Abstract
Background
Perioperative systemic glucocorticoids are frequently included in multimodal analgesia and antiemetic regimens administered to patients undergoing total hip arthroplasty (THA) and total knee arthroplasty (TKA). The objective of this systematic review was to evaluate the available randomized controlled trials (RCTs) to determine the effect of perioperative systemic glucocorticoids on postoperative nausea and vomiting (PONV), pain, narcotic consumption, antiemetic consumption, length of stay in hospital, and major complications in patients undergoing elective THA or TKA.
Methods
A predefined protocol of eligibility and methodology was used for conduct of systematic reviews. Two reviewers screened citations for inclusion, assessed methodological quality, and verified the extracted data.
Results
Six RCTs were included for analysis. Across all outcomes analyzed, patients who received glucocorticoids experienced either a benefit or no difference compared to those patients who did not receive glucocorticoids. There were no instances in which perioperative glucocorticoids had a negative impact on any of the outcomes that were analyzed. Furthermore, perioperative glucocorticoids had no effect on the rates of superficial infection, deep infection, wound complications or deep vein thrombosis (DVT).
Conclusion
The results of this systematic review support the use of perioperative systemic glucocorticoids in patients undergoing elective total hip and knee arthroplasty. Perioperative glucocorticoids have overall positive outcomes with the benefits being more robust in those patients undergoing TKA compared to THA. Glucocorticoids did not increase the occurrence of major complications. There is limited data to support the conclusion that they can reduce length of stay in hospital.
Keywords: Total knee arthroplasty, Total hip arthroplasty, Glucocorticoid, Analgesia, Systematic review
1. Introduction
Total hip and knee arthroplasty are established as effective means of improving pain and function in those suffering from hip and knee arthritis. Patient satisfaction at one year for TKA has been reported between 75 and 89.8% and satisfaction with THA is even higher.1 This has resulted in the procedures being among the most common elective orthopedic surgeries. Despite arthroplasty’s long-term success, the patients’ immediate post-operative experience can be unpleasant due to pain, nausea, and vomiting.2, 3 Not only does this lead to decreased patient satisfaction, it can also preclude participation in physiotherapy and limit early mobilization. This results in increased length of hospital stay and increased utilization of analgesic and anti-emetic medications.4 For payers of health care, this ultimately leads to increased resource utilization.
Perioperative systemic glucocorticoid administration has been shown to decrease post-operative nausea and vomiting (PONV), as well as pain in a number of surgical populations.5, 6 The addition of glucocorticoids to a multimodal analgesia regimen can also reduce the amount of anti-emetic and analgesic medications consumed in the early post-operative period.5 Theoretically, in patients undergoing arthroplasty of the hip and knee, a reduction in PONV and pain should lead to earlier mobilization and discharge from hospital. The benefits of improved patient satisfaction and decreased resource utilization must be balanced by the potential risks of glucocorticoid use. The most devastating complications following arthroplasty surgery include infection and wound complications. Glucocorticoids historically have been shown to increase the risk of both; however, this is secondary to prolonged use.7 The risk of complications following limited perioperative use of glucocorticoids in arthropasty surgery has not been clearly established.
The objective of this systematic review is to evaluate the available randomized controlled trials investigating glucocorticoid use in hip and knee arthroplasty to answer the following questions: 1) Are glucocorticoids effective at reducing PONV and pain?; 2) Do glucocorticoids reduce post-operative narcotic and anti-emetic utilization?; 3) Do patients who receive perioperative glucocorticoids have reduced length of hospital stay?; and 4) Do glucocorticoids increase the risk of major complications including superficial and deep infection, thromboembolic events, and wound healing?
2. Methods
A predefined protocol of eligibility and methodology was used for conduct of systematic review and meta-analysis of randomized controlled trials (RCT).
2.1. Literature search
A literature search was performed with the assistance of a health sciences librarian for articles published prior to and including October 3, 2015. The following databases were searched: MEDLINE and EMBASE through the OVID interface, PubMed, and the Cochrane Library. Search terms included MeSH and Emtree headings as well as keywords related to arthroplasty; glucocorticoids; and randomized controlled trials. Additionally; references of included studies were screened to identify other eligible trials.
2.2. Eligibility criteria
Inclusion and exclusion criteria were established prior to conducting the literature search. Randomized controlled trials that compared any type of perioperative systemic glucocorticoid use to placebo or standard post-operative analgesia in adult patients undergoing elective hip or knee arthoplasty were eligible for inclusion. We did not limit studies based on type, dose or timing of systemic glucocorticoid. We excluded non-randomized trials, studies investigating local administration of glucocorticoids, surgeries other than hip and knee arthroplasty, studies with co-intervention bias, and studies published in languages other than English.
2.3. Screening and assessment of eligibility
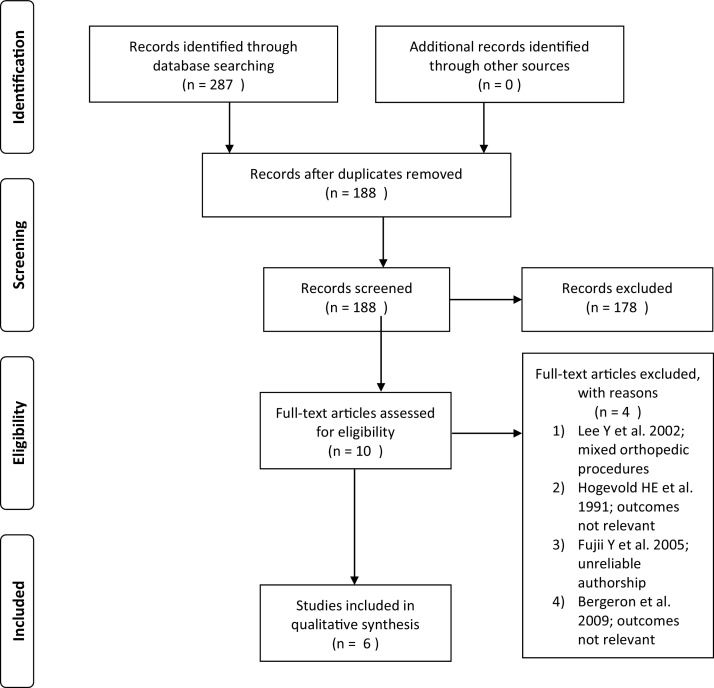
Following the literature search, duplicates were removed and the remaining pool of citations were screened for eligiblity. Two reviewers, blinded to author names, journal title and year of publication, screened all titles and abstracts to identify eligible studies. The remaining pool of studies were reviewed in full-text by both reviewers for final inclusion. Disagreements were resolved by discussion between the two reviewers; if agreement could not be reached, a third reviewer resolved the discrepancy. The PRISMA flow diagram was used to illustrate the search and study selection process (Fig. 1).
Fig. 1.
PRISMA flow diagram.
2.4. Assessment of methodological quality
Two reviewers assessed the methodological quality of the included studies using the Cochrane Collaboration’s Risk of Bias Tool. This tool is designed to evaluate randomized controlled trials for sources of bias in the following domains: selection bias, performance bias, detection bias, attrition bias, reporting bias and other sources of bias. Any disagreements in the final assessment of bias were resolved by consensus through discussion between the two reviewers.
2.5. Data extraction
One reviewer extracted the demographic and outcome data for each included study. Authors of included studies were contacted to provide missing data points as needed. The final data was entered into an electronic spreadsheet and verified by a second reviewer.
2.6. Statistical analysis
Pooling of demographic and outcome data for a meta-analysis of the included studies was not possible due to inconsistent reporting of outcome measures, differences in interventions and different follow-up times. The outcome data was reported as mean with or without standard deviation (SD) by some studies and median with interquartile range (IQR) or range by others. The mean and median pain scores were converted to a scale from 0 to 100 for consistency. Cohen’s kappa coefficient was calculated for interrater agreement between reviewers following assessment of study eligibility. Kappa values were interpreted as ≤0 indicating no agreement, 0.01–0.20 as none to slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect agreement.8
3. Results
3.1. Studies included
The electronic literature search identified 188 unique studies after 99 duplicates were removed (Fig. 1). Title and abstract screening by the 2 reviewers eliminated 178 studies, leaving 10 studies for full-text review. Screening the full-text eliminated an additional 4 studies leaving a total of 6 studies for analysis.9, 10, 11, 12, 13, 14 One of the 4 studies eliminated from the final analysis was authored by Yoshitaka Fujii.15 Although not formally retracted, serious concerns regarding the validity of the study exist due to the author’s record of numerous retractions.16, 17 Screening the references of included studies did not yield any additional studies. Agreement between the reviewers for eligibility was substantial (kappa = 0.692, 95% CI 0.44–0.95)
3.2. Risk of bias
Overall, the six studies had a low risk of bias across the 7 domains assessed by the Cochrane Risk of Bias Tool (Table 1). The study by Backes et al. was determined to be at high risk of bias under the ‘selective reporting’ domain due to consistently failing to report standard deviations (SD) for outcome data. Four studies were deemed to be at high risk of bias under the ‘selective reporting’ domain for failure to report SD, interquartile range (IQR), or range for a single outcome. One study was deemed to be at high risk of bias under the ‘other sources of bias’ domain due to a potential conflict of interest of one of the authors. The study by Mathiesen et al. had one author disclose an unrestricted research grant from Pfizer which is a supplier of pregabalin; a medication used in the study.13 Two other studies were deemed to have an unclear risk of bias under the ‘other sources of bias’ due to failure to disclose any conflicts of interest. The other five domains were rated as low risk of bias across all six studies.
Table 1.
Risk of Bias Summary.
| Author,Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Backes JR et al. [9] | + | + | + | + | + | – | ? |
| Lunn TH et al. [10] | + | + | + | + | + | – | + |
| Lunn TH et al. [11] | + | + | + | + | + | – | + |
| Koh IJ et al. [12] | + | + | + | + | + | + | + |
| Mathiesen O et al. [13] | + | + | + | + | + | – | – |
| Kardash KJ et al. [14] | + | + | + | + | + | – | ? |
1. Random sequence generation.
2. Allocation concealment
3. Blinding of participants and personnel
4. Blinding of outcome assessment
5. Incomplete outcome data
6. Selective reporting
7. Other bias.
+, Low risk of bias; −, high risk of bias; ?, unclear risk of bias.
3.3. Study and subject characteristics
The included RCTs were published between 2008 and 2013. There were a total of 655 subjects randomized in the 6 studies; 265 subjects received total hip arthroplasty and 390 subjects received total knee arthroplasty. Sample sizes ranged from 48 to 269. The average age varied from 65.1 to 72 years. Followup ranged from 24 h to one year (Table 2).
Table 2.
Study Characteristics.
| Author,Year | Surgery | Subjects (control/intervention/[intervention]) | Glucocorticoid/timing | Equivalent dexamethasone dose (mg) http://clincalc.com/Corticosteroids/ |
Anaesthesia | Pre-op analgesia | Post-op analgesia | Followup | Age (Mean) |
|---|---|---|---|---|---|---|---|---|---|
| Backes JR et al. [9] | TKA or THA | N = 120 (37/41/42) | Dexamethasone 10 mg IV/immediately prior to surgery; third group additional dose 24 h post-op | 10 | General | Oxycodone ER, celecoxib | PCA (dilaudid)x24 h, Oxycodone, hydrocodone/acetaminophen and/or dilaudid TKA patients − intra-articular pain pump (bupivicaine), celecoxib |
24 weeks | 66 |
| Lunn TH et al. [10] | TKA | N = 48 (24/24) | Methylprednisolone 125 mg IV/immediatley prior to surgery | 25 | Spinal | Gabapentin, acetaminophen, celecoxib | Periarticular injection (ropivacaine, epinephrine), celecoxib, acetaminophen, gabapentin, sufentanil in PACU, oxycodone | 30 days | 66.5 |
| Lunn TH et al. [11] | THA | N = 48 (24/24) | Methylprednisolone 125 mg IV/immediatley prior to surgery | 25 | Spinal | Gabapentin, acetaminophen, celecoxib | Celecoxib, acetaminophen, gabapentin, sufentanil in PACU, morphine | 30 days | 66 |
| Koh IJ et al. [12] | TKA | N = 291 (145/146) | Dexamethasone 10 mg IV/one hour prior to surgery | 10 | spinal, continuous femoral nerve block | Pregabalin, oxycodone ER, celecoxib, acetaminophen | Periarticular injection (ropivacaine, morphine, ketorolac, epinephrine, cefuroxime), PCA (fentanyl), continuous femoral nerve block, celecoxib, pregabalin, acetaminophen, ketoprofen | 1 year | 72 |
| Mathiesen O et al. [13] | THA | N = 126 (42/42/42) | Dexamethasone 8 mg IV/immediately prior to surgery | 8 | Spinal | Acetaminophen | PCA (morphine), acetaminophen | 24 h | 67 |
| Kardash KJ et al. [14] | THA | N = 50 (25/25) | Dexamethsone 40 mg IV/immediately prior to surgery | 40 | Spinal | None | PCA (morphine), acetaminophen, ibuprofen | 1 month | 69 |
3.4. Interventions
Two of the 6 studies used systemic methylprednisolone, whereas the remaining 4 studies used systemic dexamethasone. For comparison, the systemic glucocorticoid doses were converted to equivalent doses of intravenous dexamethasone. The intervention groups received a range of glucocorticoid doses from 8 mg to 40 mg of intravenous dexamethasone. Three studies administered the glucocorticoid immediately prior to spinal anaesthesia, one study administered it one hour prior to spinal anaethesia, one study administered it after spinal anaethesia was confirmed by decreased sensation to light touch over the greater trochanter and the final study administered it prior to the induction of general anaesthesia with one group receiving an additional dose 24 h post-operatively. Significant differences in post-operative analgesia regimens existed among the included studies as outlined in Table 2.
3.5. Outcomes
3.5.1. Narcotic consumption in 24 hours
All 6 studies evaluated the quantity of narcotic consumed in the 24 h following surgery (Table 3). Three of 6 studies found a significant reduction in the mean or median quantity of narcotic consumed over this time period in those who received perioperative glucocorticoids. For comparison the quantity of all narcotics consumed were converted to milligram equivalents of intravenous morphine. Backes et al.9 showed the largest reduction, reporting 11.3 mg less of IV morphine consumed by those patients who received perioperative steroids in the first 24 h following surgery.
Table 3.
Outcomes.
| Author,Year | Study Group | Length of Stay (Mean/SD) | Length of Stay (Median/IQR) | Narcotic consumption 0–24 h post-op (Mean[SD]) (IV morphine mg equivalent) http://clincalc.com/opioids/ |
Narcotic consumption 0–24 h post-op (Median[Range]) (IV morphine mg equivalent) http://clincalc.com/opioids/ |
# of patients requiring anti-emetics 0–24 h post-op | Anti-emetic consumption 0–24 h post op (Mean[SD]) (ondansetron mg equivalents) |
Anti-emetic consumption 0–24 h post op (Median[Range]) (ondansetron mg equivalents) |
Vomiting 0–24 h post-op (# of patients) |
|---|---|---|---|---|---|---|---|---|---|
| Backes JR et al. [9] | Group 1 (control) |
3.97 (1.14) | *23 (−) | – | – | *3.26 (−) | – | – | |
| Group 2 (dex + zofran) | *3 (0.329) | 11.7 (−) | – | – | 0.75 (−) | – | – | ||
| Group 3 (dex + zofran + dex) | **2.575 (0.087) | – | – | ||||||
| Lunn TH et al. [10] | Group 1 (control) | – | 2 (2–3) | – | 8 (3–16) | 12 | – | 2 (−) | 4 |
| Group 2 (methylpred) | – | 2 (2–3) | – | *4 (0–11) | *3 | – | *0 (−) | 2 | |
| Lunn TH et al. [11] | Group 1 (control) | – | 1 (1–2) | – | 6 (−) | 3 | – | – | 4 |
| Group 2 (methylpred) | – | 1 (1–2) | – | 4 (−) | 1 | – | – | 3 | |
| Koh IJ et al. [12] | Group 1 (Ra) | – | – | 16 (9) | – | 66 | – | – | 35 |
| Group 2 (dex + Ra) | – | – | *10 (6) | – | *36 | – | – | *9 | |
| Mathiesen O et al. [13] | Group 1 (control) | – | 4 (−) | 48.7 (27.8) | – | 15 | 2.9 (4.3) | 6 | |
| Group 2 (pregabalin) | – | 5 (−) | 23.9 (13.8) | – | 15 | 2.1 (2.9) | – | *10 | |
| Group 3 (pregabalin + dex) | – | 4 (−) | 25.2 (18.8) | – | 10 | 1 (1.9) | – | 2 | |
| Kardash KJ et al. [14] | Group 1 (control) | – | – | 28.8 (16.5) | – | 7 | – | – | – |
| Group 2 (dex) | – | – | 21.6 (11.8) | – | 1 | – | – | – | |
Note: *indicates statistically significant difference between experimental groups. **indicates statistically significant difference between experimental group (*).
3.5.2. Pain score at 4 and 24 h postoperative at rest and with ambulation
Table 4 shows the data on pain score at different time points. In the included 6 studies, pain scores were reported utilizing Visual Analog Scales (VAS) or Numeric Rating Scales (NRS) at various time points. Pain at rest 24 h following surgery was documented in all 6 studies. Three of the 6 studies found a significant reduction in mean or median pain score 24 h post-operatively in those patients receiving perioperative glucocorticoids. Pain at rest 4 h following surgery was reported in 4 studies. Only one of the 4 studies found a significant reduction in median pain score 4 h post-operative in those receiving glucocorticoids. Four of the 6 studies documented mean or median pain with ambulation 24 h following surgery. Of these 4 studies 2 found a significant reduction in pain score with ambulation 24 h post-operative in those subjects who received perioperative glucocorticoids. Pain with early mobility 4 h following surgery was investigated in 3 studies. Out of the 3 studies only one found a significant reduction in median pain score with ambulation 4 h post-operative in the group that received glucocorticoids.
Table 4.
Outcomes.
| Author,Year | Study Group | Pain Score at rest 24 h (Mean[SD]) | Pain Score at rest 24 h (Median [Range]) | Pain Score with ambulation 24 h (Mean[SD]) | Pain Score with ambulation 24 h (Median[Range]) | Pain Score at rest 4 h (Mean[SD]) | Pain Score at rest 4 h (Median[Range]) | Pain Score with ambulation 4 h (Mean[SD]) | Pain Score with ambulation 4 h (Median[Range]) |
|---|---|---|---|---|---|---|---|---|---|
| Backes JR et al. [9] | Group 1 (control) |
*39.6 (−) | – | – | – | – | – | – | – |
| Group 2 (dex + zofran) | 19.6 (−) | – | – | – | – | – | – | – | |
| Group 3 (dex + zofran + dex) | |||||||||
| Lunn TH et al. [10] | Group 1 (control) | – | 46.45 (10–76.1) | – | 69.5 (32–88.5) | – | 26.25 (10.5–68) | – | 44.8 (14–74) |
| Group 2 (methylpred) | – | *19.15 (1.5–36) | – | *26.8 (10.5–62) | – | *15.6 (0−51) | – | *19.05 (1–58.5) | |
| Lunn TH et al. [11] | Group 1 (control) | – | 10.15 (0–44.8) | 38.15 (11.6–75) | – | 6.05 (0−51.9) | – | 29.65 (1.7–64.4) | |
| Group 2 (methylpred) | – | 5.35 (0–39.4) | 22.65 (0–48) | – | 19.15 (0−47.4) | – | 26 (0–51.9) | ||
| Koh IJ et al. [12] | Group 1 (Ra) | 40 (20) | – | – | – | – | – | – | – |
| Group 2 (dex + Ra) | *24 (10) | – | – | – | – | – | – | – | |
| Mathiesen O et al. [13] | Group 1 (control) | 12.9 (12.9) | – | 31.7 (19.6) | – | 18.5 (18.2) | – | 22.4 (18.8) | – |
| Group 2 (pregabalin) | 12.8 (11.2) | – | 30.1 (21.3) | – | 16.1 (16.2) | – | 19.1 (20.7) | – | |
| Group 3 (pregabalin + dex) | 12.9 (15.8) | – | 27.6 (19.7) | – | 11.6 (18.8) | – | 14.4 (22.2) | – | |
| Kardash KJ et al. [14] | Group 1 (control) | 19 (18) | – | *69 (−) | – | 25 (20) | – | – | – |
| Group 2 (dex) | 15 (11) | – | 26 (−) | – | 21 (21) | – | – | – | |
Note: *indicates statistically significant difference between experimental groups; pain scores converted to 0–100 scale
3.5.3. Anti-emetic consumption
Five of the 6 included studies recorded the number of patients requiring anti-emetic medication in the 24 h following surgery (Table 3). Two of the 5 studies demonstrated a significant reduction in the number of patients requiring anti-emetic treatment in those who received pre-emptive glucocorticoids.
Three of the 6 studies recorded quantity of anti-emetics consumed in the 24 h following surgery. Two of the 3 studies found those patients who received glucocorticoids perioperatively had a significant reduction in the amount of anti-emetic medication consumed.
3.5.4. Emesis
Four of the included studies reported the number of patients that had an episode of emesis in the 24 h following surgery (Table 3). Two of the 4 studies found that significantly fewer patients had an episode of emesis in the those patients who received perioperative glucocorticoid group compared to the control groups. had significantly fewer subjects experience emesis.
3.5.5. Length of stay in hospital
Four of the 6 studies reported mean or median length of stay in hospital following their respective arthroplasty procedure (Table 3). Only one study found glucocorticoid administration to shorten the average length of stay in hospital. Backes et al.9 reported a statistically significant reduction in length of stay in those who received preoperative glucocorticoids and a further benefit in those who received an additional dose 24 h following surgery.
3.5.6. Complications
The occurrence of major complications, including deep and superficial infection, deep vein thrombosis (DVT) and wound healing, was rare in those who received glucocorticoids and those who did not across all 6 studies (Table 5). Complications reported by Backes et al.9 included one DVT in both the control group and the group that received a single dexamethasone dose. The group that received an additional dexamethasone dose at 24 h had one subject experience a superficial wound infection. The study by Koh et al.12 had one deep infection and two wound healing complications in the group that received glucocorticoids compared to one deep infection and three wound healing complications in the control group. The remaining four studies did not report any major complications. Other complications reported included stitch abscess,9 knee stiffness requiring manipulation,9 and hip dislocation.11
Table 5.
Outcomes.
| Author,Year | Study Group | Complications − deep infection | Complications − superficial infection | Complications − DVT | Complications − Wound Healing |
|---|---|---|---|---|---|
| Backes JR et al. [9] | Group 1 (control) |
0 | 0 | 1 | – |
| Group 2 (dex + zofran) | 0 | 0 | 1 | – | |
| Group 3 (dex + zofran + dex) | 0 | 1 | 0 | – | |
| Lunn TH et al. [10] | Group 1 (control) | 0 | 0 | 0 | 0 |
| Group 2 (methylpred) | 0 | 0 | 0 | 0 | |
| Lunn TH et al. [11] | Group 1 (control) | 0 | 0 | 0 | 0 |
| Group 2 (methylpred) | 0 | 0 | 0 | 0 | |
| Koh IJ et al. [12] | Group 1 (Ra) | 1 | 0 | – | 3 |
| Group 2 (dex + Ra) | 1 | 0 | – | 2 | |
| Mathiesen O et al. [13] | Group 1 (control) | – | – | – | – |
| Group 2 (pregabalin) | – | – | – | – | |
| Group 3 (pregabalin + dex) | – | – | – | – | |
| Kardash KJ et al. [14] | Group 1 (control) | 0 | 0 | – | 0 |
| Group 2 (dex) | 0 | 0 | – | 0 | |
4. Discussion
Perioperative glucocorticoids have been advocated due to a number of reported postoperative benefits; most commonly a reduction in post-operative nausea, vomiting and pain.18 Other reported benefits in the literature include reduced fatigue, reduced muscle weakness, improved mobility, and decreased pulmonary complications.19, 20 Use of corticosteroids prophylactically for the prevention of PONV is well established. Recent consensus guidelines by the Society for Ambulatory Anesthesiology includes dexamethasone or methylprednisolone as an intervention to prevent PONV in those patients identified as moderate risk.21 The use of perioperative glucocorticoids to reduce postoperative pain is also well established across a range of surgeries. De Oliveira et al. conducted a meta-anaysis on the use of perioperative dexamethasone to reduce postoperative pain in patients undergoing a variety of surgeries.22 They concluded that intermediate and high-dose dexamethasone can decrease post-operative opiod consumption and pain. These benefits were greater when dexamethasone was administered preoperatively versus intraoperatively. In another meta-analysis, Waldron et al. evaluated the impact of perioperative dexamethasone and found patients, undergoing a variety of surgeries, treated with dexamethasone experienced less postoperative pain, required less postoperative opioids, had longer time to first analgesic dose, needed less rescue analgesia, and had shorter recovery room stays.5
Total hip and knee arthroplasty are major orthopedic surgeries in which patients can experience intense pain and unpleasent nausea and vomiting; subsequently, length of stay in hospital may be prolonged.2 This systematic review is the first to evaluate the effects of perioperative systemic glucocorticoids in patients undergoing hip and knee arthroplasty exclusively. An earlier systematic review evaluated the benefits and risks associated with any type of perioperative glucocorticoid, systemic or local, in any type of elective hip or knee surgery.23 The authors found that both low and high dose systemic glucocorticoids have anti-emetic effects but only at high doses do glucocorticoids exhibit an analgesic effect (low and high doses of glucocorticoids were defined as ≤10 mg and >10 mg of dexamethasone, respectively). Since the publication of this systematic review, two additional randomized controlled trials, which are included in this review, have been published with a combined 411 subjects undergoing hip or knee arthroplasty and receiving systemic glucocorticoids.9, 12
The results of this systematic review support, although not strongly, the use of perioperative glucocorticoids during elective hip and knee arthroplasty. Across all outcomes analyzed, patients who received glucocorticoids experienced either a benefit or no difference compared to those patients who did not receive glucocorticoids. There were no instances in which perioperative glucocorticoids had a negative impact on any of the outcomes that were analyzed. Reduction in pain at rest and ambulation was greater at 24 h compared to 4 h. Half of the studies that reported on narcotic and anti-emetic consumption as well as the incidence of emesis found a benefit with glucocorticoid use. Only one study demonstrated a reduction in length of stay in hospital due to the administration of perioperative glucocorticoids. In the studies that investigated total knee arthroplasty, patients that received perioperative glucocorticoids had nearly universal positive outcomes. In contrast, patients undergoing hip arthroplasty experienced far fewer positive outcomes with glucocorticoid administration.
It is well known that glucocorticoids can have adverse effects on wound healing and increase the risk of infection. This risk is proportional to the duration and dose of corticosteroid; with long term, higher doses posing the greatest risk.19 It has been shown in the literature that the use of short-term perioperative glucocorticoids in healthy patients is safe and low risk.24 The results of our review support the safe use of perioperative glucocorticoids in patients undergoing hip and knee arthroplasty. The followup for the included studies in our systematic review ranged from 24 h to 1 year. During this followup, there were no differences in the rates of superficial infection, deep infection, wound complications or deep vein thrombosis. These findings in an arthroplasty population are consistent with three meta-analyses that found no increased complication rate in patients who received perioperative glucocorticoids compared to those who did not while undergoing a variety of surgeries.5, 6, 19
The strengths of this systematic review include a thorough and comprehensive review of the literature with multiple reviewers involved in screening, assessment, and data verification. This review process identified six good quality randomized controlled trials from which we were able to draw our conclusions. This is a strength as, compared to other study designs, randomized controlled trials are less prone to bias. Another strength is that we included only studies whose subjects underwent total knee or hip arthoplasty making our findings generalizable to a specific population of interest.
There are a number of limitations to our systematic review. Although we utilized sound and control-biased methodology we were unable to quantitatively synthesize the findings from these RCTs due to heterogeneity and inconsistent reporting of outcome measures. The inability to pool data for quantitative analysis left only qualitative analysis to draw conclusions. Another limitation was the variability in glucocorticoid type, dosing, and timing, as well as the pre- and post-operative analgesia regimens across studies. The effect of the glucocorticoid may be minimized if the dose is too small or if it is masked or impaired by another analgesic or combination of analgesics. Based on this systematic review we cannot make any strong recommendations regarding optimal glucocorticoid type, dosing or timing. Our conclusions regarding the safety of glucocortoid use in hip and knee arthroplasty are also limited, as pooling of the post-operative complications data would have been unreliable due to the rarity of their occurrence in this small number of trials. In light of this, caution should be taken in interpreting the safety results of this systematic review. Finally, our attempt in obtaining all the required data on outcome measures by contacting the authors was unsuccessful.
5. Conclusion
Perioperative glucocorticoids administered to patients undergoing total hip and knee arthroplasty have overall positive outcomes including reduction in pain, PONV, and narcotic and anti-emetic consumption. The benefits appear more robust in those patients undergoing total knee arthroplasty compared to total hip arthroplasty. In no instances did glucocorticoids have a negative impact on the outcomes analyzed nor did they cause an increase in serious postoperative complications. There is limited data to support the conclusion that they can reduce length of stay in hospital.
Future directions
Healthcare resources are growing increasingly finite and clinicians must continue to find ways to improve patient outcomes in the peri and postoperative phase to minimize demands on the system. Perioperative steroids have shown some promise in improving postoperative pain and decreasing nausea and vomiting but the results did not strongly support an improvement in overall length of stay. Additional high quality RCTs assessing the impact of perioperative steroids are needed to help identify the dose and timing needed for glucocorticoid administration to optimize the patients’ postoperative course.
Conflict of interest
The authors have none to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosures
On behalf of all authors, the corresponding author states that there is no conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors thank Dr. Mitchell Winemaker and Dr. Justin De Beer of the Hamilton Arthroplasty Group for their constructive feedback and assistance in preparing the manuscript.
Contributor Information
Jeffrey Hartman, Email: jeffrey.hartman@medportal.ca.
Vickas Khanna, Email: khanna.vickas@gmail.com.
Anthony Habib, Email: anthony.habib@medportal.ca.
Forough Farrokhyar, Email: farrokh@mcmaster.ca.
Muzammil Memon, Email: memon.muzammil@hotmail.com.
Anthony Adili, Email: adilia@mcmaster.ca.
References
- 1.Lau R., Gandhi R., Mahomed S., Mahomed N. Patient satisfaction after total knee and hip arthroplasty. Clin Geriatr Med. 2012;28:349–365. doi: 10.1016/j.cger.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Grosu I., Lavand’homme P., Thienpont E. Pain after knee arthropasty: an unresolved issue. Knee Surg Sports Traumatol Arthrosc. 2014;22:1744–1758. doi: 10.1007/s00167-013-2750-2. [DOI] [PubMed] [Google Scholar]
- 3.Macario A., Weinger M., Carney S., Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652–658. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 4.McCracken G., Houston P., Lefebvre G. Guideline for the management of postoperative nausea and vomiting. J Obstet Gynaecol Can. 2008;209:600–607. doi: 10.1016/s1701-2163(16)32895-x. [DOI] [PubMed] [Google Scholar]
- 5.Waldron N.H., Jones C.A., Gan T.J., Allen T.K., Habib A.S. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth. 2013;110(2):191–200. doi: 10.1093/bja/aes431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Oliveira G., Castro-Alves L.J.S., Ahmad S., Kendall M.C., McCarthy R.J. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg. 2013;116(1):58–74. doi: 10.1213/ANE.0b013e31826f0a0a. [DOI] [PubMed] [Google Scholar]
- 7.Cutolo M., Seriolo B., Pizzorni C. Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008;8:153–155. doi: 10.1016/j.autrev.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 8.McHugh M.L. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22(3):276–282. [PMC free article] [PubMed] [Google Scholar]
- 9.Backes J.R., Bentley J.C., Politi J.R., Chambers B. Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial. J Arthroplasty. 2013;28:11–17. doi: 10.1016/j.arth.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 10.Lunn T.H., Kristensen B.B., Anderson L.O. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth. 2011;106(2):230–238. doi: 10.1093/bja/aeq333. [DOI] [PubMed] [Google Scholar]
- 11.Lunn T.H., Anderson L.O., Kristensen B.B. Effect of high-dose preoperative methylprednisolone on recovery after total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Br J Anaesth. 2013;110(1):66–73. doi: 10.1093/bja/aes345. [DOI] [PubMed] [Google Scholar]
- 12.Koh I.J., Chang C.B., Lee J.H., Jeon Y.T. Preemptive low-dose dexamethasone reduces postoperative emesis and pain after TKA: a randomized controlled study. Clin Orthop Relat Res. 2013;471:3010–3020. doi: 10.1007/s11999-013-3032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathiesen O., Jacobsen L.S., Holm H.E. Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth. 2008;101(4):535–541. doi: 10.1093/bja/aen215. [DOI] [PubMed] [Google Scholar]
- 14.Kardash K.J., Sarrazin F., Tessler M.J., Velly A.M. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg. 2008;106:1253–1257. doi: 10.1213/ANE.0b013e318164f319. [DOI] [PubMed] [Google Scholar]
- 15.Fujii Y., Nakayama M. Effects of dexamethasone in preventing postoperative emetic symptoms after total knee replacement surgery: a prospective, randomized, double-blind, vehicle-controlled trial in adult Japanese patients. Clin Ther. 2005;27(6):740–745. doi: 10.1016/j.clinthera.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Carlisle J.B. The analysis of 168 randomized controlled trials to test data integrity. Anaesthesia. 2012;67:521–537. doi: 10.1111/j.1365-2044.2012.07128.x. [DOI] [PubMed] [Google Scholar]
- 17.Kranke P., Apfel C., Roewer N. Reported data on granisetron and postoperative nausea and vomiting by Fujii, et al Are incredibly nice! Anesth Analg. 2000;90(4):1004–1006. [PubMed] [Google Scholar]
- 18.Jakobson J. Preoperative single-dose intravenous dexamethason during ambulatory surgery: update around the benefit versus risk. Curr Opin Anesthesiol. 2010;23:682–686. doi: 10.1097/ACO.0b013e32833ff302. [DOI] [PubMed] [Google Scholar]
- 19.Sauerland S., Nagelschmidt M., Mallmann P., Neugebauer E.A.M. Risk and benefits of preoperative high dose methylprednisolone in surgical patients: a systematic review. Drug Saf. 2000;23(5):449–461. doi: 10.2165/00002018-200023050-00007. [DOI] [PubMed] [Google Scholar]
- 20.Murphy G.S., Szokol J.W., Greenberg S.B. Preoperative dexamethasone enhances quality of recovery after laparoscopic cholecystectomy: effect on in-hospital and post-discharge recovery outcomes. Anesthesiology. 2011;114:882–890. doi: 10.1097/ALN.0b013e3181ec642e. [DOI] [PubMed] [Google Scholar]
- 21.Gan T.J. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 22.De Oliveira G.S., Almeida M.D., Benzon H.T., McCarthy R.J. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115:575–588. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]
- 23.Lunn T.H., Kehlet H. Perioperative glucocorticoids in hip and knee surgery −benefit vs. harm? A review of randomized clinical trials. Acta Anaesthesiol Scand. 2013;57:823–834. doi: 10.1111/aas.12115. [DOI] [PubMed] [Google Scholar]
- 24.Salerno A., Hermann R. Efficacy and safety of steroid use for postoperative pain relief: update and review of the medical literature. J Bone Jt Surg Am. 2006;88(6):1361–1372. doi: 10.2106/JBJS.D.03018. [DOI] [PubMed] [Google Scholar]