The article by Medlock et al. (1) reminds us that HIV infection is still the epidemic of our times. Currently, 36.7 million persons are living with HIV, and annual deaths from HIV are still upward of 1 million persons per year (aidsinfo.unaids.org). Because a wide variety of studies have demonstrated enhanced survival and quality of life with the early detection of HIV and the timely initiation of antiretroviral therapy (ART) (2, 3), much of this continuing global morbidity and mortality is due to the inability of our health care systems to scale up these interventions. Among countries that have steadily increased access to antiretrovirals, we have at last seen a “bending of the curve,” as life expectancy increases in adults and HIV infections are averted in infants (Fig. 1A). The reversal of mortality rates from the early years of the epidemic when ART was not readily available has given both hope and impetus to try and “end AIDS.” In 2014, the Joint United Nations Program on HIV and AIDS (UNAIDS) outlined a three-part program entitled “90-90-90” as a commitment to improve access to ART as a life-saving intervention, a transmission prevention measure, and a human rights endeavor (4). These targets include (i) successfully diagnosing 90% of all HIV-positive people, (ii) delivering ART to 90% of those people diagnosed, and (iii) achieving viral suppression for 90% of those people on treatment. The goal of this program is to markedly reduce the global morbidity and mortality due to HIV-related causes by 2020 as a first step in the global control of the epidemic.
Fig. 1.
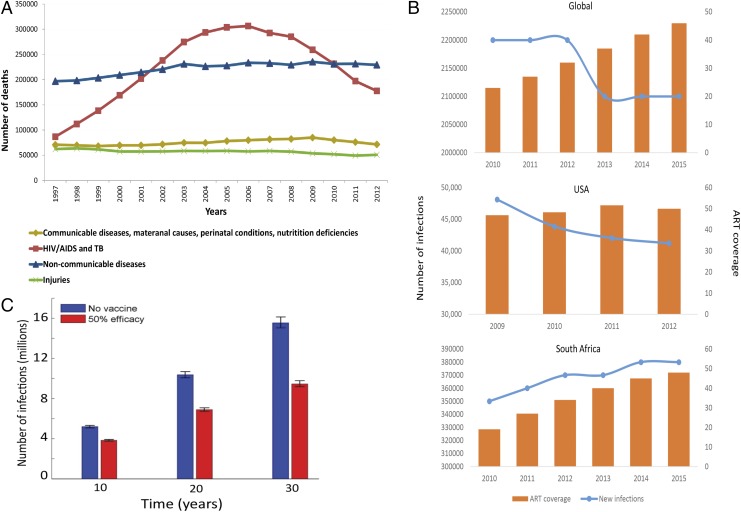
(A) Mortality rates in South Africa 1997–2012. HIV-related deaths dropped from 300,000 in 2006 to 153,000 in 2012 coincident with large-scale roll-out of ART (14). (B) Association between new HIV acquisitions and ART coverage. The number of new infections each year (left y axis) is depicted by the blue line. ART coverage (right y axis), depicted by the orange bars, is defined as the number of people living with HIV divided by the number of people receiving ART. A global graph (Top; aidsinfo.unaids.org), graph of the United States (Middle; ref. 15), and graph of South Africa (Bottom; aidsinfo.unaids.org) are shown. (C) Projected number of HIV infections over 10, 20, and 30 y in South Africa. Adapted with permission from ref. 11.
Although there is enormous rationale for the UNAIDS program, the logistics of reaching the goals are significant. One important reality check for the 90-90-90 program is the fragmented health care systems, inadequate human resources, and lack of sustainable funding for ART in almost all of the most affected countries with extensive HIV epidemics, making the goals outlined by the UNAIDS for 2020 mostly aspirational. Currently, only Sweden has achieved the UNAIDS three-part target of the 90-90-90 program (5). A recent study evaluating the treatment cascades of 69 countries showed that for target 1, The Netherlands had achieved 87% of the 90% target set, and for targets 2 and 3, Switzerland, at 71%, had the highest rate of people on treatment, but of these people, only 68% had achieved viral suppression (6), demonstrating the enormous difficulties in achieving these targets even in high-income countries. In contrast, where the burden of disease is much greater, such as in eastern and southern Africa, only 56% of HIV-infected persons know their status, with only 54% of these persons on treatment and only 45% of these persons virally suppressed (7). Although 87% of HIV-infected persons are estimated to know their status in the United States, only 52% of HIV-infected persons are on treatment, with 82% having full viral suppression, illustrating the difficulty of maintaining not only early diagnosis but the sustained medical and social efforts required to achieve optimal therapeutic results (1).
A key concept in the HIV model used by Medlock et al. (1) is that the reduction in viremia achieved by ART translates into reduced infectiousness that decreases HIV transmission to sexual partners (secondary prevention). Although this concept has been demonstrated among discordant couples in a randomized control study [HIV Prevention Trials Network 052] (8), the translation of this finding to reduce transmission on a population basis is much less apparent. Despite an ever-increasing number of people on ART, now numbering well over 17 million persons globally, we are only making a substantial dent in the prevention of transmission of HIV from mother to child. ART has been successful for prevention of mother-to-child transmission because exposure to HIV is defined for a relatively short time period from birth through the months of breast feeding, the health care system has enabled the integration of this intervention into routine antenatal care and primary health care, and maternal and health care worker motivation drives fidelity to the intervention. The detailed analyses by Medlock et al. (1) of the current and projected epidemics in nearly all 127 countries affected by HIV indicate that there are substantial differences in population effects between secondary versus primary prevention, and bending the acquisition curve requires more than saturating people with ART. The logistics of identifying 90% of persons with HIV infection in a country, especially before they are out of the high-risk transmission period of their infection, are likely the explanation for the inability of our current programs to achieve the substantial reductions in HIV acquisition required to control the HIV epidemic. In our opinion, the likelihood of altering this observation over time is not high, due to the almost invariable subclinical acquisition and transmission of HIV. These observations indicate that the quest to end this epidemic must concentrate on developing biological interventions to prevent the acquisition of HIV and reduce the number of persons living with HIV. This reduction in HIV-1 acquisition is where the major frontier of HIV innovation must concentrate to reduce the number of persons with HIV infection, as well as the long-term medical and economic burden of the pandemic. This is the second message from the article by Medlock et al. (1): that a biomedical intervention to prevent the acquisition of HIV itself, like an HIV vaccine, is critical to augment the effects of ART on the HIV epidemic.
Globally, there are still 2 million new HIV infections per year, despite the rapid scale-up of ART (Fig. 1B). One of the most staggering projections of Medlock et al. (1) is the estimate that with the current rate of ART initiation, 49 million more new HIV infections will occur by 2035. Importantly, in hyperendemic regions, such as KwaZulu-Natal in South Africa, there has been no reduction in the incidence rate of HIV in young women in the past decade despite the biggest ART program in the world rolled out by the South African Government (9), indicating that the correlation between improving survival and reducing HIV incidence is far from a straight-line association (Fig. 1B, Bottom). If the 90-90-90 level is reached globally, ∼22 of the projected 49 million new infections might be averted, and an additional 3.3 million might be averted if the 95-95-95 goal (an increase in the three targets) is achieved. In contrast, over 17 million new infections are averted with a 50% effective HIV vaccine.
The model of Medlock et al. (1) illustrates that among countries with increasing per capita incidence of HIV, such as South Africa, achieving the UN targets reduces the number of persons living with HIV infection. However, reduction is incomplete, and in absolute lives saved, the addition of a partially effective vaccine is substantive. For example, a detailed mathematical model based upon an ongoing vaccine trial in South Africa called HIV Vaccine Trials Network (HVTN) 702 indicates that a substantial number of new cases of HIV can be averted in the first decade if this vaccine achieves its 50% vaccine efficacy and if its initial implementation is directed at targeting young women.* Other models evaluating the impact of a partially efficacious vaccine implemented in South Africa predict a 52% reduction in mean HIV-1 incidence (10, 11) (Fig. 1C). Conversely, among countries where per capita incidence of HIV is already declining, such as the United States and India, emphasis on secondary prevention through ART provides high benefit for improving survival but little benefit in reducing the incidence of infection. Globally, the message is that combining vaccination with effective population-based antiretroviral coverage is synergistic and that the synergy is predicted to be most substantial where UN targets are insufficient to reverse the epidemiological trajectory of infection, such as most countries in sub-Saharan Africa.
These data clearly show that the unsolved biomedical research question for the global HIV epidemic is how to develop an effective system of primary HIV prevention. How do we develop effective population-based strategies to reduce acquisition of HIV infection over the entire lifetime of persons at risk, or if long-term durability is not achievable, how do we maximize preventative measures over the years of maximal/highest risk for the 15- to 30-y-old women, as well as men who have sex with men (MSM) or those recent users of i.v. drugs (IDUs)? In South Africa, data suggest that close to 25% of all new infections occur in women aged 15–24 y, emphasizing this group as a major driver of the epidemic and highlighting the value of targeted approaches to populations that are most vulnerable to HIV acquisition.
Both test and treat interventions and vaccines of partial efficacy leave holes in our ability to contain the epidemic. Inadequate levels of diagnosis and viral suppression result in low rates of prevention, as does a partially effective vaccine of less than prolonged durability. Both approaches achieve the best effect when used together. It is likely the initial biomedical interventions for reducing HIV acquisition will not have lifetime durability; hence, strategies to maximize their impact need to be devised. One approach is to direct the implementation of both primary and secondary prevention programs to populations that are vulnerable to HIV acquisition.
We would recommend that more attention be directed to evaluating the impact of a targeted approach to HIV vaccine roll-out to evaluate saturating certain key populations, such as female sex workers, MSM, IDUs, prisoners, or young women in highly endemic areas. A more immediate impact of both vaccination and test and treat strategies may be to concentrate the program and resources on key populations compared with a traditional mass scale-up of vaccination on a population basis. This directed approach is likely to induce more rapid inflections in both cost and reductions in HIV. A recent modeling exercise indicated that HIV vaccination of adolescents in South Africa reduced both mortality and HIV incidence (12), as well as demonstrating cost efficacy. National implementation of this targeted intervention was estimated to result in a cumulative gain of 23.6 million years of life among adolescents aged 10–19 y.
Although vaccination has been the technology most discussed in reducing HIV-1, prevention using both long-acting injectable antivirals as well as the direct administration of monoclonal antibodies with broadly neutralizing activity to the globally diverse array of HIV strains is under active investigation. These injectable approaches are currently being evaluated in proof-of-concept efficacy trials and may compete favorably with a vaccine in their ability to prevent HIV infection. There have been major advances in the technology to isolate highly potent, broadly neutralizing antibodies to HIV: modify them so that they are manufacturable in high quantity as well as possessing a pharmokinetic profile that allows subcutaneous injections that may only have to be given at 4- to 6-mo intervals. The levels of neutralizing activity are, to date, far greater than the neutralizing titers seen with any candidate HIV vaccine currently in development (13). This approach of antibody-mediated prevention may emerge as a major technology for primary prevention, one that is likely to be more costly than a traditional vaccine but may be a highly successful tool for the highest risk period for high-risk populations and, as such, a useful and cost-effective strategy until a highly effective vaccine is developed.
The article by Medlock et al. (1) is a stark reminder that the silent epidemic of HIV continues unabated throughout the world and that the scientific community must continue to push forward with the development of new technologies to prevent HIV acquisition. The gains from ART are remarkable, and have led to increased life expectancy, reduced morbidity, and a reduction in the number of children infected with HIV. However, the gain that ART may have in reducing infectiousness, as well as its impact on the onward transmission of HIV, has not yet been realized and is at best only partial. It is evident that we cannot treat ourselves out of this epidemic and that the investment in biomedical interventions like HIV vaccines is critical to turning the tide on HIV, particularly in high-burden regions. Early introduction of HIV prevention strategies should be directed at specific populations with the highest HIV incidence. In southern and eastern Africa, it is clear that young women should be the beneficiaries of such an intervention. We also recommend that should any HIV vaccine be found to be efficacious, irrespective of durability or level of effectiveness, bridging studies be rapidly conducted to evaluate correlates of protection in other populations with high HIV incidences, such as young MSM and IDUs, to maximize the impact of HIV vaccination. It is clear an ounce of prevention is worth more than a pound of therapy, and that preventing HIV through an effective durable vaccine or other biomedical prevention strategies, such as the administration of long-acting monoclonal antibodies or antivirals, is the only path toward an AIDS-free generation.
Acknowledgments
We appreciate the editorial and technical assistance of Mindy Miner, PhD, who provided invaluable insight in both the construction of the article and its figure. We are funded by NIH/National Institute of Allergy and Infectious Diseases Grant 5UM1AI068614-11.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4017.
*Selinger C, et al. (2017) Conference on Retroviruses and Opportunistic Infections (CROI 2017), February 13–17, 2017, Seattle, WA (abstr 1036).
References
- 1.Medlock J, et al. Effectiveness of UNAIDS targets and HIV vaccination across 127 countries. Proc Natl Acad Sci USA. 2017;114:4017–4022. doi: 10.1073/pnas.1620788114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundgren JD, et al. INSIGHT START Study Group Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danel C, et al. TEMPRANO ANRS 12136 Study Group A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS 2014 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. Available at www.unaids.org/en/resources/documents/2014/90-90-90. Accessed March 10, 2017.
- 5.Gisslén M, et al. Sweden, the first country to achieve the Joint United Nations Programme on HIV/AIDS (UNAIDS)/World Health Organization (WHO) 90-90-90 continuum of HIV care targets. HIV Med. 2017;18:305–307. doi: 10.1111/hiv.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levi J, et al. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Global Health. 2016;1:e000010. doi: 10.1136/bmjgh-2015-000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNAIDS 2016 Global AIDS Update 2016. Available at www.who.int/hiv/pub/arv/global-aids-update-2016-pub/en/. Accessed March 10, 2017.
- 8.Cohen MS, et al. HPTN 052 Study Team Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moorehouse M. Closer to zero: Reflections on ten years of ART rollout. South Afr J HIV Med. 2014;15:9. [Google Scholar]
- 10.Phillips AN, et al. Potential future impact of a partially effective HIV vaccine in a southern African setting. PLoS One. 2014;9:e107214. doi: 10.1371/journal.pone.0107214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitrov D, Kublin JG, Ramsey S, Corey L. Are clade specific HIV vaccines a necessity? An analysis based on mathematical models. EBioMedicine. 2015;2:2062–2069. doi: 10.1016/j.ebiom.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moodley N, Gray G, Bertram M. Projected economic evaluation of the national implementation of a hypothetical HIV vaccination program among adolescents in South Africa, 2012. BMC Public Health. 2016;16:330. doi: 10.1186/s12889-016-2959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledgerwood JE, et al. VRC 602 Study Team Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol. 2015;182:289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Msemburi W, et al. Second National Burden of Disease Study for South Africa: Cause of Death Profile for South Africa, 1997–2012. South African Medical Research Council; Cape Town, South Africa: 2016. [Google Scholar]
- 15. Centers for Disease Control and Prevention (2015) Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2013. HIV Surveillance Supplemental Report 20(2). Available at https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html/. Accessed March 10, 2017.