS isolates, randomly selected 198 (E. coli -103 and Klebsiella spp. - 95) isolates were tested for MIC of carbapenems and 150 were confirmed (E. coli - 73 and Klebsiella spp. - 77) as ETP NS isolates. These isolates were recovered from 71 (47.3%) male and 79 (52.7%) female patients with the median age of 42 years (range - one day to 78 yr).
Forty-eight (24.2%) of 198 ETP NS isolates detected by disc diffusion method were found susceptible by agar dilution method. Further, 33 (16.7%) were identified as intermediate resistant. Hence, only 117 of 198 isolates were confirmed as ETP resistant (Table).
Table.
Carbapenem resistance by minimum inhibitory concentration in Escherichia coli and Klebsiella spp.
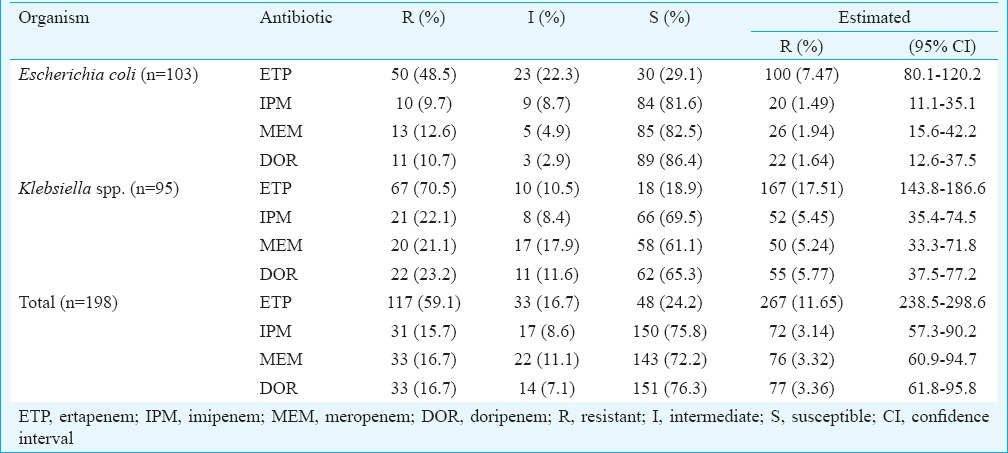
Although the disc diffusion method revealed 19.4 per cent (444/2292) ETP NS isolates, these included a considerable proportion of susceptible isolates, which were later identified by agar dilution method. Hence, the presence of CRE was estimated based on the MIC results of sample data (n = 103 for E. coli and 95 for Klebsiella spp.). The estimated carbapenem resistance was about three per cent with the notable exception of ETP, which had 4-fold higher resistance rate (~12%) when compared with other carbapenems13. Further, the resistance was higher among Klebsiella spp. and the difference was significant (P<0.05). The resistance of ETP [17.51 vs. 7.47%; odds ratio (OR) 2.63; 95% confidence interval (95% CI) 2.01-3.42] and MEM (5.24 vs. 1.94%; OR 2.79; 95% CI 1.72-4.52) was 2-fold higher in Klebsiella spp.; besides, the resistance of IPM (5.45 vs. 1.49%; OR 3.80; 95% CI 2.25-6.41) and DOR (5.77 vs. 1.64%; OR 3.66; 95% CI 2.22-6.04) was 3-fold higher in Klebsiella spp. when compared with E. coli14. The carbapenem resistance seen in the present study was not considerably different from that reported by Gupta et al15 in New Delhi. However, according to a recent report based on the systematic literature obtained from the Asian countries14, the resistance rate of IPM and MEM was, respectively, 0.2 and 0.5 per cent in E. coli, and 1.9 and 2.4 per cent in Klebsiella spp. The current study showed higher carbapenem resistance in both E. coli and Klebsiella spp. when compared with the average of Asian countries necessitating timely detection and appropriate infection control measures to contain the spread of CRE in this region.
In conclusion, the present study documented carbapenem resistance in about three per cent clinically important members of Enterobacteriaceae from south India. The newer carbapenem ETP had 4-fold higher resistance rate. Further, molecular investigations need to be done to understand the mechanism of resistance.
Acknowledgment
Authors thank the Indian Council of Medical Research, New Delhi, India, for financial support, and Shrimati Pandiyan Thenmozhi and Shrimati Thangam Devisri for technical assistance.
Footnotes
Conflicts of Interest: None.
References
- 1.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Temkin E, Adler A, Lerner A, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci. 2014;1323:22–42. doi: 10.1111/nyas.12537. [DOI] [PubMed] [Google Scholar]
- 3.McKenna M. Antibiotic resistance: the last resort. Nature. 2013;499:394–6. doi: 10.1038/499394a. [DOI] [PubMed] [Google Scholar]
- 4.Dortet L, Poirel L, Nordmann P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res Int 2014. 2014:249856. doi: 10.1155/2014/249856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13:785–96. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization. 2014. [accessed on January 24, 2017]. Available from: apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf .
- 7.Rahman M, Shukla SK, Prasad KN, Ovejero CM, Pati BK, Tripathi A, et al. Prevalence and molecular characterisation of New Delhi metallo-ß-lactamases NDM-1, NDM-5, NDM-6 and NDM-7 in multidrug-resistant Enterobacteriaceae from India. Int J Antimicrob Agents. 2014;44:30–7. doi: 10.1016/j.ijantimicag.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Kazi M, Drego L, Nikam C, Ajbani K, Soman R, Shetty A, et al. Molecular characterization of carbapenem-resistant Enterobacteriaceae at a tertiary care laboratory in Mumbai. Eur J Clin Microbiol Infect Dis. 2015;34:467–72. doi: 10.1007/s10096-014-2249-x. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disk susceptibility tests: Approved standard. 11th ed. CLSI document M02-A11. Wayne, PA: CLSI; 2012. [Google Scholar]
- 10.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: Twenty second informational supplement. CLSI document M100-S22. Wayne, PA: CLSI; 2012. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: Twenty fifth informational supplement. CLSI document M100-S25. Wayne, PA: CLSI; 2015. [Google Scholar]
- 12.Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard. 9th ed. CLSI document M07-A9. Wayne, PA: CLSI; 2012. [Google Scholar]
- 13.Wang JT, Wu UI, Lauderdale TL, Chen MC, Li SY, Hsu LY, et al. Carbapenem-nonsusceptible Enterobacteriaceae in Taiwan. PLoS One. 2015;10:e0121668. doi: 10.1371/journal.pone.0121668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Gu B, Huang M, Liu H, Xu T, Xia W, et al. Epidemiology of carbapenem resistant Enterobacteriaceae (CRE) during 2000-2012 in Asia. J Thorac Dis. 2015;7:376–85. doi: 10.3978/j.issn.2072-1439.2014.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta E, Mohanty S, Sood S, Dhawan B, Das BK, Kapil A. Emerging resistance to carbapenems in a tertiary care hospital in North India. Indian J Med Res. 2006;124:95–8. [PubMed] [Google Scholar]


