Abstract
Conventional anticancer therapies such as radiotherapy and chemotherapies are associated with oxidative stress generating reactive oxygen species (ROS) and reactive aldehydes like 4-hydroxynonenal in cancer cells that govern them to die. The main mechanism activated due to exposure of the cell to these reactive species is the Nrf2-Keap1 pathway. Although Nrf2 was firstly perceived as a tumor suppressor that inhibits tumor initiation and cancer metastasis, more recent data reveal its role also as a pro-oncogenic factor. Discovery of the upregulation of Nrf2 in different types of cancer supports such undesirable pathophysiological roles of Nrf2. The upregulation of Nrf2 leads to activation of cytoprotective genes thus helping malignant cells to withstand high levels of ROS and to avoid apoptosis, eventually becoming resistant to conventional anticancer therapy. Therefore, new treatment strategies are needed for eradication of cancer and in this review, we will explore two opposing approaches for modulation of Nrf2 in cancer treatments.
Keywords: Cancer, Nrf2, Oxidative stress, Cancer therapy, Growth regulation, 4-hydroxynonenal
Graphical abstract
Highlights
-
•
Nrf2-Keap1 pathway is the main pathway activated upon redox imbalance.
-
•
Nrf2 and oxidative stress products (e.g. ROS, HNE) play dual role in carcinogenesis.
-
•
Nrf2 is both, a tumor suppressor and a proto-oncogene.
-
•
Usage of Nrf2 activators or inhibitors depends upon the stage of the cancer.
1. Introduction
Cancer still remains one of the leading causes of death worldwide with alarming expectancy to rise a further 75% over the next two decades [1]. Hence, there is a constant need of finding new treatment strategies and drugs that will combat cancer or at least will improve survival rate and quality of life of patients. Although many different types of cancer can be distinguished, each being unique as is the ill person unique, more generally cancer is described as an uncontrollable growth of abnormal cells persisting even after the cease of its etiological cause that can happen in any type of tissue and can spread all over the body giving rise to metastatic disease. The complexity of cancer was reviewed by Hanahan & Weinberg in [2], who proposed eight hallmarks of cancer with two enabling characteristics, which are acquired by cancer cells during the multistep process of development of human tumors. These include: sustaining proliferative signaling, evading growth suppressors, activating invasion and metastasis, enabling replicative immortality, inducing angiogenesis, resisting cell death, deregulating cellular energetics, avoiding immune destruction, genome instability and mutation, and tumor-promoting inflammation [2]. The authors based their explanation of cancer phenotypes on the premises adopted by the somatic mutation theory, while some critics advocate the tissue organization field theory, in which cancer is viewed as a tissue-based disease similar to "development gone awry”, as a better alternative [3], [4]. Though new theories are emerging each day [5], [6], [7]. Irrespective of each preference to certain theory, different signaling pathways affected by diverse growth factors, transcriptional factors and other signaling molecules such as reactive oxygen species (ROS) or their consequential end-products (e.g. 4-hydroxynonenal (HNE)) are important in cancer development (reviewed in [2], [8], [9], [10], [11]). In this review paper, we will focus on the role of nuclear factor erythroid 2 [NF-E2]-related factor 2 (Nrf2) in carcinogenesis and on two different approaches arisen in the cancer therapy based on Nrf2 modulation.
2. Nrf2-Keap1 pathway
To defend themselves from stress conditions induced by oxidative stress and xenobiotics, cells have evolved defense mechanisms that will keep them alive. The main mechanism activated in such conditions is Nrf2 – Keap1 (Kelch-like ECH-associated protein 1) pathway.
Nrf2 is a basic leucine zipper (bZip) transcription factor with a Cap “n” Collar (CNC) structure [12]. In homeostatic conditions, Nrf2 is bound to its repressor Keap1 and thus subjected to ubiquitin-dependent degradation in proteasomes. However, in stress conditions, Nrf2 becomes released from Keap1 and translocated into nucleus where it forms heterodimers with small Maf proteins and binds to antioxidant response element (ARE) of the target genes guiding their activation and thus cytoprotection of the cell (Fig. 1) [13]. Target genes activated by Nrf2 are involved in: 1) synthesis of glutathione (Glutamate-cysteine ligase, catalytic subunit (Gclc), glutamate-cysteine ligase, modifier subunit (Gclm)), 2) elimination of ROS (Thioredoxin reductase 1 (Txnrd1), Peroxiredoxin 1 (Prdx1), 3) detoxification of xenobiotics (NAD(P)H dehydrogenase, quinone 1 (Nqo1), Glutathione S-transferase (Gst) gene family) and 4) drug transport (Multidrug resistance-associated protein (Mrp) gene family) [13].
Fig. 1.
Schematic overview of the Nrf2 pathway. In homeostatic conditions, transcription factor Nrf2 is bound to its repressor Keap1 and thus subjected to ubiquitin-dependent degradation in proteasomes. On the other hand, in stress conditions, Nrf2 released from Keap1 is translocated into nucleus where it forms heterodimers with small Maf proteins and activates cytoprotective genes through antioxidant response element (ARE).
3. Role of oxidative stress and Nrf2-Keap1 pathway in carcinogenesis
For a long time, radical species, such as ROS, emerged during oxidative stress have been considered as harmful, co-carcinogenic factors of cancer development. For instance, in infection-associated cancers such as human papilloma virus-induced cervical cancer, hepatitis B virus-positive hepatocarcinoma, and Helicobacter pylori-positive gastric cancer, chronic infection leads to generation of ROS as the first line of antimicrobial defense, which in turn can initiate cancer development [14]. ROS comprise mostly of hydrogen peroxide (H2O2), superoxide anion (O2•-) and hydroxyl radical (•OH). Both, endogenous (mitochondrial electron transport chain, xanthine oxidase (XO), nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), peroxisomes and cytochrome P450) and exogenous sources (ultraviolet light, ionizing radiation, pharmaceuticals, environmental agents, and industrial chemicals) contribute to their generation [8], [15]. ROS are highly reactive by nature and as such cause oxidation of the cellular macromolecules (DNA, proteins, lipids) thus contributing to chromosome instability, genetic mutation, and/or modulation of cell growth [8], [9], [15], [16]. It has been postulated that cancer cells have higher levels of ROS than normal cells. Yet, they can withstand these levels by activation of certain pathways that drive their proliferation and survival. These pathways include activation of antioxidant machinery to reduce ROS, but also reprogramming of metabolic pathways that can generate additional ROS and make cancer cells more vulnerable to forthcoming stress. Indeed, recent findings by Harris et al. suggested that glutathione (GSH) and thioredoxin play important role in carcinogenesis, with GSH being important in cancer initiation while in already established cancers thioredoxin is a key player that abolishes detrimental levels of ROS and governs cancer progression [17], [18].
Therefore, antioxidant defense (endogenous and exogenous) together with ROS have been known for their dual role in carcinogenesis. It is noteworthy to mention that ROS are short lived and their oxidation of lipids is generating longer living molecules known as second messengers of oxidative stress [19]. One of the most intensively studied among them is 4-hydroxynonenal (HNE). HNE is a highly reactive aldehyde, more stable than ROS, which can easily react with cellular macromolecules such as proteins, DNA and phospholipids. HNE can also act as signaling molecule affecting cell proliferation, differentiation, and apoptosis and as such has been implicated in different cancers [20], [21], [22]. HNE is a potent inducer of Nrf2 either by covalently binding to cysteine sites in Keap1 protein and thus disrupting Keap1-dependent degradation of Nrf2, or by activating Nrf2 through activation of upstream kinases such as PKC, ERK and PI3K [23], [24], [25], [26]. Furthermore, HNE is metabolized through activation of Nrf2 as well.
The main signaling pathway activated to amend disturbed redox balance is Nrf2-Keap1 pathway. Current research recognizes dual role of Nrf2 in carcinogenesis: protective in early stages and detrimental in later stages (Fig. 2). When present in lower levels that are sufficient to maintain homeostasis, Nrf2 is eliminating ROS, carcinogens, and other DNA-damaging agents, which leads to inhibition of tumor initiation and cancer metastasis. Supporting Nrf2 protective role is evidence that a single nucleotide polymorphism in the human Nrf2 upstream promoter region (rs6721961), which may result in reduced Nrf2 gene expression, is increasing a risk of lung cancer in current and even in former smokers [27].
Fig. 2.
Activation of Nrf2 has dual role in carcinogenesis. In carcinogenesis Nrf2 was recognized to have a dual role: protective in early stages and detrimental in later stages. Indeed, in early stages, sufficient Nrf2 levels maintain homeostasis by eliminating carcinogens, such as ROS and other DNA-damaging agents, and thus inhibit tumor initiation and cancer metastasis. Whereas, in cancer, Nrf2 is usually upregulated leading to activation of cytoprotective genes and thus helping malignant cells to endure high levels of ROS and avoid apoptosis.
In cancer, Nrf2 is usually upregulated, thus helping malignant cells to withstand high levels of ROS and avoid apoptosis through activation of metabolic and cytoprotective genes that contribute to enhanced cell proliferation [12], [28], [29], [30], [31], [32], [33], [34]. Different mechanisms lead to observed increased Nrf2 activity in cancers, such as: 1) somatic mutations in Nrf2, Keap1, or Cul3; 2) epigenetic silencing of Keap1; 3) microRNA-mediated regulation of Nrf2 and Keap1; 4) disruption of Nrf2-Keap1 interaction by aberrantly accumulated proteins; 5) transcriptional up-regulation of Nrf2 through oncogene-dependent signaling; and 6) modification of Keap1 by metabolic intermediates [12], [29], [30]. For instance, recent data revealed the necessity of Nrf2 in sustaining proliferation in pancreatic cancer through the regulation of mRNA translation. The loss of Nrf2 led to defected autocrine signaling of epidermal growth factor receptor (EGFR) and to oxidation of specific proteins of translational machinery, which resulted in impaired cap-dependent and cap-independent mRNA translation [35]. In human leukemia, Nrf2 was found to be constitutively activated, and in acute myeloid leukemia (AML), the activation of Nrf2 is a result of upstream constitutive activation of nuclear factor-κB (NF-κB) and not of somatic mutation of Nrf2 or its inhibitor Keap1 [36]. Moreover, recent data showed increased expression of Nrf2 in non-small cell lung cancers, such as squamous cell carcinoma (SCC) and adenocarcinoma (AC) with different involvement of oxidative stress-induced lipid mediators between these two subtypes: more pronounced involvement of lipid peroxidation in AC and endocannabinoid system in SCC, respectively [37]. In addition, prostate cancer cell lines have shown different sensitivity to HNE-mediated inhibition of cell growth and apoptosis. In particular, DU145 cells exhibited lower levels of Keap1, higher levels of Nrf2, GSH and GST A4 and greater GSH/GST-mediated HNE detoxification and thus were less sensitive than PC3 and LNCaP cells [38]. Furthermore, Nrf2 was recognized as “a key regulator in chemotherapeutic resistance under hypoxia through ROS-Nrf2-GCLC-GSH pathway” in breast cancer cells [39]. Therefore, better understanding of mediators of oxidative stress with their possible clinical relevance [40], together with improved knowledge of the role of Nrf2 and its downstream targets could contribute to future, more effective, anticancer therapy.
While specific role of Nrf2 in cancer still remains controversial, since Nrf2 is considered as both, a tumor suppressor and a proto-oncogenic factor, some researchers postulate that its precise role is dependent on the stage of tumorigenesis [34], [41].
4. Cancer therapy and Nrf2
Nowadays drugs affecting redox processes have been recognized as “a significant expansion of the chemotherapeutic armamentarium”, as reviewed in [42]. Majority of conventional therapy against cancer (radiotherapy and many chemotherapeutics) relies on generation of ROS and consequently HNE, resulting in cancer cell death. Unfortunately, there are limitations in their clinical use because they are designed to destroy rapidly dividing non-malignant cells, hence, while not all cancer cells are eliminated, some normal cells are. Besides, these therapies exert toxicity also well known as undesirable side effects. For a long time it was considered that antioxidant supplementation during anticancer therapies could reduce side effects of such therapies, but recent data suggest more caution is needed due to their observed unfavorable role when used during cancer therapy [8], [43].
Cancer cells are known to adapt to the negative environment by boosting their pro-survival responses which combined with the decrease of therapy-induced ROS by antioxidants, below the critical threshold required for cell death, may explain observed findings [8]. Perceived resistance of cancer cells to therapy is initiated by a range of potentially complementary mechanisms, such as the mutation or overexpression of the drug target, inactivation of the drug, or its elimination from the cell by ATP-binding cassette (ABC) transporters and RLIP76, etc. [44], [45], [46]. Response mechanisms to the increased redox imbalance during radio- and/or chemotherapy requires involvement of the Nrf2-Keap1 pathway, which is the main regulator of cytoprotective responses to resulting stress. As already mentioned, Nrf2 had been recognized for its dual role in carcinogenesis; beneficial in the early stages where activation of the Nrf2 pathway leads to activation of cytoprotective genes and thus generation of antioxidant machinery that removes ROS, HNE, and xenobiotics from the cells thus restoring the redox balance and avoiding unwanted DNA mutations and cancer initiation; but detrimental in later stages of cancer by increasing resistance to conventional radio- and/or chemotherapy.
Therefore, open question remains: How does or can antioxidative defense of cancer cells, activated mainly through Nrf2-Keap1 pathway, affect anticancer therapy?
4.1. Nrf2 activators
A variety of natural and synthetic compounds have been recognized as activators of Nrf2 and implicated in prevention of cancer development including sulforaphane, curcumin, resveratrol, lycopene, oleanane triterpenoid, etc. [29], [31], [47], [48]. Sulforaphane (SFN) is one of the most intensively studied natural compounds that target the Nrf2–Keap1 signaling. SFN is an isothiocyanate found in cruciferous vegetables such as broccoli [12]. Several epidemiological studies linked consumption of broccoli with a lower incidence of cancer including breast, prostate, lung, stomach and colon cancer [49]. A phase II clinical trial study in China evaluating the chemo-preventive properties of broccoli sprout preparations containing >60% glucoraphanin, a precursor of SFN, determined that SFN reduced afla-toxin–DNA adducts and thus protect against hepatocellular carcinoma [12], [50]. Additionally, in a review paper by Sharma et al., authors suggest that HNE plays a crucial role in the mechanisms of the biological activities of SFN which include selective cytotoxicity to cancer cells by targeting signaling pathways that provide their specific killing as well as upregulation of defense mechanisms that protect normal cells against oxidative/electrophilic stress [51].
Synthetic oleanane triterpenoid, RTA 405 is an antioxidant inflammation modulator (AIM) that displays its antitumor activity by binding to Keap1 leading to inhibition of Nrf2 degradation, thus increasing Nrf2 activity. Probst et al. compared the influence of RTA 405 on Keap1 with a constitutive activation of Nrf2 by Keap1 deletion and found these are indeed different mechanisms that differently affect cancer cell growth [52]. In particular, cancers with Keap1 loss or mutation usually have increased levels of Nrf2 and other oncogenic proteins such as IKKβ and Bcl2, while this was not the case upon RTA 405 treatment where only levels of Nrf2 were increased but not levels of IKKβ and Bcl2. Moreover, RTA 405 directly inhibited IKKβ, leading to decreased NF-κB activity, and consequently, suppression of cancer cell survivor and promotion of apoptosis. In addition, cancer cells, pretreated with RTA 405, were not protected from growth inhibition mediated by doxorubicin or cisplatin. All these finding led to conclusion that genetic activation of Nrf2 and pharmacological activation of Nrf2 by RTA 405 are distinct and that RTA 405-mediated activation of Nrf2 does not promote growth or survival of cancer cells [52].
Caution with Nrf2 activators was raised in case of diabetic patients. Notably, Wang et al. have shown that common classes of drugs used for treatment of diabetes mellitus type 2, saxagliptin and sitagliptin (the dipeptidyl peptidase-4 (DPP-4) inhibitors) and α-lipoic acid (ALA) (for treating diabetic neuropathy), known for their antioxidative properties, induce prolonged activation of Nrf2 and although they do not enhance cancer incidence they do increase the risk of metastasis development in diabetic patients who already have cancer [53].
4.2. Nrf2 inhibitors
Since the oncogenic activity of Nrf2 has also been revealed, concerns about usage of Nrf2 activators was been raised, too. Namely, Wang et al. have evaluated expression profile of Nqo1, which is an Nrf2-downstream target gene, in tissue samples of lung cancer patients. The tissue samples were ranged from normal to more advanced cancer stages (stages I, II and III). The elevated expression of Nqo1 was present in patients with stage II and stage III lung cancer. Moreover, they were interested in what role does Nrf2 have in response to drug treatments in different cancers (lung carcinoma, breast adenocarcinoma and neuroblastoma). They found that upregulation of Nrf2 increases resistance of cancer cells while downregulation of Nrf2 makes cancer cells more sensitive to chemotherapeutic drugs such as cisplatin, doxorubicin and etoposide. Therefore, to increase the efficacy of anticancer drugs in cancer patients, they suggested the usage of Nrf2 inhibitors that will inhibit the Nrf2-dependent response [54]. Hence, current research is trying to find Nrf2 inhibitors and elucidate their role in cancer therapy. We will list just some of them.
Malabaricone-A (MAL-A) is a pro-oxidant compound suggested to likely be more effective in leukemia than solid tumors and its mode of action is directed on targeting Nrf2 antioxidant pathway, by decreasing levels of Nrf2 and increasing redox imbalance [55]. Moreover, MAL-A induces peroxidation of cardiolipin [55], a source of HNE [56]. Since HNE is known as a second messenger of oxidative stress and in high concentrations it governs cancer cell to apoptosis, we can postulate that HNE together with the decrease of Nrf2 may have been employed in this increased chemosensitivity. In favor of this assumption are the most recent data from in vitro and clinical study that revealed a gradual decrease of cardiolipin in tissue samples of hepatocellular carcinoma in comparison to non-cancerous tissue leading to decreased oxidation of cardiolipin and consequently lesser formation of HNE which might be a possible adaptation mechanism of the cancer cells to avoid apoptosis [57].
Another mechanism adopted by the cancer cells in hepatocellular carcinoma that helps them to resist to chemotherapeutic agents is linked to overexpression of Nrf2 and its downstream targets (multidrug resistance-associated protein 5 and AKR1B10 – a member of aldo-keto reductase superfamily) in comparison to adjacent non-cancerous tissue [58]. Therefore, Gao et al. conducted concomitant in vitro and in vivo study to explore inhibitory potential of apigenin (a natural bioflavonoid found in many fruits and vegetables) to Nrf2 pathway. In particular, apigenin inhibited Nrf2 pathway, observed as decrease of Nrf2 at both transcriptional and translational levels, which occurred via downregulation of PI3K/Akt pathway. Moreover, apigenin sensitized doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin, and in BEL-7402 xenografts, cotreatment of apigenin and doxorubicin was more effective that doxorubicin treatment alone observed as inhibition in tumor size [58].
All trans-retinoic acid (ATRA) is another proposed specific Nrf2 inhibitor in which presence Nrf2 is forming a complex with retinoic acid receptor alpha (RARα). These complexes do not bind to ARE sequences and the activation of the pathway is blocked [29], [47]. Indeed, retinoic acid has been identified as an inhibitor of Nrf2 in human breast cancer (MCF-7) cells transfected with an ARE-luciferase reporter construct [59].
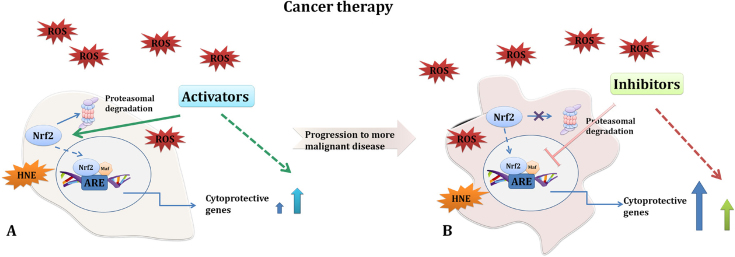
A simplified scheme of the role of Nrf2 activators and inhibitors is presented in Fig. 3, although we have to highlight that the specific effect is a compound-used directed since different compounds affect also other pathways. It is well known that cancer cells are not uniform and reprogramming of cancer cells contributing to progression of the disease further impact their heterogeneity. Therefore, the usage of specific Nrf2 activators or inhibitors during the cancer therapy was shown to be highly associated with cancer stage.
Fig. 3.
Simplified schematic overview of interaction of Nrf2 activators and inhibitors in anticancer therapy. Majority of conventional therapy against cancer relies on generation of ROS resulting in cancer cell death. Yet, not all cancer cells are affected and they tend to become resistant. Although, diverse mechanisms contribute to perceived resistance, this therapy-induced increase of redox imbalance requires involvement of the Nrf2-Keap1 pathway which is the main regulator of cytoprotective responses. Observation that a single nucleotide polymorphism in the human Nrf2, leading to reduced Nrf2 gene expression, increases risk of lung cancer supported Nrf2 protective role. In addition, due to the fact that Nrf2 inhibits tumor initiation and cancer metastasis by eliminating carcinogens, ROS and other DNA-damaging agents, a variety of its natural and synthetic activators have been implicated in prevention of cancer development (e.g. sulforaphane, oleanane triterpenoid RTA 405, etc.). Some of them have also shown their beneficial role during cancer therapy by affecting other mechanism. Examples are: RTA405 which was found to decrease NF-κB activity and thus suppress cancer cell survival and promote apoptosis and sulforaphane (SFN) which selective cytotoxicity of cancer cells involves generation of HNE (A). Yet, caution in using Nrf2 activators was suggested since they have been implicated in enhanced cancer cell resistance. Due to the fact that in many cancers, especially in later stages of the disease, Nrf2 and Keap1 are mutated leading to Nrf2 increased activity, inhibitors of Nrf2 were suggested to be a promising tool that will sensitize cancer cells and increase efficacy of conventional therapy (B). Though, both, Nrf2 activators and inhibitors have shown to be beneficial, e.g. activators mainly in prevention of cancer incidence and inhibitors during progression of the disease, there is a lot of uncertainties that is pushing us to unveil all the modalities by which Nrf2 can be used as a therapeutic target.
Noteworthy, Leinonen et al. introduced an alternative approach, known as suicide gene therapy, using thymidine kinase (TK) containing Nrf2-driven vectors (ARE-HSV-TK) for targeting cancer cells with high constitutive Nrf2 expression. They evaluated this approach in human lung adenocarcinoma cells and found it effective in both in vitro and in vivo, offering an additional promising treatment that could be used in conjunction with traditional therapies [30], [60].
5. Conclusion
Although much effort has been invested, cancer still remains a puzzle. Current research is trying to elucidate why we are still losing in our fight against cancer. While conventional therapy has its benefits it also has its limitations observed as cancer ability to resist to it. Therefore, researchers intensively work on trying to find mechanisms involved in cancer adaptation and resistance. The Nrf2-Keap1 pathway was found to be one of the key mechanisms in these processes. Therefore, its role is being highly investigated. Though Nrf2 was firstly perceived as a tumor suppressor, nowadays, it is also recognized as a proto-oncogene. Hence, along with the research of Nrf2 activators that inhibit cancer development, an increasing field of research is pursuing to find inhibitors of Nrf2 that will combat cancer resistance to conventional therapy.
Thus, a continuous need for better understanding of the modalities by which Nrf2 can be used as a therapeutic target, still remains to be carried complementary with the research of clinical relevance and involvement of mediators of oxidative stress especially of HNE, in cancer and in the anticancer therapy.
Dedication
This review is dedicated to those who suffer from cancer and to those who try to help them.
Acknowledgement
The authors wish to express the gratitude to Prof. Liudimila Korkina for support and constructive suggestions for the manuscript preparation.
Contributor Information
Lidija Milkovic, Email: lidija.milkovic@irb.hr.
Neven Zarkovic, Email: zarkovic@irb.hr.
Luciano Saso, Email: luciano.saso@uniroma1.it.
References
- 1.Stewart B.W., CP W., editors. World Cancer Report 2014, International Agency for Research on Cancer. WHO; Lyon, France: 2014. [Google Scholar]
- 2.D. Hanahan, R.A. Weinberg, Chapter 2: Hallmarks of Cancer: An Organizing Principle for Cancer Medicine, in: pp. 1–51, 2011.
- 3.Soto A.M., Sonnenschein C. The tissue organization field theory of cancer: a testable replacement for the somatic mutation theory. Bioessays. 2011;33:332–340. doi: 10.1002/bies.201100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonnenschein C., Soto A.M. The aging of the 2000 and 2011 Hallmarks of Cancer reviews: a critique. J. Biosci. 2013;38:651–663. doi: 10.1007/s12038-013-9335-6. 〈http://www.ncbi.nlm.nih.gov/pubmed/23938395〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonnenschein C., Soto A.M., Rangarajan A., Kulkarni P. Competing views on cancer. J. Biosci. 2014;39:281–302. doi: 10.1007/s12038-013-9403-y. 〈http://www.ncbi.nlm.nih.gov/pubmed/24736160〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker S.G. A cancer theory kerfuffle can lead to new lines of research. J. Natl. Cancer Inst. 2015;107:1–8. doi: 10.1093/jnci/dju405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonnenschein C., Soto A.M. Carcinogenesis explained within the context of a theory of organisms. Prog. Biophys. Mol. Biol. 2016;122:70–76. doi: 10.1016/j.pbiomolbio.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milkovic L., Siems W., Siems R., Zarkovic N. Oxidative stress and antioxidants in carcinogenesis and integrative therapy of cancer. Curr. Pharm. Des. 2014;20:6529–6542. doi: 10.2174/1381612820666140826152822. 〈http://www.ncbi.nlm.nih.gov/pubmed/25341930〉 [DOI] [PubMed] [Google Scholar]
- 9.Panieri E., Santoro M.M. ROS homeostasis and metabolism: a dangerous liaison in cancer cells. Cell Death Dis. 2016;7:e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sever R., Brugge J.S. Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 2015;5:1–2. doi: 10.1101/cshperspect.a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acharya A., Das I., Chandhok D., Saha T. Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid. Med. Cell. Longev. 2010;3:23–34. doi: 10.4161/oxim.3.1.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaramillo M.C., Zhang D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 14.De Luca C., Kharaeva Z., Korkina L. Is there a role for antioxidants in the prevention of infection-associated carcinogenesis and in the treatment of infection-driven tumors? Curr. Top. Med. Chem. 2015;15:120–135. 〈http://www.benthamdirect.org/pages/all_b_bypublication.php%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed13&NEWS=N&AN=2015893760%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/25496269〉 [PubMed] [Google Scholar]
- 15.Klaunig J.E., Kamendulis L.M., Hocevar B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 16.Liou G.-Y., Storz P. Reactive oxygen species in cancer. Free Radic. Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris I.S., Treloar A.E., Inoue S., Sasaki M., Gorrini C., Lee K.C., Yung K.Y., Brenner D., Knobbe-Thomsen C.B., Cox M.A., Elia A., Berger T., Cescon D.W., Adeoye A., Brüstle A., Molyneux S.D., Mason J.M., Li W.Y., Yamamoto K., Wakeham A., Berman H.K., Khokha R., Done S.J., Kavanagh T.J., Lam C., Mak T.W. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27:211–222. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Schumacker P. Reactive oxygen species in cancer: a dance with the devil. Cancer Cell. 2015;27:156–157. doi: 10.1016/j.ccell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Guéraud F., Atalay M., Bresgen N., Cipak A., Eckl P.M., Huc L., Jouanin I., Siems W., Uchida K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010;44:1098–1124. doi: 10.3109/10715762.2010.498477. [DOI] [PubMed] [Google Scholar]
- 20.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. 〈http://www.ncbi.nlm.nih.gov/pubmed/1937131〉 [DOI] [PubMed] [Google Scholar]
- 21.Milkovic L., Cipak Gasparovic A., Zarkovic N. Overview on major lipid peroxidation bioactive factor 4-hydroxynonenal as pluripotent growth-regulating factor. Free Radic. Res. 2015;49:850–860. doi: 10.3109/10715762.2014.999056. [DOI] [PubMed] [Google Scholar]
- 22.Zarkovic N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Asp. Med. 2003;24:281–291. doi: 10.1016/s0098-2997(03)00023-2. 〈http://www.ncbi.nlm.nih.gov/pubmed/12893006〉 [DOI] [PubMed] [Google Scholar]
- 23.Levonen A.-L., Landar A., Ramachandran A., Ceaser E.K., Dickinson D.A., Zanoni G., Morrow J.D., Darley-Usmar V.M. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Numazawa S., Ishikawa M., Yoshida A., Tanaka S., Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am. J. Physiol. Cell Physiol. 2003;285:C334–C342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y., Li W., Kong A.-N.T. Anti-oxidative stress regulator NF-E2-related factor 2 mediates the adaptive induction of antioxidant and detoxifying enzymes by lipid peroxidation metabolite 4-hydroxynonenal. Cell Biosci. 2012;2:40. doi: 10.1186/2045-3701-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Łuczaj W., Gęgotek A., Skrzydlewska E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2016 doi: 10.1016/j.freeradbiomed.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T., Shibata T., Takaya K., Shiraishi K., Kohno T., Kunitoh H., Tsuta K., Furuta K., Goto K., Hosoda F., Sakamoto H., Motohashi H., Yamamoto M. Regulatory nexus of synthesis and degradation deciphers cellular Nrf2 expression levels. Mol. Cell. Biol. 2013;33:2402–2412. doi: 10.1128/MCB.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geismann C., Arlt A., Sebens S., Schäfer H. Cytoprotection “gone astray”: Nrf2 and its role in cancer. Onco Targets Ther. 2014;7:1497–1518. doi: 10.2147/OTT.S36624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menegon S., Columbano A., Giordano S. The dual roles of NRF2 in cancer. Trends Mol. Med. 2016;22:578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Kansanen E., Kuosmanen S.M., Leinonen H., Levonenn A.L., Levonen A. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kensler T.W., Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J., Keum Y. NRF2, a key regulator of antioxidants with two faces towards cancer. Oxid. Med. Cell. Longev. 2016;2016:2746457. doi: 10.1155/2016/2746457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau A., Villeneuve N.F., Sun Z., Wong P.K., Zhang D.D. Dual roles of Nrf2 in cancer. Pharmacol. Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sporn M.B., Liby K.T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Publ. Gr. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chio I.I.C., Jafarnejad S.M., Ponz-Sarvise M., Park Y., Rivera K., Palm W., Wilson J., Sangar V., Hao Y., Öhlund D., Wright K., Filippini D., Lee E.J., Silva B. Da, Schoepfer C., Wilkinson J.E., Buscaglia J.M., DeNicola G.M., Tiriac H., Hammell M., Crawford H.C., Schmidt E.E., Thompson C.B., Pappin D.J., Sonenberg N., Tuveson D.A. NRF2 promotes tumor maintenance by modulating mRNA translation in pancreatic cancer. Cell. 2016;166:963–976. doi: 10.1016/j.cell.2016.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdul-Aziz A., MacEwan D.J., Bowles K.M., Rushworth S.A. Oxidative stress responses and NRF2 in human leukaemia. Oxid. Med. Cell. Longev. 2015;2015:1–7. doi: 10.1155/2015/454659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gęgotek A., Nikliński J., Žarković N., Žarković K., Waeg G., Łuczaj W., Charkiewicz R., Skrzydlewska E. Lipid mediators involved in the oxidative stress and antioxidant defence of human lung cancer cells. Redox Biol. 2016;9:210–219. doi: 10.1016/j.redox.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettazzoni P., Ciamporcero E., Medana C., Pizzimenti S., Dal Bello F., Minero V.G., Toaldo C., Minelli R., Uchida K., Dianzani M.U., Pili R., Barrera G. Nuclear factor erythroid 2-related factor-2 activity controls 4-hydroxynonenal metabolism and activity in prostate cancer cells. Free Radic. Biol. Med. 2011;51:1610–1618. doi: 10.1016/j.freeradbiomed.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Syu J., Chi J., Kung H. Nrf2 is the key to chemotherapy resistance in MCF7 breast cancer cells under hypoxia. Oncotarget. 2016;7:14659–14672. doi: 10.18632/oncotarget.7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frijhoff J., Winyard P.G., Zarkovic N., Davies S.S., Stocker R., Cheng D., Knight A.R., Taylor E.L., Oettrich J., Ruskovska T., Gasparovic A.C., Cuadrado A., Weber D., Poulsen H.E., Grune T., Schmidt H.H.H.W., Ghezzi P. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015;23:1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shelton P., Jaiswal A.K. The transcription factor NF-E2-related factor 2 (Nrf2): a protooncogene? FASEB J. 2013;27:414–423. doi: 10.1096/fj.12-217257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wondrak G.T. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid. Redox Signal. 2009;11:3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasueda A., Urushima H., Ito T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: a systematic review. Integr. Cancer Ther. 2015 doi: 10.1177/1534735415610427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vatsyayan R., Lelsani P.C.R., Awasthi S., Singhal S.S. RLIP76: a versatile transporter and an emerging target for cancer therapy. Biochem. Pharmacol. 2010;79:1699–1705. doi: 10.1016/j.bcp.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Housman G., Byler S., Heerboth S., Lapinska K., Longacre M., Snyder N., Sarkar S. Drug resistance in cancer: an overview. Cancers (Basel) 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cort A., Ozben T., Saso L., De Luca C., Korkina L. Redox control of multidrug resistance and its possible modulation by antioxidants. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/4251912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magesh S., Chen Y., Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012;32:687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korkina L.G., De Luca C., Kostyuk V.A., Pastore S. Plant polyphenols and tumors: from mechanisms to therapies, prevention, and protection against toxicity of anti-cancer treatments. Curr. Med. Chem. 2009;16:3943–3965. doi: 10.2174/092986709789352312. [DOI] [PubMed] [Google Scholar]
- 49.Ullah M. Sulforaphane (SFN): an isothiocyanate in a cancer chemoprevention paradigm. Medicines. 2015;2:141–156. doi: 10.3390/medicines2030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kensler T.W., Chen J.G., Egner P.A., Fahey J.W., Jacobson L.P., Stephenson K.K., Ye L., Coady J.L., Wang J.B., Wu Y., Sun Y., Zhang Q.N., Zhang B.C., Zhu Y.R., Qian G.S., Carmella S.G., Hecht S.S., Benning L., Gange S.J., Groopman J.D., Talalay P. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo Township, Qidong, People's Republic of China. Cancer Epidemiol. Biomark. Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 51.Sharma R., Sharma A., Chaudhary P., Sahu M., Jaiswal S., Awasthi S., Awasthi Y.C. Role of 4-hydroxynonenal in chemopreventive activities of sulforaphane. Free Radic. Biol. Med. 2012;52:2177–2185. doi: 10.1016/j.freeradbiomed.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Probst B.L., McCauley L., Trevino I., Wigley W.C., Ferguson D.A. Cancer cell growth is differentially affected by constitutive activation of NRF2 by KEAP1 deletion and pharmacological activation of NRF2 by the synthetic triterpenoid, RTA 405. PLoS One. 2015;10:e0135257. doi: 10.1371/journal.pone.0135257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H., Liu X., Long M., Huang Y., Zhang L., Zhang R., Zheng Y., Liao X., Wang Y., Liao Q., Li W., Tang Z., Tong Q., Wang X., Fang F., de la Vega M.R., Ouyang Q., Zhang D.D., Yu S., Zheng H. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci. Transl. Med. 2016;8:334ra51. doi: 10.1126/scitranslmed.aad6095. [DOI] [PubMed] [Google Scholar]
- 54.Wang X.J., Sun Z., Villeneuve N.F., Zhang S., Zhao F., Li Y., Chen W., Yi X., Zheng W., Wondrak G.T., Wong P.K., Zhang D.D. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manna A., De Sarkar S., De S., Bauri A.K., Chattopadhyay S., Chatterjee M. The variable chemotherapeutic response of Malabaricone-A in leukemic and solid tumor cell lines depends on the degree of redox imbalance. Phytomedicine. 2015;22:713–723. doi: 10.1016/j.phymed.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Liu W., Porter N.A., Schneider C., Brash A.R., Yin H. Formation of 4-hydroxynonenal from cardiolipin oxidation: intramolecular peroxyl radical addition and decomposition. Free Radic. Biol. Med. 2011;50:166–178. doi: 10.1016/j.freeradbiomed.2010.10.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong H., Xiao M., Zarkovic K., Zhu M., Sa R., Lu J., Tao Y., Chen Q., Xia L., Cheng S., Waeg G., Zarkovic N., Yin H. Mitochondrial control of apoptosis through modulation of cardiolipin oxidation in hepatocellular carcinoma: a novel link between oxidative stress and cancer. Free Radic. Biol. Med. 2017;102:67–76. doi: 10.1016/j.freeradbiomed.2016.10.494. [DOI] [PubMed] [Google Scholar]
- 58.Gao A., Ke Z., Wang J., Yang J., Chen S.-Y., Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34:1806–1814. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- 59.Broekgaarden M., Weijer R., van Gulik T.M., Hamblin M.R., Heger M. Tumor cell survival pathways activated by photodynamic therapy: a molecular basis for pharmacological inhibition strategies. Cancer Metastas-. Rev. 2015;34:643–690. doi: 10.1007/s10555-015-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leinonen H.M., Ruotsalainen A.K., Määttä A.M., Laitinen H.M., Kuosmanen S.M., Kansanen E., Pikkarainen J.T., Lappalainen J.P., Samaranayake H., Lesch H.P., Kaikkonen M.U., Ylä-Herttuala S., Levonen A.L. Oxidative stress-regulated lentiviral TK/GCV gene therapy for lung cancer treatment. Cancer Res. 2012;72:6227–6235. doi: 10.1158/0008-5472.CAN-12-1166. [DOI] [PubMed] [Google Scholar]