Significance
Dynamic demethylation of histone residues plays a crucial role in the regulation of gene expression. Lysine Specific Demethylase 1 (LSD1) can remove both transcriptionally permissive and repressive histone marks. How these activities are controlled is not clearly understood. Here, we show that the estrogen-related receptor α (ERRα) induces LSD1 to erase repressive marks in vitro. Through such a mechanism, LSD1 and ERRα commonly activate a set of transcriptional targets that include genes involved in the cellular capacity to invade the extracellular matrix. This process is a hallmark of cancer progression, to which high expression of both LSD1 and ERRα are strongly correlated.
Keywords: LSD1, histone demethylation, ERRα, transcriptional regulation, cell migration
Abstract
Lysine Specific Demethylase 1 (LSD1) removes mono- and dimethyl groups from lysine 4 of histone H3 (H3K4) or H3K9, resulting in repressive or activating (respectively) transcriptional histone marks. The mechanisms that control the balance between these two antagonist activities are not understood. We here show that LSD1 and the orphan nuclear receptor estrogen-related receptor α (ERRα) display commonly activated genes. Transcriptional activation by LSD1 and ERRα involves H3K9 demethylation at the transcriptional start site (TSS). Strikingly, ERRα is sufficient to induce LSD1 to demethylate H3K9 in vitro. The relevance of this mechanism is highlighted by functional data. LSD1 and ERRα coregulate several target genes involved in cell migration, including the MMP1 matrix metallo-protease, also activated through H3K9 demethylation at the TSS. Depletion of LSD1 or ERRα reduces the cellular capacity to invade the extracellular matrix, a phenomenon that is rescued by MMP1 reexpression. Altogether our results identify a regulatory network involving a direct switch in the biochemical activities of a histone demethylase, leading to increased cell invasion.
Understanding how defined transcription factors (TFs) can promote diverse transcriptional programs in a tissue-specific manner is a fundamental goal in biology. A substantial body of work showed that the chromatin environment plays a central role in these specific activities—hence, in transcriptional outputs of TFs. Histone N-terminal tails are decorated by posttranslational modifications (methylation, acetylation, ubiquitylation, sumoylation, glycosylation, etc.) that are crucial for transcriptional regulation (1). The histone code is regulated by histone modifiers that can write or erase posttranslational modifications in a dynamic manner (2–5). Intriguingly, it has been shown that TFs can regulate the target gene specificity of histone modifiers to promote their defined transcriptional program. Therefore, an intricate transcriptional network is required to induce physiological as well as pathological processes. In particular, cancer progression has been related to specific chromatin states (6–9).
Lysine Specific Demethylase 1 (LSD1/KDM1A) has been the first histone demethylase identified and can remove mono- and dimethyl groups on the lysine 4 of histone H3 (H3K4), resulting in transcriptional repressive marks (10). LSD1 interacts with CoREST, which enhances its demethylase activities toward H3K4 in vitro and in vivo (11, 12). Moreover, LSD1 is a subunit of the NuRD corepressor complex, which inhibits gene transcription through the cooperation of histone deacetylation and demethylation (12). In addition to H3K4 demethylation, LSD1 is also capable of demethylating H3K9, resulting in transcriptional activation. For instance, LSD1 interacts with the Androgen Receptor (AR) to promote the transcription of at least a subset of AR target genes through these activities (13). Whether LSD1 retains H3K4 demethylation abilities at these promoters is unclear, as contradictory results have been published (14, 15). In addition, a direct effect of AR on LSD1 biochemical activities in vitro has not been published. LSD1 recruitment by AR mostly occurs at enhancer sites [i.e., distal to the transcriptional start sites (TSSs)]. However, recent data have shown that the nuclear respiratory factor 1 (NRF1) cardinal TF can tether LSD1 to proximal promoter elements resulting in either transcriptional activation or repression (16, 17). How the activities (repressive or activating) of LSD1 are determined and what consequences this control has on the biological functions of this comodulator are currently unknown.
LSD1 regulates numerous physiological processes such as the balance between embryonic stem cell self-renewal and differentiation (18, 19) or differentiation and activity of adipose tissue (17, 20). High expression of LSD1 is also a factor of poor prognosis in various cancer types, where it promotes such phenomena as proliferation, cell survival, and epithelial-to-mesenchymal transition (21–25). How these regulations are precisely exerted at the molecular level and, in particular, through which TFs are presently unclear.
Nuclear receptors (NRs) constitute a large family of ligand-dependent TFs that regulate a vast number of biological processes including metabolism, differentiation, development, and proliferation (26). NRs interact with multisubunit cofactor complexes that promote or repress their activities through chromatin modifications (27, 28). The NR family also comprises orphan members—that is, for which no natural ligand has been identified to date (29). This is the case of the estrogen-related receptor α (ERRα), which activates its target genes in a ligand-independent manner and whose activities are regulated through interactions with transcriptional comodulators (30). For instance, interactions of ERRα with members of the peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1α and β) family is highly involved in the metabolic regulations exerted by the receptor (31, 32). These effects can be inhibited by various factors such as the prospero-related homeobox 1 (Prox1) protein or the NCoR1 corepressor (33, 34), suggesting a wide variety of potential cofactor complexes controlling the activities of ERRα. Whereas the ERRα–PGC-1α axis is extensively studied, in particular for its impact on metabolic pathways, little is known about ERRα interactions with chromatin-associated cofactors especially in a PGC-1–independent context. High expression of ERRα is also a factor of poor prognosis in various cancer types (35). This includes breast tumors where elevated levels of ERRα correlate with the establishment of metastasis and reduced disease-free survival (36). Consistently, the receptor promotes several traits of cancer progression, including cell migration and invasion (37–41).
Here we show that LSD1 and ERRα interact together and display common transcriptional targets, as identified by RNA-sequencing approach. Focusing on positively regulated genes, we show that inactivation of LSD1 or ERRα results in increased H3K9me2 deposition at the TSS, with no variation in H3K4me2, suggesting that these proteins are specifically involved in H3K9 demethylation. Importantly, ERRα, but not NRF1, is sufficient to induce LSD1 to demethylate H3K9 in vitro, suggesting that ERRα plays a central role in regulating LSD1 demethylation activities in vivo. Through this mechanism, LSD1 and ERRα enhance the expression of the matrix metalloprotease MMP1. This leads to increased cell ability to invade the extracellular matrix and possibly accounts for the capacities of LSD1 and ERRα to potentiate tumor progression.
Results
Common and Distinct ERRα–LSD1 Targets.
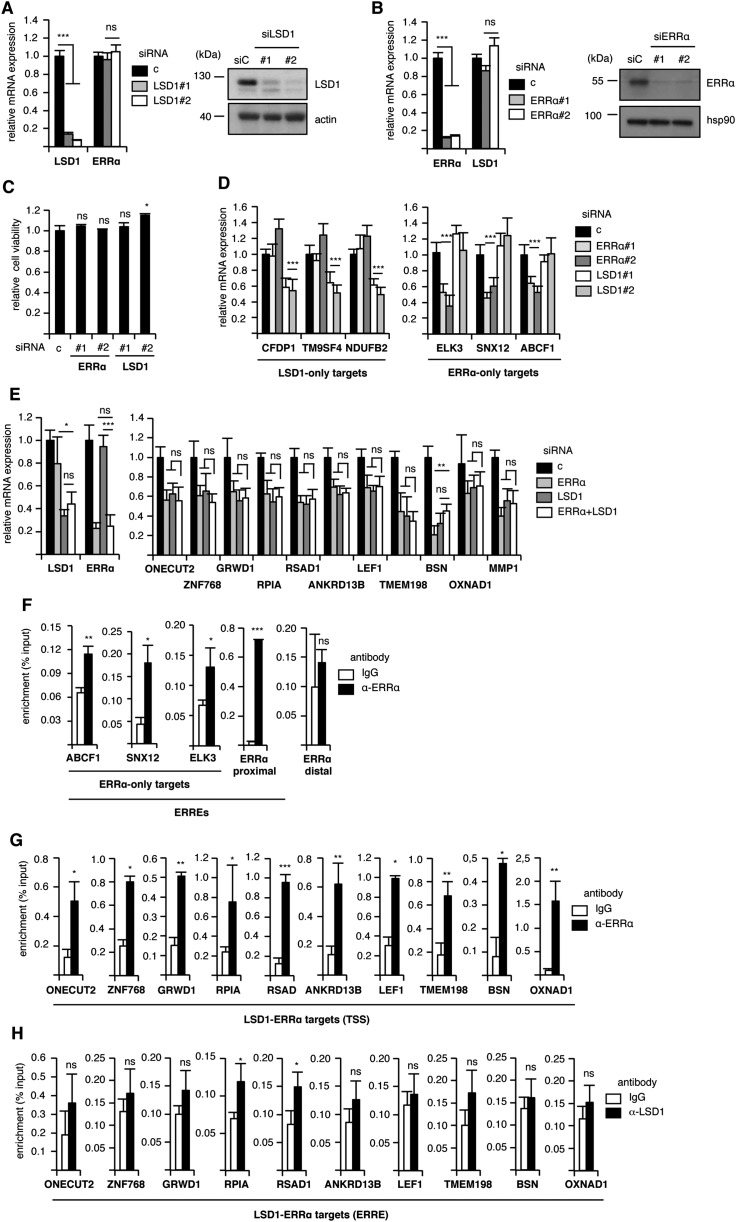
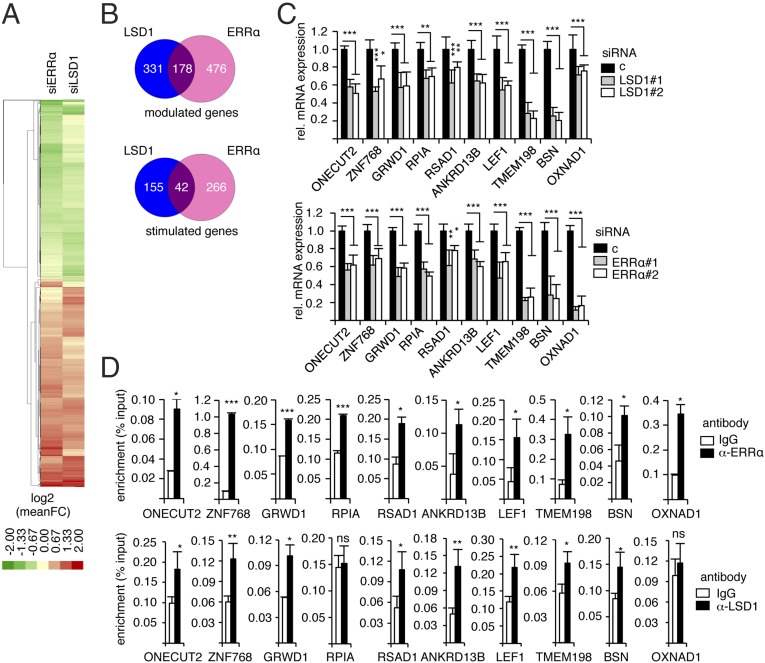
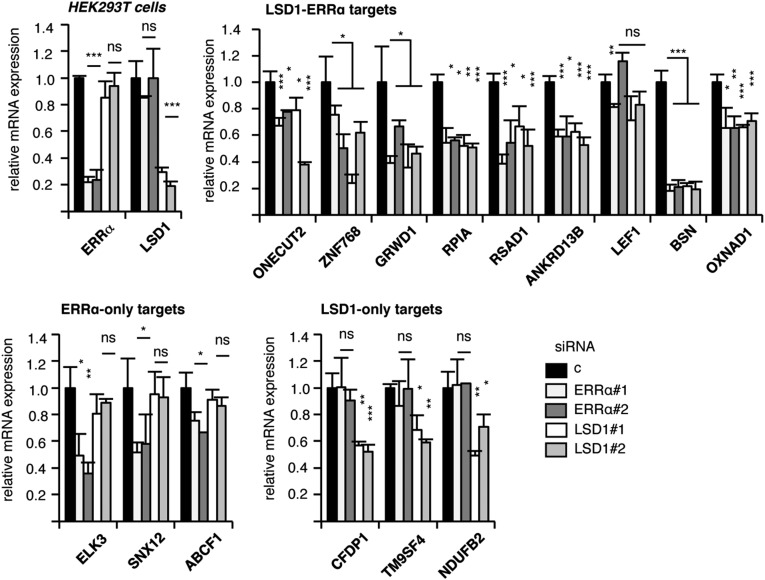
To investigate the mechanisms through which LSD1 positively regulates the expression of its target genes, we first determined the transcriptome of this factor in a model cell. To this end, MDA-MB231 breast cancer cells were transfected with siRNAs directed against LSD1 (Fig. S1A), and RNA sequencing was performed. We found that the expression of 509 genes was deregulated, among which 197 were down-regulated upon siRNA treatment (i.e., stimulated by LSD1) (Dataset S1). Quick inspection of this list suggested targets that are common with the ERRα TF. We thus performed a thorough analysis comparing the genes regulated by LSD1 to those regulated by ERRα, which we recently published (38) (Dataset S1). Hierarchical clustering revealed genes that are commonly modulated by both factors (Fig. 1A). Interestingly modulated genes are up- or down-regulated by ERRα and LSD1 in the same direction, suggesting a common regulation. A total of 178 genes were modulated by both ERRα and LSD1, among which 42 were stimulated by both factors (Fig. 1B). Although this may indicate that ERRα and LSD1 mainly exert repressive activities, we focused on stimulated genes to investigate mechanisms of the transcriptional activation driven by LSD1. We first validated the RNA-sequencing results on extracts from cells treated by siRNAs directed against LSD1 or ERRα and that do not affect cell viability (Fig. S1 A–C). We selected 10 genes that are commonly regulated by LSD1 and ERRα (referred to as LSD1–ERRα genes), for which a direct binding of ERRα has been observed in ChIP-Seq experiments performed on mouse liver (42). In addition, genes that are regulated by either one or the other factor (referred to as LSD1-only and ERRα-only) were also selected and used as controls. RT-qPCR confirmed the expected deregulation of the expression of these genes upon siRNA treatment (Fig. 1C and Fig. S1D). Simultaneous invalidation of LSD1 and ERRα did not result in increased deregulation of target gene expression, suggesting that both factors act in the same pathway (Fig. S1E). To investigate a possible direct regulation of the LSD1–ERRα target genes, ChIP experiments were performed (Fig. 1D). ERRα protein was found enriched on predicted cognate response elements (ERREs; Table S1) of all its target genes as well as at the TSSs of LSD1–ERRα genes (Fig. 1D and Fig. S1 F and G). LSD1 protein was detected at the TSSs of the majority of the common genes but not at the ERREs (Fig. 1D and Fig. S1H). A similar gene response pattern was also observed in HEK293T cells upon siRNA-mediated depletion of LSD1 or ERRα (Fig. S2), suggesting a general LSD1–ERRα connection.
Fig. S1.
Common transcriptional targets of ERRα and LSD1. (A and B) Cells transfected with siRNAs directed against LSD1 (A) or ERRα (B) were analyzed for expression of the indicated genes by RT-qPCR (Left) or Western blots (Right). RT-qPCR results are presented relative to control conditions with bars representing mean ± SEM of three independent experiments performed in triplicate. hsp90 and actin were used as a loading control in Western blots. (C) Cell viability was determined 48 h after transfection with the indicated siRNAs. Data are expressed relative to control-transfected cells. Bars represent mean ± SEM of two independent experiments in triplicate. (D) Expression of the indicated genes after siERRα or siLSD1 transfection analyzed by RT-qPCR and presented relative to control conditions. Bars represent mean ± SEM of three independent experiments performed in triplicate. (E) Cells were transfected with siLSD1 or siERRα, alone or in combination. Expression of the indicated genes was analyzed by RT-qPCR and is presented relative to control conditions. Bars represent mean ± SEM of three independent experiments performed in triplicate. Significance is shown for individual siRNA (siLSD1 or siERRα) relative to combined siRNA (siLSD1 + siERRα). (F) Binding of ERRα on the ERREs of the indicated genes analyzed by ChIP followed by qPCR. Percent enrichment relative to input is shown using anti-ERRα or IgG as a control. Bindings of ERRα on a proximal (but not distal) element of its own promoter were used as positive and negative controls, respectively. (G) Binding of ERRα on the TSSs of the indicated genes analyzed by ChIP followed by qPCR. Percent enrichment relative to input is shown using anti-ERRα or IgG as a control. (H) Binding of LSD1 on the ERREs of the indicated genes analyzed by ChIP followed by qPCR. Percent enrichment relative to input is shown using anti-LSD1 or IgG as a control. *P < 0.05; **P < 0.01; ***P < 0.005; ns, nonsignificant.
Fig. 1.
Common transcriptional targets of ERRα and LSD1. (A) Heatmap of the 985 genes over- or underexpressed upon both siERRαs or both siLSD1s using log twofold changes (scale indicated). (B) Venn diagrams schematizing the number of genes modulated (up or down, Top) or activated (Bottom) by ERRα (pink) and/or LSD1 (blue). See Dataset S1 for complete gene lists. (C) Expression of the indicated genes analyzed by RT-qPCR after transfection with the indicated siRNA, relative to control conditions. Values are mean ± SEM of three independent experiments performed in triplicate. (D) ChIP experiments using anti-ERRα (Upper) and anti-LSD1 (Lower) antibody or IgG. Percent enrichments relative to input were measured by qPCR, amplifying a region encompassing the TSS for LSD1 or putative ERREs for ERRα. Bars represent mean ± SEM of three independent experiments performed in duplicate. Significance is shown relative to control conditions. *P < 0.05; **P < 0.01; ***P < 0.005; ns, nonsignificant.
Table S1.
Identification of putative ERREs in the promoters of the indicated genes using oPossum online software (opossum.cisreg.ca/oPOSSUM3/)
| Genes ID | Chr | ERRE position relative to TSS | ERRE strand | % score | TFBS sequence |
| GRWD1 | 19 | −1378 | — | 88 | GGGCCAAGGTCT |
| RSAD1 | 17 | −4004 | — | 87 | ATCACAAGGACA |
| LEF1 | 4 | 1742 | — | 94 | CAGGCAAGGTCA |
| BSN | 3 | 541 | + | 87 | AGCGCAGGGTCA |
| RPIA | 2 | 4600 | — | 77 | TCAGGAAGTTCA |
| ZNF768 | 16 | −321 | + | 96 | AGACCAAGGTCA |
| TMEM198 | 2 | −2644 | + | 85 | TGCACAGGGTCA |
| ANKRD13B | 17 | −5349 | — | 85 | CTCCCAAGTTCA |
| ONECUT2 | 18 | −1859 | — | 85 | AAACAAGGGCA |
| OXNAD1 | 3 | −286 | + | 82 | AGTCCGAGGCCA |
| ELK3 | 12 | 704 | + | 86 | CCGCCCAGGTCA |
| SNX | X | 469 | — | 83 | AATTCAAGGAGA |
| ABCF1 | 6 | −8454 | — | 80 | TAGGGAATGTCA |
Localization is shown relative to TSS. Conservation of the response elements relative to consensus is indicated with % score.
Fig. S2.
Transcriptional targets of LSD1 and ERRα in HEK293T cells. Expression of the indicated genes in HEK293T cells after siERRα or siLSD1 treatment analyzed by RT-qPCR and expressed relative to 36b4. Note that all studied genes respond to ERRα and LSD1 in a similar manner in HEK293T and MDA-MB231 cells with the exception of LEF1 (not regulated by either factor) and TMEM198, the expression of which was not detected in HEK293T cells. *P < 0.05; **P < 0.01; ***P < 0.005; ns, nonsignificant.
ERRα and LSD1 Induce H3K9 Demethylation at Target Promoters.
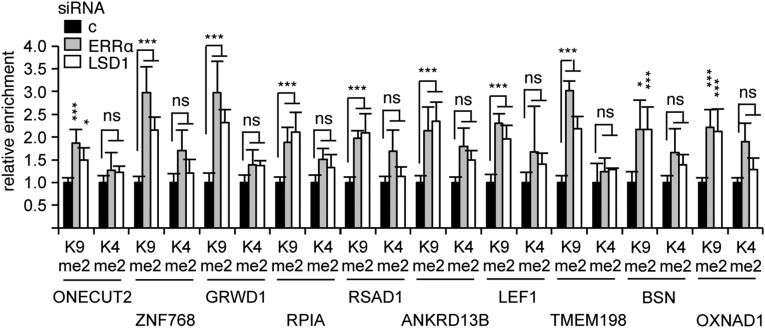
The results above suggest a functional interplay between LSD1 and ERRα that can lead to transcriptional activation. Transient transfections were then performed using isolated ERREs driving the expression of the luciferase reporter gene (Fig. S3A). We observed that the activity of ERRα on its response element was blunted upon siRNA-mediated LSD1 inactivation, indicating that LSD1 is required for ERRα transcriptional effect, at least on artificial systems. Depending on its target promoters, LSD1 has been shown to exert two possible activities, demethylating either H3K4me2 or H3K9me2, leading to transcriptional repression or activation, respectively. Thus, inactivation of LSD1 should result in an accumulation of the dimethylated lysine residue that is affected by the demethylase on these promoters. To determine the mechanisms through which LSD1 and ERRα activate gene expression, we investigated the histone methylation status at the TSSs of their common target genes. Consistent with transcriptionally active promoters, a strong enrichment of H3K4me2 mark, together with low abundance of H3K9me2, was first detected by ChIP experiments on the TSSs of LSD1–ERRα, as well as ERRα-only, targets (Fig. S3B). SiRNA-mediated inactivation of LSD1 resulted in an increased level of H3K9me2 histone mark at the TSSs of all 10 LSD1–ERRα promoters (Fig. 2) without any significant change in H3K4me2 levels, indicating that LSD1 exerts H3K9 demethylase activities at these promoters. Strikingly, inactivation of ERRα resulted in identical variations in histone marks on these TSSs. In contrast, no increase in the methylation status at the ERREs was observed upon LSD1 or ERRα inactivation (Fig. S3C). ERRα-only promoters did not show any change in H3K9me2 marks upon LSD1 or ERRα inactivation, although H3K4me2 variations were erratically observed (Fig. S3D). This suggests that a common LSD1–ERRα complex mediates H3K9me2 demethylation at the TSSs of positively regulated target genes.
Fig. S3.
ERRα transcriptional activity depends on LSD1 and controls for ChIP assays. (A) Cells inactivated for LSD1 (siLSD1#1 and #2) or not (siC) were transfected with ERRE-luciferase plasmid together with increasing amounts of ERRα-encoding plasmid. Results were normalized to samples transfected by ERRE-luciferase only. Bars show the mean ± SEM of three independent experiments performed in triplicate. (B) Detection of H3K4me2 and H3K9me2 at the TSSs of the indicated genes analyzed by ChIP. IgG was used as a control. Enrichments are presented relative to total H3 with bars representing mean ± SEM of three independent experiments performed in duplicate. (C) Detection of H3K9me2 at the ERREs of the indicated gene after siRNA-mediated inactivation of ERRα or LSD1. Results are expressed relative to control conditions with bars showing the mean of two independent experiments performed in triplicate. (D) Same as Fig. 2, analyzing the TSSs of the indicated genes. Significance is indicated relative to control conditions. *P < 0.05; ***P < 0.005; ns, nonsignificant.
Fig. 2.
ERRα induces H3K9 demethylase activities of LSD1. ChIP experiments were performed using antibodies against H3K9me2, H3K4me2, or H3 on chromatin from MDA-MB231 cells treated with the indicated siRNAs (c, control siRNA). qPCRs were performed using primers specifically amplifying the TSSs of the indicated genes. Enrichments are shown relative to H3 and to control conditions. Bars represent the mean ± SEM of three independent experiments performed in duplicate. Significance is shown relative to control. *P < 0.05; ***P < 0.005; ns, nonsignificant.
ERRα Interacts with LSD1 and Induces Its H3K9 Demethylase Activity.
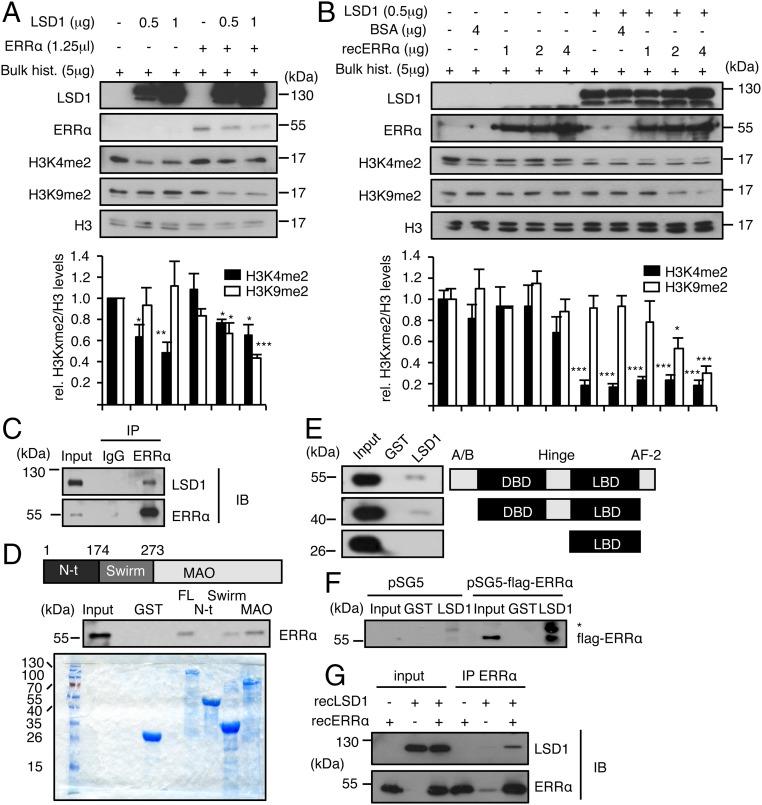
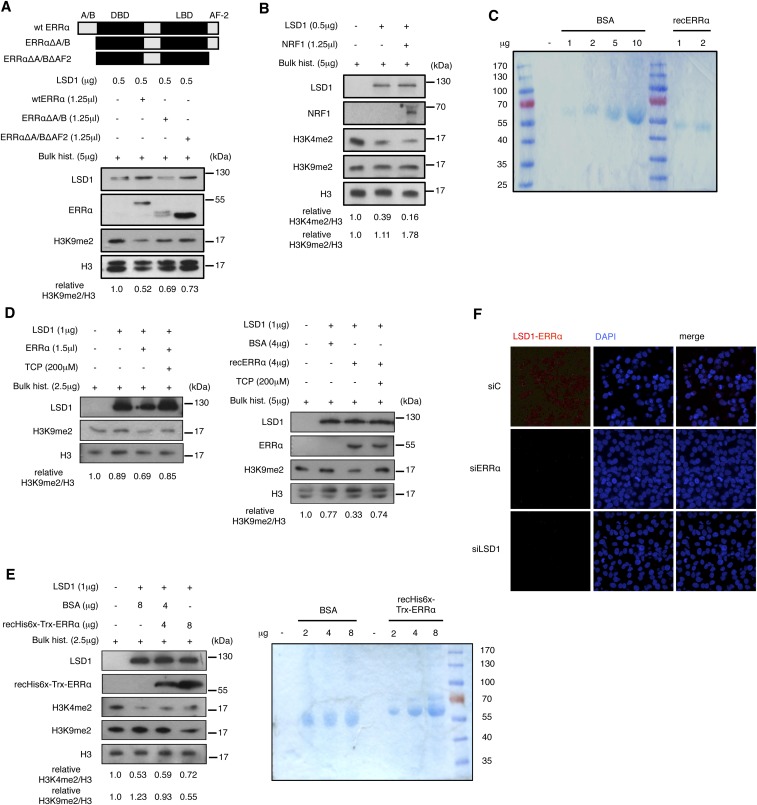
Despite its reported dual activity on target promoters, LSD1 has only been shown to demethylate H3K4me2 in in vitro assays (11). This raises the possibility that additional cellular compounds induce LSD1 to demethylate H3K9me2. Incubation of recombinant LSD1 with bulk histones in in vitro demethylation assays resulted in a dose-dependent reduction of H3K4me2 levels without altering those of H3K9me2 (Fig. 3A). Strikingly, demethylation of H3K9me2 by LSD1 was observed upon supplementation with in vitro translated ERRα protein. This activity of ERRα is independent of its intrinsic transcriptional activation domains. Indeed, an ERRα derivative deleted from its N-terminal A/B domain together with its C-terminal end (AF2 domain) still displayed an impact, albeit more moderately, on LSD1 activity (Fig. S4A). Recent publications indicate that the positive transcriptional regulation exerted by LSD1 often involves the NRF1 TF, which binds close to the TSS and tethers LSD1 to promoter regions (16, 17). However, in sharp contrast to ERRα, supplementation of the in vitro demethylation assay with NRF1 did not induce LSD1 to demethylate H3K9me2 (Fig. S4B), indicating the specificity of ERRα in this process. It is possible that additional factors present in the in vitro translation mixture cooperate with the receptor to induce H3K9me2 demethylation by LSD1. To exclude this possibility, we next used recombinant ERRα, in a preparation that does not contain any detectable additional protein (Fig. S4C). When added in demethylation assays, recombinant ERRα dose-dependently induced recombinant LSD1 to demethylate H3K9me2 (Fig. 3B). Tranylcypromine, an LSD1 inhibitor, blocked LSD1-induced H3K9 demethylation promoter by in vitro translated and recombinant ERRα (Fig. S4D). Recombinant ERRα produced in bacteria and purified to homogeneity also induced LSD1-driven H3K9 demethylation (Fig. S4E), further showing that the receptor is sufficient for such an activity.
Fig. 3.
ERRα interacts with LSD1 and promotes H3K9 demethylase activity. (A and B) Bulk histones were incubated with recombinant LSD1 in demethylation buffer. Reactions were supplemented with ERRα translated in reticulocyte lysates (A) or recombinant ERRα protein (B). Unprogramed reticulocyte lysate (A) or BSA (B) was used as controls in the absence of ERRα. Levels of H3K4me2, H3K9me2, and H3 were analyzed by Western blot. Quantifications of histone marks were determined using ImageJ software and are expressed relative to H3 and to control conditions. Bars represent the mean ± SEM of four independent experiments. Significance is shown relative to control. *P < 0.05; **P < 0.01; ***P < 0.005. (C) Coimmunoprecipitation of endogenous proteins in MDA-MB231 cells with anti-LSD1 or anti-ERRα antibodies and rabbit IgG used as a control. IB, immunoblotting; IP, immunoprecipitation. (D) Pull-down assay using the indicated GST-fused LSD1 derivatives and nuclear extract from HeLa cells. FL, full-length; MAO, MonoAmine Oxidase domain; N-ter, N-terminal domain. Coomassie blue staining below shows the expression of GST-fused proteins used. (E) Pull-down experiment using GST-LSD1 (full-length) and nuclear extract from HeLa cells transfected with the indicated flag-tagged ERRα derivatives. (F) In vitro interaction assay using GST-LSD1 (full-length) and in vitro translated flagged ERRα or empty vector (pSG5). Western blots were probed with flag antibody. *, nonspecific band. (G) Recombinant LSD1 and recombinant ERRα were incubated in demethylation buffer. Immunoprecipitation was then performed using anti-ERRα antibody. Immunoblots (IBs) were probed with the indicated antibodies. Shown are 20% inputs.
Fig. S4.
Controls for demethylation and interaction assays. (A) Demethylation assay detecting H3K9me2 similar to Fig. 3A using the indicated flagged ERRα deletion mutants produced in reticulocyte lysates. ERRα mutants were detected in Western blot using flagM2 antibody. (B) In vitro demethylation assay with the same representation as Fig. 3A with supplementation by in vitro translated NRF1. (C) Control of the purity of recombinant ERRα (recERRα) used in Fig. 3B. Shown is a Coomassie blue staining of a gel loaded with the indicated amounts of recombinant ERRα or BSA used as a control. (D) Demethylation assay detecting H3K9me2 similar to Fig. 3A (for Left) and Fig. 3B (for Right) with supplementation of tranylcypromine (TCP), an LSD1 inhibitor. (E) Demethylation assay detecting H3K4me2 and H3K9me2, using recombinant LSD1 and ERRα (recHis6x-Trx-ERRα) purified from bacteria. Right shows a Coomassie blue staining of a gel loaded with the indicated amounts of recombinant ERRα and BSA used as a control. (F) Proximity ligation assays using anti-LSD1 and anti-ERRα antibodies under the indicated conditions. All quantifications represent the mean of three independent experiments.
Our results predict that LSD1 and ERRα likely interact with each other. This is indeed the case for endogenous cellular proteins, as using an anti-ERRα antibody allowed for immunoprecipitation of LSD1 (Fig. 3C). As indicated by proximity ligation assays, this interaction takes place in the nucleus (Fig. S4F). We next used pull-down experiments to examine which domains of the proteins are involved in these contacts. GST-fused full-length LSD1 protein interacted with ERRα originating from MDA-MB231 nuclear extracts (Fig. 3D). Interaction was also detected when considering GST-fused Swirm (involved in protein–protein interactions) or Monoamine Oxidase domains (MAO; catalytic domain) but not the LSD1 N-terminal domain. GST-fused full-length LSD1 was next used to pull down nuclear extracts from MDA-MB231 cells transfected with flag-tagged ERRα derivatives (Fig. 3E). Full-length ERRα interacted with LSD1. An ERRα deletion mutant in which both the N-terminal A/B and the AF2 domains were deleted still retained the capacity to contact LSD1. In contrast, the putative ERRα ligand-binding domain (LBD) alone did not interact with LSD1, indicating that the DNA binding-hinge domains are required for physical contacts. In addition, in vitro translated flagged ERRα also interacted with GST-LSD1 (Fig. 3F). Furthermore, coimmunoprecipitation experiments show that recombinant LSD1 and recombinant ERRα physically interact when incubated in demethylation assay buffer (Fig. 3G). Together with the demethylation results above, these data indicate that direct interaction with the DBD and/or hinge regions of ERRα is sufficient to induce LSD1 to demethylate H3K9.
LSD1 and ERRα Promote Cell Invasion in an MMP1-Dependent Manner.
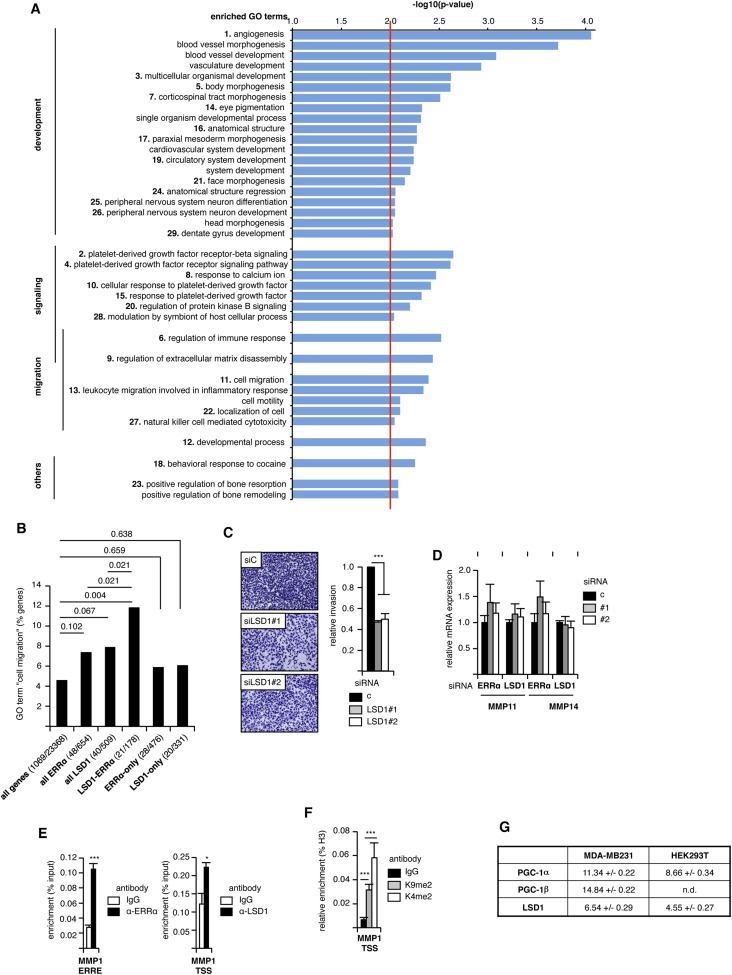
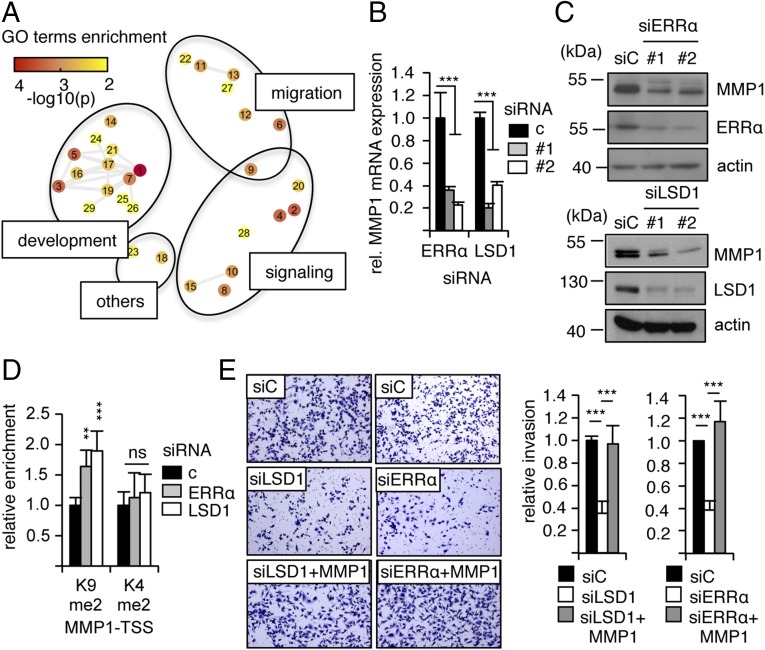
We next investigated the physiopathological consequences of these transcriptional processes. To this end, the list of genes commonly modulated by LSD1–ERRα was submitted to Gene Ontology (GO) analysis. Several GO terms were found significantly enriched (Fig. S5A) and were ordered according to semantic similarities using the REVIGO software. This resulted in a clustering into three major groups (“development,” “signaling,” and “migration”; Fig. 4A). We and others have previously shown that inactivation of ERRα inhibits cell migration and invasion (37–39). On the other hand, inhibition of LSD1 results in a similar phenotype (43, 44). Noteworthy, the GO term “cell migration” is significantly enriched when considering LSD1–ERRα genes but not all LSD1 nor all ERRα genes (Fig. S5B). This suggests that the promigratory effect of LSD1 and ERRα depends on both factors acting together. We thus focused on this process as a possible phenotypic outcome of LSD1–ERRα interaction. SiRNA-mediated inactivation of LSD1 strongly reduced cell invasion capacities as evaluated in 3D invasion assays (Fig. S5C). To investigate the mechanisms through which LSD1 and ERRα regulate cell invasion, genes appearing under the enriched GO terms related to migration were examined. This revealed MMP1 (Matrix MetalloProteinase 1), whose product is a secreted protein involved in extracellular matrix degradation and cell invasion (45), as an LSD1–ERRα target. RT-qPCR experiments (Fig. 4B) as well as Western blot analysis (Fig. 4C) showed that siRNA-mediated inactivation of LSD1 or ERRα led to reduced expression of MMP1-corresponding mRNA and protein. In contrast, neither MMP11 nor MMP14 were regulated by any of these factors, indicating a specific regulation of MMP1 (Fig. S5D). ChIP experiments revealed LSD1 binding at the MMP1 TSS and ERRα binding on an ERRE proximal to the TSS (Fig. S5E). SiRNA-mediated inactivation of LSD1 or ERRα resulted in increased representation of H3K9me2, without any change in H3K4me2 (Fig. S5F and Fig. 4D), indicating that both factors activate MMP1 expression through H3K9me2 demethylation. This also suggests that MMP1 is an effector of LSD1–ERRα in their regulation of cell invasion. This possibility was evaluated by invasion assays. Strikingly, reintroduction of MMP1 in cells in which LSD1 or ERRα had been inactivated by siRNA treatment resulted in rescued invasion potential (Fig. 4E). Together we conclude that LSD1–ERRα contributes to reduce H3K9 dimethylation at the MMP1 TSS, leading to enhanced expression of this factor, which in turn results in increased cell invasion potential.
Fig. S5.
ERRα and LSD1 induce cell migration in an MMP1-dependent manner. (A) Extended list of enriched GO terms in ERRα–LSD1 common targets. Numbers correspond to the representation in Fig. 4A. –log10(P value) is indicated as enrichment level. Only enrichments >2 (red line) were considered significant. (B) Percentage of genes found under GO term cell migration (GO:0016477) in the whole genome (all genes) or within the genes modulated by LSD1 and/or ERRα, as indicated. Number of genes is displayed under parentheses. Significance is shown as P values above the bars. (C) Similar to Fig. 4E using cells treated with the indicated siRNAs. (D) Similar to Fig. 4B, analyzing the expression of MMP11 and MMP14 after treatment with the indicated siRNA. No variation (relative to control siRNA) was found significant. (E) Binding of ERRα or LSD1 on the indicated MMP1 promoter regions analyzed by ChIP. Percent enrichment is shown relative to input with IgG used as a control. Bars represent mean ± SEM of three independent experiments performed in duplicate. (F) Detection of H3K4me2 and H3K9me2 on the TSS of MMP1 gene. Conditions and representation are similar to Fig. S3B. (G) Expression of the indicated genes in MDA-MB231 or HEK293T cells determined by RT-qPCR is shown relative to that of the housekeeping gene 36b4 (values are in Ct of the indicated gene minus that of 36b4). Experiments were performed in triplicate. Values are mean ± SD. n.d., not detected. *P < 0.05; ***P < 0.005.
Fig. 4.
ERRα and LSD1 induce cell migration in an MMP1-dependent manner. (A) ERRα–LSD1 coregulated genes were analyzed by GO. Network of enriched GO terms obtained with REVIGO software after removing redundant terms is shown. Nodes represent GO terms that are gathered according to their semantic similarity. GO terms are coded by numbers (Fig. S4A for correspondence). Colors indicate the P value. (B) Expression of MMP1 analyzed by RT-qPCR after transfection with the indicated siRNAs, relative to control conditions. Values are mean ± SEM of three independent experiments performed in triplicate. Significance is shown relative to control. ***P < 0.005. (C) Expression of MMP1 protein analyzed by Western blot after treatment with the indicated siRNAs. (D) ChIP experiments performed using H3K9me2, H3K4me2, or H3 on chromatin from MDA-MB231 cells treated with the indicated siRNAs (c, control siRNA). qPCRs were performed using primers specifically amplifying the TSSs of the MMP1 gene. Enrichments are shown relative to H3 and to control conditions. Bars represent the mean ± SEM of three independent experiments performed in duplicate. Significance is shown relative to control. **P < 0.01; ***P < 0.005; ns, not significant. (E) Cells transfected with the indicated siRNAs supplemented or not with transfected MMP1 were allowed to invade Matrigel on Boyden chamber assays for 48 h. Microphotographs are displayed on Left. Quantifications were performed on whole well using ImageJ software. Bars represent the mean ± SEM of three independent experiments. ***P < 0.005.
Discussion
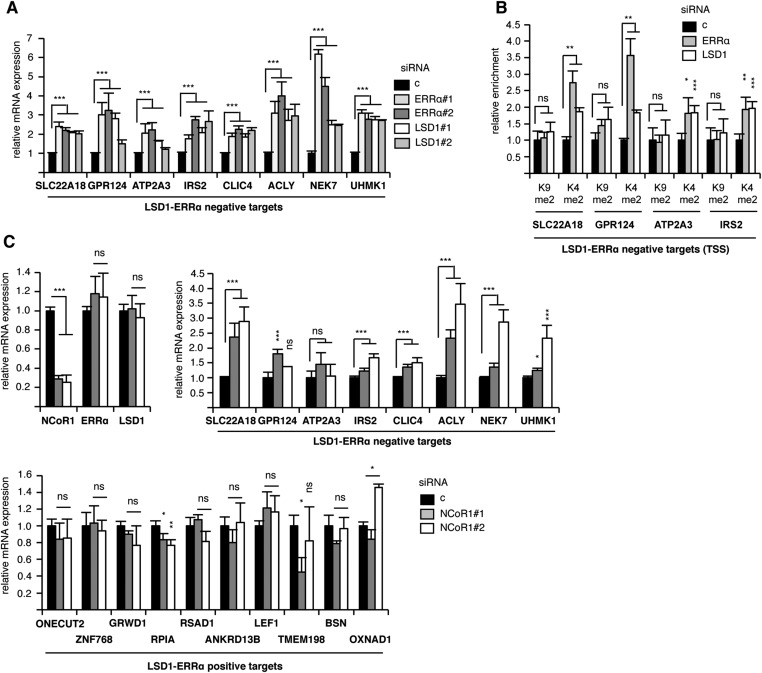
LSD1 has initially been shown to demethylate H3K4 residues on local chromatin, resulting in transcriptional repression (10). However, it has rapidly been demonstrated that this enzyme could also contribute to transcriptional activation. For instance, the hormone-dependent recruitment of LSD1 by the AR or the Estrogen Receptor α (ERα) (at AR- or ER-response elements, respectively) leads to the transcriptional activation of a subset of AR- or ER-responsive genes (13, 46–48). This has been associated with hypomethylated H3K9, suggesting that LSD1 can also demethylate this residue at specific loci. LSD1 still retains H3K4 demethylating activity on AR-LSD1 responsive genes, indicating a dual repressing/activating function of the demethylase (15). However, enzymatic assays have shown that, on its own, LSD1 demethylates H3K4 in vitro but not H3K9 (10). This suggests that specific chromatin context may change the specificity of LSD1 toward H3K9 demethylation. Indeed, AR-controlled phosphorylation of H3T6 and H3T11 by PKCβ1 and PKN, respectively, switches the specificity of LSD1 from H3K4 to H3K9 demethylation and may thus contribute to LSD1 coactivation functions (14, 49). Alternatively, it is possible that additional compounds may autonomously induce LSD1 to demethylate H3K9. In this respect, a possible effect of AR has not been reported. ERα does not induce such an activity unless it is supplemented by the Pelp1 coactivator (50). Results of our in vitro assays show that the ERRα orphan receptor is actually sufficient to unmask H3K9 demethylation activity in LSD1. This activity depends on ERRα domains that are involved in interaction with LSD1. The molecular mechanism through which this is achieved is unknown. ERRα interacts with the SWIRM-MAO domains, which are actually in close proximity (51). One could hypothesize that interaction with ERRα promotes an allosteric change in LSD1 structure that allows H3K9 demethylation. Consistently, inactivation of LSD1 or ERRα led to an increase of H3K9me2 deposition at the TSSs, but not enhancers, of common positive target genes. This is in contrast with the AR situation, where alterations of H3K9me2 status mainly occur at enhancers (15). Knockdown of LSD1 or ERRα did not alter the local H3K4 methylation levels on common positive targets, suggesting that LSD1 does not demethylate H3K4 at these TSSs. This is in striking contrast with LSD1–ERRα negative targets, on the TSSs of which both LSD1s mediate H3K4 demethylation (Fig. S6 A and B). This negative activity may depend on the recruitment of NCoR1, which has been shown to repress ERRα activities (34). Indeed, inactivation of this corepressor leads to increased expression of negative (but not positive) targets (Fig. S6C).
Fig. S6.
Features of LSD1–ERRα-repressed genes. (A) Similar to Fig. 1C, analyzing the expression of the LSD1–ERRα-repressed genes. (B) Similar to Fig. 2, analyzing histone marks at the TSS of the indicated genes. (C) Similar to Fig. 1C, analyzing the effect of siRNA-mediated NCoR1 inactivation on the indicated genes. Values are mean ± SEM (three independent experiments). Significance is shown relative to control conditions. *P < 0.05; **P < 0.01; ***P < 0.005; ns, nonsignificant.
The relevance of an LSD1–ERRα interconnection is highlighted by our functional assays. ERRα is highly involved in the regulation of energetic metabolism, including in breast cancer cells, an activity that strongly depends on members of the PGC-1 family of coactivators (31, 32). However, PGC-1s are very poorly expressed in MDA-MB231 cells compared with LSD1 (Fig. S5G), which possibly accounts for the lack of any enrichment in metabolic-related pathways in LSD1–ERRα-regulated genes. This suggests that this complex is not involved in metabolism but rather in other processes. The literature indicates that LSD1 and ERRα display similar physiopathological activities. For instance, epidemiological data have shown that high expression of LSD1 or ERRα constitute a factor of poor prognosis and is associated with decreased survival in breast cancer (23, 35, 36, 52–54). This suggests that these proteins promote parameters of cancer progression. Indeed, LSD1 increases the capacities of various cancer cell types to migrate and invade the extracellular matrix, which are essential determinants of cancer aggressiveness (ref. 43, 44; this report). These activities have also been reported for ERRα (37–39). Importantly, GO analysis of the genes that are commonly regulated by LSD1 and ERRα show a significant enrichment for terms related to cell migration. This enrichment is, however, not significant when considering all genes modulated by LSD1 or all genes modulated by ERRα. Furthermore, genes regulated by either one or the other factor (i.e., excluding genes regulated by both LSD1 and ERRα) do not show any significant enrichment for migratory functions. The promigratory functions of one factor (LSD1 or ERRα) may thus depend on the other. In particular, the MMP1 metalloprotease is positively regulated by both factors through H3K9 demethylation. Importantly, re-expression of MMP1 is sufficient to rescue the invasive defect observed in the absence of LSD1 or ERRα. This indicates that this metalloprotease is a key element in the regulation of the invasive process driven by LSD1–ERRα. Altogether this suggests that these three factors build a common network to promote cancer progression, at least through the induction of cell invasion in an MMP1-dependent manner. Negatively targeting one of these factors or their interaction capacities may be a promising approach to reduce cell invasion.
Materials and Methods
MDA-MB231 and HEK293T cells were cultured in DMEM supplemented with 10% FCS, 10 U/mL penicillin, and 10 µg/mL streptomycin. For siRNA transient transfection, 3×105 cells per mL were seeded in six-well plates, and 25 pmol/mL of siRNAs against LSD1, ERRα (Dharmacon and Invitrogen), or control (medium GC Stealth RNA interference negative control duplexes, Invitrogen) were transfected with INTERFERin (Polyplus Transfection) according to the manufacturer’s protocol. Plasmid transfections were performed with JetPRIME (Polyplus Transfection). For luciferase assays, cells were cotransfected with CMV-βGal plasmid. Luciferase activity was normalized to that of β-galactosidase. Cells were harvested 48 h after transfection. SiRNA sequences are shown in Table S2. ERRα deletion mutants as well as ERRE-Luciferase plasmids have been described elsewhere (55). Detailed materials and methods are provided in SI Materials and Methods.
Table S2.
Oligonucleotides and siRNAs used in this study
| GenesID | Forward | Reverse |
| Oligonucleotides for expression studies | ||
| 36b4 | GTCACTGTGCCAGCCCAGAA | TCAATGGTGCCCCTGGAGAT |
| KDM1A | ACCACAACAGACCCAGAAGG | CTCGGTGGACAAGCACAGTA |
| ESRRA | CAAGCGCCTCTGCCTGGTCT | ACTCGATGCTCCCCTGGATG |
| ONECUT2 | AAATCTGGCAGGGAGACCTT | GGTTCTTGCTCTTTGCGTTT |
| ZNF768 | GGGTACCTCAGAGGCAACAT | GGGTTCAAACTCTGGGCTTT |
| GRWD1 | TGGTCACCGACTGAGAACAC | GCTCCAGCTGATGACATTGA |
| RPIA | TAGTCGCTTCATCGTGATCG | GATTCCCTTGTGCCACTGAT |
| RSAD1 | CGCAGCTGAGATGTACCAGA | AACGCCAAGGTACTGACCAC |
| ANKRD13B | GGCAAGGTCAAAGGCTGTAA | GTGATCAGGGTCCCATTTTG |
| LEF1 | TGGAAAACGAAGCTCATTCC | GGGTTGGCAGTGATTGTCTT |
| TMEM198 | GTGCTGTTTGTTTGGAGTCG | TCTCGGTAGCAGAGGAGGAA |
| BSN | CCAGCCAAACTTCAACACCT | AGCCCTCTGCATCTGACAGT |
| OXNAD1 | CAGCAGCTAAGGTGTGTGGA | AACCCACCAACCACAGAGAC |
| ABCF1 | CAGTGCCAACCAGTGATGAG | GGCAGGCTTAGGAGGATGTT |
| SNX12 | CCGAGGAGATGAAGGGATCT | TGTAGGCAGCGTTCATTCTG |
| ELK3 | CCAAAGGCTTGGAAATCTCA | CGGAGTCAGAAGCAATCCAT |
| CFDP1 | GTTCCTTCAGCTCTGCCATC | TGAAGCTCTCCCAGTCCAGT |
| TM9SF4 | CCAGAACGATCCCGTAGAAA | TCTCAGCACCTCTCCCAGAT |
| NDUFB2 | ACTGCTGGAGATGGTGGAGT | CACATGAGTCCGCTGAAGAA |
| SLC22A18 | GTGGATCCCTGGCTTAGTGA | ACAGCTTGGGTTGTGGTGAG |
| GPR124 | CTGGAGAAGCTGGACCTGAG | AAGGTCTCGGAGGTGAGACA |
| ATP2A3 | TTGCACGAGTTCACCATCTC | TAGTCCAGAGCCGAGTCGTT |
| IRS2 | AGACGCTCAGCACTGTTTCA | AACAAGGGAAAGAGGCAGGT |
| CLIC4 | CCTTTTCCCAGAGGCTCTTC | CTGGCTTCCTTTTCAGGTCA |
| ACLY | TGCCGACTACATCTGCAAAG | GGTTCAGCAAGGTCAGCTTC |
| NEK7 | TGGATGAGCAATCACAAGGA | TAGCCCATATCCGGTCGTAA |
| UHMK1 | TGGCCTGCAGAGTGATACAG | AGCAGAACTGTTTGCCTTCC |
| NCOR1 | CGGAGTCCCTGATTATCGTT | TCTTGAGGCCTGTCAGAACC |
| MMP1 | AGGTCTCTGAGGGTCAAGCA | CTGGTTGAAAAGCATGAGCA |
| MMP11 | ATGATCGACTTCGCCAGGTA | ATCCCCTTCTCGGTGAGTCT |
| MMP14 | GCAGAAGTTTTACGGCTTGC | TAGCGCTTCCTTCGAACATT |
| Oligonucleotides for ChIP on TSS | ||
| ONECUT2 | GCCCTGATGGACTGAATGAA | GTGCCCAGACTTTCCATTGT |
| ZNF768 | TTCTTCCTCAGGCTTTGGAG | CAGCAACAGCAAAGCACTTC |
| GRWD1 | GATTAGCGGTCCCAGGAGTT | CTGCTGACACCCGGTAAGAG |
| RPIA | CGGAGAGCATTATGGGATTG | GGGTCTTGCTGGGAAATGTA |
| RSAD1 | AGTTCTGAGCCAGGCACATC | CCTCCTGTCGGTTCTTTCC |
| ANKRD13B | TCCTCTCTCCCCATCATCC | GGTCCCCACAGCTGCATA |
| LEF1 | ACACACCCCAAAACCAAGAC | GAAGGAGGTGGTGATTGAGG |
| TMEM198 | GGGTTGGCAGTGATTGTCTT | TCTGCTCCTGACGTCACTC |
| BSN | CAGAGGCCGCTTCTAGTGG | CGCGGTTGTATCATGCTG |
| OXNAD1 | TGGAGATGGCTGAACTAGGG | GCGAGCCCCACATTCTATT |
| ABCF1 | AGGCTTCCCAGACAGTCGTA | GAGCTTCGCCGTCATCTC |
| SNX12 | TGTGCTGCTAGGCAGGGTAT | GAGTGGAGGCCATATTCAGG |
| ELK3 | GGCGGAAAAGCCTGTTTA | GAGCAGGAAGTTGGAGCAGT |
| MMP1 | AGGAATCCATAAGGGGAGGA | CGGTGTCACCAGTGCTATCT |
| SLC22A18 | TTCCCACATTCCCTCAGAAC | GTAGCCAGGGAACTGGTTGA |
| GPR124 | ACTCGGCAGAGGGTCTAGG | CATGGATGGAGGCAGCTC |
| ATP2A3 | CAGATCCCACCCCTAGCAG | TGCAGAGGGGAAACTGAGAC |
| IRS2 | CTGTGTGTGCCTGCGTAAC | CGCACAGTGAGTAACACATCG |
| Oligonucleotides for ChIP on ERREs | ||
| ONECUT2 | GGCAGAGCTCGGAATACTGT | CGGCAGAAAAGGTCACTGAG |
| ZNF768 | TGTTCTAGGTCTCCGTCCCT | CTTCTCGCTGACCTTGGTCT |
| GRWD1 | CACAGTCTGGAGTCGGATGA | AAACTGTGACCCCAGACCTT |
| RPIA | AAGCAGGGCCACAACTAAAA | AGGCCTAGGGGCTGACTTTA |
| RSAD1 | CCCCTCAGTGTCTTCCCTTC | CACATTCCCCTGTCTGTCCT |
| ANKRD13B | CATCTCCTCCACCCTTGAGA | TGCTGTCCCTCTGCCTCTAT |
| LEF1 | CCCTACCCATCCTCACTGTC | GGATGCAAGCACGAGAAGAG |
| TMEM198 | AGAGGGAAAGTTGGGGTCAC | CCTTTGTCCACTGTCCTTGC |
| BSN | GATTGGCGTGAACTGGGTG | CGTTGGGGAGAAGCGAATTT |
| OXNAD1 | TGGGCAGGGATAGAAATACG | CTGACCATGGCAGTGTTTCA |
| ABCF1 | AATCCAAAGGCAGGGAGAGT | CCTACCCCCAATGTTGTCAG |
| SNX12 | CCCATGAGGAAGTGAAGCAT | CAGCCGTGATTCCTATCTCC |
| ELK3 | AAAGCCGGTGGTCTCTATTG | TCGAAGGTAAACAGGGATGG |
| ESRRA prox. | CCATCCGAGTGGAATTTGAGTCCTAAAG | GAACCGTAGACCCAGTAGCCCCACAGAG |
| ESRRA dist. | GTGGCCCACAGGTGTCGCTCAAGTCTTC | GGATGCAGTGTCCTTCTCCCCAGATTG |
| siRNAs | ||
| ERRα#1 | GGCAGAAACCUAUCUCAGGUU | CCUGAGAUAGGUUUCUGCCUC |
| ERRα#2 | GAAUGCACUGGUGUCACAUCUGCUG | CAGCAGAUGAGACACCAGUGCUUC |
| LSD1#1 | ACUUUGUAACUGUCGAGCUGC | GCAGCUCGACAGUUACAAAGU |
| LSD1#2 | CCACGGAGCGACAGAGCGAGC | GCUCGCUCUGUCGCUCCGUGG |
| NCOR1#1 | CUCGUCUCAUCUCCGUUAA | UUAACGGAGAUGAGACGAG |
| NCOR1#2 | CGAGUCAAGUUCAUUAACA | UGUUAAUGAACUUGACUCG |
SI Materials and Methods
Cell Culture and Transfection.
For proliferation assays, 104 siRNA-transfected cells were seeded in 96-well plates. Cell viability was determined 48 h after transfection using CellTiterGlo kit (Promega) under the manufacturer’s recommendations. For invasion assays, 5×104 cells were suspended in 200 µL DMEM/2% FCS and seeded on top of matrigel invasion chambers (Corning). Cells were allowed to migrate toward the lower chamber containing DMEM/10% FCS for 48 h. Matrigel was removed using cotton buds, and cells were fixed 1 h with 4% formaldehyde, colored with 0.1% crystal violet, and microphotographed. Invading cells were counted on whole well using ImageJ.
Interaction Assays.
For coimmunoprecipitation assays, cells were harvested in PBS, and pellets were resuspended in Nonidet P-40 buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40) supplemented with protease inhibitor mixture (Sigma-Aldrich). We precleared 800 µg to 1 mg of proteins for 2 h on Sepharose-protein A (GE-Healthcare), and 3 µg of antibodies were added for 4 h at 4 °C with rotation (ERRα, PP-H5844-00, R&D; and LSD1, 05–939, Millipore). Beads were then added to the extract and incubated for 1 h, washed 5 times with Nonidet P-40 buffer, and finally resuspended in Laemmli buffer for immunoblotting analysis. We analyzed 50 µg of whole-cell lysate as input fraction.
For GST pull-down assays, GST-tagged proteins were produced from BL-21 (DE3) bacterial strain, purified on Gluthatione-Agarose beads (Sigma), and quantified by Coomassie staining. In vitro interaction assays were performed with equal amounts of GST or GST fusion proteins in affinity buffer (20 mM Hepes, 10 mM ZnCl2, 0.1% Triton, 2 mM EDTA) supplemented with NaCl, PMSF, DTT, and protease inhibitor mixture. HeLa nuclear extracts or ERRα produced in vitro with rabbit reticulocyte lysate (Promega) were subjected to interaction assays for 2 h at 4 °C under mild rotation. Bound proteins were washed 4 times and resuspended in Laemmli buffer for Western blot analysis. Ten percent of the interaction mix was loaded as an input fraction.
For proximity ligation assays, cells cultured on coverslips were fixed with 4% paraformaldehyde (Merck) for 10 min at room temperature, washed with PBS, and analyzed with the Duolink PLA kit (O-link; Bioscience) according to the recommendations provided by the manufacturer using anti-LSD1 or anti-ERRα antibodies. Samples were Dapi-counterstained. Images were acquired using a Zeiss AxioImager microscope.
In Vitro Demethylation Assay.
In vitro demethylation assays were performed on bulk histones (Sigma-Aldrich), using purified LSD1 (Enzo-Lifesciences; cat. no. BML-SE544) with or without ERRα translated in vitro using the reticulocyte lysate system (Promega) or recombinant ERRα produced as described (56). For production in insect cells, full-length ERRα (2–422) was cloned into pDEST8 vector containing a C-terminal hexahistidine tag and expressed in Sf9 or Sf21 insect cells using the baculovirus technology. For production in bacteria, full-length ERRα was cloned into pnEAvHX vector containing an N-terminal hexahistidine tag as well as a TrxA fusion moiety and expressed in BL21 Escherichia coli. For purification, harvested cells were resuspended in lysis buffer (20 mM Tris⋅HCl, pH 8, 400 mM NaCl, 10% glycerol, 2 mM CHAPS, 5 mM imidazole, cOmplete EDTA-free protease inhibitor mixture tablet; Roche Applied Science), sonicated, and centrifuged. The supernatant was loaded on 5 mL HisTrapp FF crude column (GE Healthcare). The protein was eluted at 250 mM imidazole and further purified by SEC on Superdex S200 (16/60 and 10/300, GE Healthcare) column. Purity and homogeneity of the protein were assessed by SDS/PAGE, and complex formation was monitored by native PAGE. For the demethylation assays, bulk histones were incubated with purified LSD1 and/or ERRα and NRF1 in the demethylase activity assay buffer (50 mM Tris, pH8.8, 50 mM KCl, 5 mM MgCl2, 0.5% BSA, 5% glycerol) for 18 h at 30 °C. The demethylase activity of LSD1 was analyzed by Western blotting using methylated H3K4- and H3K9-specific antibodies. Where required, Tranylcypromine (Sigma) was added at 200 µM.
Western Blot Analysis.
For Western blot analysis, cells were lysed in Nonidet P-40 or RIPA buffer supplemented with Protease Inhibitor Mixture (Sigma-Aldrich). Proteins (25–50 µg) were resolved on 8–15% SDS/PAGE, blotted onto PVDF membrane (GE-Healthcare), and probed with specific antibodies after saturation. The antibodies (and their dilution) used in this study were as follows: ERRα (GTX108166, Genetex, 1/5,000), hsp90 (API-SPA-830, Enzo Life Sciences, 1/3,000), LSD1 (ab17721, Abcam, 1/1,000), NRF1 (ab55744, Abcam, 1/3,000), MMP1 (AB6002, Millipore, 1/1,000), flag-M2 (F3165, Sigma, 1/3,000), β-actin (A5060, Sigma, 1/10,000), H3K4me2 (07-030, Millipore, 1/5,000), H3K9me2 (07-441, Millipore, 1/1,000), and histone3 (ab1791, Abcam, 1/5,000).
RNA Extraction and Real-Time PCR.
Total RNAs were extracted by guanidinium thiocyanate/phenol/chloroform. We converted 1 µg of RNA to first strand cDNA using IScript cDNA synthesis kit (Biorad). Real-time PCR was performed in a 96-well plate using the IQ SYBR Green Supermix (Biorad). Data were quantified by the ∆∆-Ct method and normalized to 36b4 expression. Sequences of the primers used in this study are shown in Table S2.
RNA Sequencing and Bioinformatic Analysis.
RNAs were extracted from two independent replicates from MDA-MB231 cells (transfected with siRNAs) using Qiagen RNA extraction kit. RNA quality was assessed using BioAnalyzer 2100TM (Agilent Technologies). Sequencing was performed by ProfileXpert platform with HiSEq. 2000 Illumina. Sequences were aligned on the human genome (hg19, GRCh37 version) using TopHat. Read counts were determined using HT-seq. Differentially expressed genes were identified with DESeq2 R package, using an adjusted P value (padj) < 0.05. Genes showing significantly modified expression in at least one siRNA experiment were gathered together for hierarchical clustering (Cluster 3.0) and heatmap representation (Java TreeView 1.1.6r4). Clustering used the euclidian distance and the average linkage method applied to log twofold changes. The complete study (jmvanack_files.zip) has been deposited on the GEO/NCBI website (https://www.ncbi.nlm.nih.gov/gds). The list of LSD1-regulated genes is presented in Dataset S1. Lists of regulated genes obtained for each siRNA were intersected. GO analysis was performed using R-GOSeq package.
ChIP.
We cross-linked 10 × 106 cells with 1% formaldehyde and quenched them for 5 min in 0.125 M Glycine. After centrifugation, cell pellets were resuspended in lysis buffer (1% SDS, 50 mM Tris⋅HCl, pH 8, 10 mM EDTA). Sonication was performed with Bioruptor (Diagenode). Lysates from 5 × 106 cells were incubated with 5 μg of antibody overnight at 4 °C on rotation and then for 1 h with 40 μL of Dynabead-protein G (Life Technology). Beads were washed with TSE-150 (0.1% SDS, 1% Triton, 2 mM EDTA, 20 mM Tris, pH 8.1, 150 mM NaCl), TSE-500 (as TSE-150 with 500 mM NaCl), LiCl detergent (0.25 M LiCl, 1% Nonidet P-40, 1% Sodium Deoxycholate, 1 mM EDTA, 10 mM Tris, pH 8.1), and Tris-EDTA (5–1 mM). Elution was performed in SDS/NaHCO3 buffer twice for 20 min at 65 °C. Cross-linking was reversed with RNase and NaCl overnight at 65 °C. DNA fragments were purified using NucleoSpin (Macherey-Nagel) and diluted to 1/100 for input and to half for immunoprecipitated fractions. qPCRs were performed using 2 μL of DNA in duplicate, and enrichment was calculated relative to input or Histone H3 values. For ChIP experiments, we used the antibodies ERRα (GTX108166, Genetex), LSD1 (ab17721, Abcam), Histone H3 (ab1791, Abcam), H3K4me2 (07-030, Millipore), and H3K9me2 (39753, Activemotif).
Statistical Significance and Quantifications.
Statistical analyses were performed with a one-way ANOVA test. Protein expression levels were quantified with ImageJ software, and analysis revealed the ratio of the protein of interest relative to a housekeeping gene (actin, hsp90, or histone3 for chromatin mark).
Supplementary Material
Acknowledgments
This work was funded by Ligue contre le Cancer (comité Drôme, Puy de Dôme and Rhône) and JoRiss/ENS research programs. J.C. is funded by Association pour la Recherche sur le Cancer (ARC). L.Z. is funded by the Chinese Scolarship Council (CSC) and Région Rhône-Alpes.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=dfsrrmigeekoeby&acc=GSE49110 and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=sbyhmcgyhvefpof&acc=GSE58492 (accession nos. GSE49110 and GSE58492).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614664114/-/DCSupplemental.
References
- 1.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128(4):707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: A coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20(11):1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 4.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol Cell. 2007;25(1):1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Cloos PA, Christensen J, Agger K, Helin K. Erasing the methyl mark: Histone demethylases at the center of cellular differentiation and disease. Genes Dev. 2008;22(9):1115–1140. doi: 10.1101/gad.1652908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kampranis SC, Tsichlis PN. Histone demethylases and cancer. Adv Cancer Res. 2009;102:103–169. doi: 10.1016/S0065-230X(09)02004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim S, Metzger E, Schüle R, Kirfel J, Buettner R. Epigenetic regulation of cancer growth by histone demethylases. Int J Cancer. 2010;127(9):1991–1998. doi: 10.1002/ijc.25538. [DOI] [PubMed] [Google Scholar]
- 9.Greer EL, Shi Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13(5):343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Shi YJ, et al. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19(6):857–864. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138(4):660–672. doi: 10.1016/j.cell.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 13.Metzger E, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437(7057):436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 14.Metzger E, et al. Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature. 2010;464(7289):792–796. doi: 10.1038/nature08839. [DOI] [PubMed] [Google Scholar]
- 15.Cai C, et al. Lysine-specific demethylase 1 has dual functions as a major regulator of androgen receptor transcriptional activity. Cell Reports. 2014;9(5):1618–1627. doi: 10.1016/j.celrep.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benner C, et al. Decoding a signature-based model of transcription cofactor recruitment dictated by cardinal cis-regulatory elements in proximal promoter regions. PLoS Genet. 2013;9(11):e1003906. doi: 10.1371/journal.pgen.1003906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duteil D, et al. LSD1 promotes oxidative metabolism of white adipose tissue. Nat Commun. 2014;5:4093. doi: 10.1038/ncomms5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamo A, et al. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol. 2011;13(6):652–659. doi: 10.1038/ncb2246. [DOI] [PubMed] [Google Scholar]
- 19.Whyte WA, et al. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature. 2012;482(7384):221–225. doi: 10.1038/nature10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musri MM, et al. Histone demethylase LSD1 regulates adipogenesis. J Biol Chem. 2010;285(39):30034–30041. doi: 10.1074/jbc.M110.151209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahl P, et al. Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res. 2006;66(23):11341–11347. doi: 10.1158/0008-5472.CAN-06-1570. [DOI] [PubMed] [Google Scholar]
- 22.Schulte JH, et al. Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: Implications for therapy. Cancer Res. 2009;69(5):2065–2071. doi: 10.1158/0008-5472.CAN-08-1735. [DOI] [PubMed] [Google Scholar]
- 23.Lim S, et al. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis. 2010;31(3):512–520. doi: 10.1093/carcin/bgp324. [DOI] [PubMed] [Google Scholar]
- 24.Lin T, Ponn A, Hu X, Law BK, Lu J. Requirement of the histone demethylase LSD1 in Snai1-mediated transcriptional repression during epithelial-mesenchymal transition. Oncogene. 2010;29(35):4896–4904. doi: 10.1038/onc.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayami S, et al. Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. Int J Cancer. 2011;128(3):574–586. doi: 10.1002/ijc.25349. [DOI] [PubMed] [Google Scholar]
- 26.Laudet V, Gronemeyer H. The Nuclear Receptor Factbook. Academic; San Diego, CA: 2002. [Google Scholar]
- 27.Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Deconstructing repression: Evolving models of co-repressor action. Nat Rev Genet. 2010;11(2):109–123. doi: 10.1038/nrg2736. [DOI] [PubMed] [Google Scholar]
- 28.Kato S, Yokoyama A, Fujiki R. Nuclear receptor coregulators merge transcriptional coregulation with epigenetic regulation. Trends Biochem Sci. 2011;36(5):272–281. doi: 10.1016/j.tibs.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Giguère V. Orphan nuclear receptors: From gene to function. Endocr Rev. 1999;20(5):689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- 30.Horard B, Vanacker JM. Estrogen receptor-related receptors: Orphan receptors desperately seeking a ligand. J Mol Endocrinol. 2003;31(3):349–357. doi: 10.1677/jme.0.0310349. [DOI] [PubMed] [Google Scholar]
- 31.Giguère V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29(6):677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 32.Villena JA, Kralli A. ERRalpha: A metabolic function for the oldest orphan. Trends Endocrinol Metab. 2008;19(8):269–276. doi: 10.1016/j.tem.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charest-Marcotte A, et al. The homeobox protein Prox1 is a negative modulator of ERRα/PGC-1α bioenergetic functions. Genes Dev. 2010;24(6):537–542. doi: 10.1101/gad.1871610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez-Schindler J, et al. The corepressor NCoR1 antagonizes PGC-1α and estrogen-related receptor α in the regulation of skeletal muscle function and oxidative metabolism. Mol Cell Biol. 2012;32(24):4913–4924. doi: 10.1128/MCB.00877-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ariazi EA, Jordan VC. Estrogen-related receptors as emerging targets in cancer and metabolic disorders. Curr Top Med Chem. 2006;6(3):203–215. doi: 10.2174/1568026610606030203. [DOI] [PubMed] [Google Scholar]
- 36.Fradet A, et al. Dual function of ERRα in breast cancer and bone metastasis formation: Implication of VEGF and osteoprotegerin. Cancer Res. 2011;71(17):5728–5738. doi: 10.1158/0008-5472.CAN-11-1431. [DOI] [PubMed] [Google Scholar]
- 37.Dwyer MA, et al. WNT11 expression is induced by estrogen-related receptor alpha and beta-catenin and acts in an autocrine manner to increase cancer cell migration. Cancer Res. 2010;70(22):9298–9308. doi: 10.1158/0008-5472.CAN-10-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sailland J, et al. Estrogen-related receptor α decreases RHOA stability to induce orientated cell migration. Proc Natl Acad Sci USA. 2014;111(42):15108–15113. doi: 10.1073/pnas.1402094111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tribollet V, et al. miR-135a inhibits the invasion of cancer cells via suppression of ERRα. PLoS One. 2016;11(5):e0156445. doi: 10.1371/journal.pone.0156445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bianco S, Sailland J, Vanacker JM. ERRs and cancers: Effects on metabolism and on proliferation and migration capacities. J Steroid Biochem Mol Biol. 2012;130(3-5):180–185. doi: 10.1016/j.jsbmb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Deblois G, Giguère V. Oestrogen-related receptors in breast cancer: Control of cellular metabolism and beyond. Nat Rev Cancer. 2013;13(1):27–36. doi: 10.1038/nrc3396. [DOI] [PubMed] [Google Scholar]
- 42.Chaveroux C, et al. Molecular and genetic crosstalks between mTOR and ERRα are key determinants of rapamycin-induced nonalcoholic fatty liver. Cell Metab. 2013;17(4):586–598. doi: 10.1016/j.cmet.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari-Amorotti G, et al. Inhibiting interactions of lysine demethylase LSD1 with snail/slug blocks cancer cell invasion. Cancer Res. 2013;73(1):235–245. doi: 10.1158/0008-5472.CAN-12-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao G, et al. Lysine-specific demethylase 1 mediates epidermal growth factor signaling to promote cell migration in ovarian cancer cells. Sci Rep. 2015;5:15344. doi: 10.1038/srep15344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Bassets I, et al. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell. 2007;128(3):505–518. doi: 10.1016/j.cell.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wissmann M, et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9(3):347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- 48.Perillo B, et al. DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science. 2008;319(5860):202–206. doi: 10.1126/science.1147674. [DOI] [PubMed] [Google Scholar]
- 49.Metzger E, et al. Phosphorylation of histone H3 at threonine 11 establishes a novel chromatin mark for transcriptional regulation. Nat Cell Biol. 2008;10(1):53–60. doi: 10.1038/ncb1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nair SS, et al. PELP1 is a reader of histone H3 methylation that facilitates oestrogen receptor-alpha target gene activation by regulating lysine demethylase 1 specificity. EMBO Rep. 2010;11(6):438–444. doi: 10.1038/embor.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y, et al. Crystal structure of human histone lysine-specific demethylase 1 (LSD1) Proc Natl Acad Sci USA. 2006;103(38):13956–13961. doi: 10.1073/pnas.0606381103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serce N, et al. Elevated expression of LSD1 (Lysine-specific demethylase 1) during tumour progression from pre-invasive to invasive ductal carcinoma of the breast. BMC Clin Pathol. 2012;12:13. doi: 10.1186/1472-6890-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derr RS, et al. High nuclear expression levels of histone-modifying enzymes LSD1, HDAC2 and SIRT1 in tumor cells correlate with decreased survival and increased relapse in breast cancer patients. BMC Cancer. 2014;14:604. doi: 10.1186/1471-2407-14-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagasawa S, et al. LSD1 overexpression is associated with poor prognosis in basal-like breast cancer, and sensitivity to PARP inhibition. PLoS One. 2015;10(2):e0118002. doi: 10.1371/journal.pone.0118002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanacker JM, et al. Transcriptional activities of the orphan nuclear receptor ERR alpha (estrogen receptor-related receptor-alpha) Mol Endocrinol. 1999;13(5):764–773. doi: 10.1210/mend.13.5.0281. [DOI] [PubMed] [Google Scholar]
- 56.Petoukhov MV, et al. Reconstruction of quaternary structure from X-ray scattering by equilibrium mixtures of biological macromolecules. Biochemistry. 2013;52(39):6844–6855. doi: 10.1021/bi400731u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.